Abstract
Background: Tolvaptan can promote water clearance without a deterioration of serum electrolytes in HF patients, but its efficacy and safety were unclear. We performed a meta-analysis of randomized controlled trials (RCTs) to investigate the efficacy and safety of tolvaptan in the treatment of patients hospitalized for heart failure (HF). Methods: In Oct 2014, a literature search was started and found all studies conducted from 2000 to 2014. We systematically searched the literature through the MEDLINE database and EMBASE database. Quality assessments were evaluated with Jadad quality scale. Data were extracted considering the characteristics of efficacy and safety designs. Result: Eight RCTs enrolling 13453 participants satisfying the inclusion criteria were finally analyzed. There were significant decreases of body weight (MD=-0.87, 95% CI=-0.94 to -0.80, P<0.001) among all subgroups. Significant increase of serum sodium was found between tolvaptan and placebo groups at day 1 (MD=2.93, 95% CI=2.70 to 3.16, P<0.001) and at day 7 or discharge (MD=3.10, 95% CI=2.78 to 3.42, P<0.001). There were significant differences between the day 1 subgroup and day 7 or discharge subgroup (MD=2.99, 95% CI=2.80 to 3.18, P<0.001). A statistical significant improve in dyspnea (RR=1.10, 95% CI=1.07 to 1.13, P<0.001) and edema (RR=1.05, 95% CI=1.02 to 1.08, P<0.001) occurred, whereas there was no difference in rales (RR=2.38, 95% CI=0.89 to 6.38, P=0.08) and pulmonary congestion (RR=1.02, 95% CI=0.71 to 1.45, P=0.93). Pooled effect measure in the outcome of common adverse event (RR=1.08, 95% CI=0.99 to 1.18, P=0.08) and serious adverse (RR=0.96, 95% CI=0.88 to 1.04, P=0.29) event both show no significant occurrence. Conclusion: Tolvaptan decreases body weight, increases serum sodium, and improves congestion without significant increasing adverse events in HF patients.
Keywords: Heart failure, meta-analysis, tolvaptan
Introduction
Heart failure (HF) is one of the most important causes of morbidity and mortality in the world [1]. Approximately 5.3 million men and women (2.5% of the adult American population) were suffered from HF in the United State [2]. Various types of therapeutic agents are advanced for HF; unfortunately, the number of annual hospitalizations for HF and the rate of annual mortality among HF remain high in the worldwide [3].
Arginine vasopressin (AVP) is secreted from the posterior pituitary in response to elevation in plasma osmolality and decreases in arterial pressure in patients with HF and left ventricular systolic dysfunction [4]. Elevated AVP plasma concentrations could activate the V2 receptor that is expressed on the basolateral membranes of the renal collecting duct principal cells, increase the expression of AQP channels, and cause fluid overload and hyponatremia [5,6]. Fluid overload has detrimental effect in patients with HF, as a result of increasing congestion, while hyponatremia is associated with increased poor outcomes. Loop and thiazide diuretics are widely used as the standard therapy for improving symptoms associated with volume overload in HF but could lead to several adverse clinical effects, including electrolyte imbalance (hyponatremia, hypokalemia), neurohormonal activation, renal dysfunction, and possibly increased mortality [7-10]. Existence of an unmet need for more efficacy and safety strategies, therefore, HF represents a therapeutic challenge for health care providers now.
Tolvaptan is an oral, once-daily, non-peptide, selective antagonist of the vasopressin V2 receptor whose action on the renal collecting ducts inhibits vasopressin-mediated water reabsorption [11,12]. Accordingly, tolvaptan is successfully to promote an increase in free water clearance without a deterioration of serum electrolytes in HF patients in who poorly respond to conventional diuretics or who are susceptible to decreasing of plasma electrolyte concentrations. Currently, a large number of short or long trials have been reported that this compound can decrease fluid and increase sodium levels [13-16]; however, its efficacy and safety were mixed. Here we conducted a meta-analysis of randomized controlled trials (RCTs) of the efficacy and safety of the AVP antagonist tolvaptan on the treatment of HF, which would provide clinicians with new strategy of HF pharmacological therapeutics.
Methods
Search strategy
A meta-analysis of the available published researches about intervention studies of vasopressin antagonist Tolvaptan on HF was performed. In Oct 2014, a literature search was conducted at Department of Cardiology, the First College of Clinical Medical Sciences, Institute of Cardiovascular Diseases, China. Three Gorges University. Articles were preselected, which had been published between 2000 and 2014. To identify all prospective RCTs of Tolvaptan in the treatment of heart failure in patients with symptomatic HF, we systematically searched the literature through the MEDLINE database and EMBASE database. We also searched clinicaltrals.gov to make sure there is no bias caused by the unpublished data. Searches of MEDLINE database and EMBASE database included terms “heart failure” and “tolvaptan”, which are based on English only. Ethical approval was obtained from the Scientific Research Committee of the Three Gorges University.
Inclusion and exclusion criteria
The inclusion criteria are as follows: 1) study design: double-blinded randomized controlled trial, 2) type of participants: patients with symptomatic HF, 3) intervention: vasopressin antagonist tolvaptan, 4) comparator: placebo, 5) outcomes: the changes of serum sodium, the changes of weight, the improvement of congestive symptoms, adverse events. We excluded trails that did not report any of the out comes mentioned above. The identifying of titles and abstracts and extracting of data were independently screened by two reviewers. Corresponding author is responsible for the potential of disagreement and discordance between the two reviewers.
Quality assessment and data abstraction
Quality assessments were evaluated with Jadad quality scale (a numerical score between 0 and 7, with 0 being the weakest and 7 being the strongest), including the following: 1) Random sequence generation 2) Allocation concealment 3) Double blinding 4) Description of withdrawals and drop-out. Extracting of data from each study is following: patients’ number, tolvatan dose, means age, baseline medication, inclusion criteria, follow-up and primary endpoint.
Statistical synthesis
All statistical analyses were performed using RevMan 5.0 that is provided by The Cochrane Collaboration. The effect measure of risk ratio (RR) with 95% confidence interval (CI) and the statistical method of Mantel-Haenszel were used for dichotomous data, while standardized mean differences (MD) and Inverse Variance for continuous data. We used the fixed analysis model to calculate pooled analyses initially, but if there was evidence of significant heterogeneity, the random analysis model would be replaced. Heterogeneity was assessed using the chi-square statistic and a P value of less than 0. 05 were considered to represent significant heterogeneity between trails. The statistic strength was identified by overall effect size Z and heterogeneity index I2. Additionally, Sensitivity analyses that were conducted to determine the stability of the overall effects were performed by the random effects model. Furthermore, we utilized Begg’s funnel plots to examine the potential publication bias. All the statistical significance was set at 0.05.
Result
Search results and study characteristics
There were 221 relevant reports identified by the search, 19 full text articles were retrieved for detailed evaluation. In total, 8 RCTs [17-24] enrolling 13453 participants satisfying the inclusion criteria were finally analyzed. Figure 1 shows the flow diagram of the study selection process in the Meta-Analysis. Characteristics of the Clinical Trials Included are shown in Table 1. According to the Jadad quality scale, all trials included are of high quality which is shown in Table 2.
Figure 1.
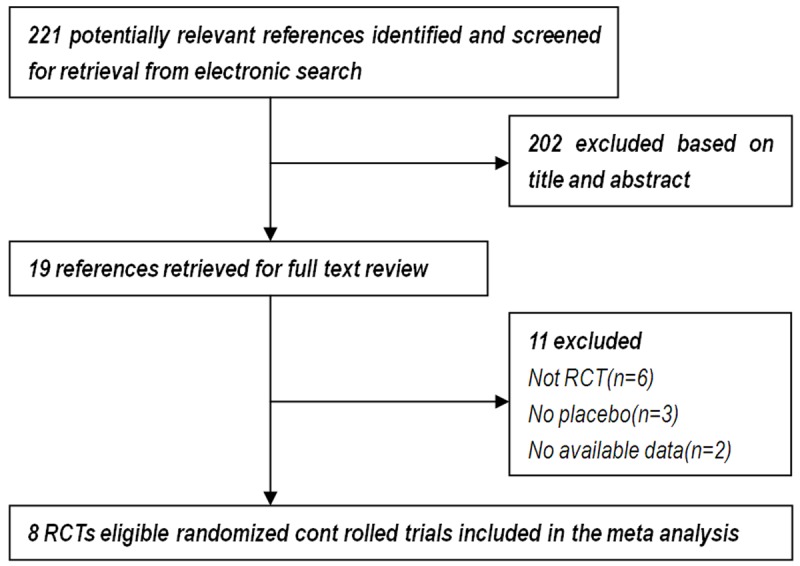
Flow diagram of the study selection process in the Meta-Analysis.
Table 1.
Characteristics of the Clinical Trials Included in the Meta-analysis
| Trials | Patients Number | Tolvaptan dose | Mean age | Baseline medication | Inclusion criteria | Follow-up | Primary endpoint |
|---|---|---|---|---|---|---|---|
| Gheorghiade 2003 [17] | 254 | 30 mg/d | 67.6±10.9 | ACEI/ARB, digoxin, β-blockers, hydralazine/nitrates, furosemide | Irrespective of LVEF, signs of volume overload, (rales, JVD, edema) | 25 days | Body weigh changes, adverse event |
| 45 mg/d | 65.6±13.1 | ||||||
| 60 mg/d | 68.5±13.6 | ||||||
| 65.1±12.9 (placebo) | |||||||
| Gheorghiade 2004 [18] | 319 | 30 mg/d | 62.0±14.0 | ACEI/ARB, digoxin, β-blockers, diuretics, calcium channe blockers, furosemide, intravenous inotropes | LVEF≤40%, systemic congestion evidences (rales, JVD, edema), NHYA classes III to IV | 60 days | Body weigh changes, adverse event |
| 60 mg/d | 62.0±13.0 | ||||||
| 90 mg/d | 62.0±14.0 | ||||||
| 60.0±14.0 (placebo) | |||||||
| Gheorghiade 2007 [19] | TralA 2048 | 30 mg/d | 65.8±11.7 | ACEI/ARB, digoxin, β-blockers, hydralazine/nitrates, furosemide, diuretics, aldosterone-blocking agent, calcium channe blockers, nesirtide, intravenous inotropes | LVEF≤40%, signs of congestion (dyspnea, JVD, edema) | NG | Global clinical status changes |
| 65.6±11.9 (placebo) | |||||||
| TralB 2085 | 30 mg/d | 66.0±11.7 | |||||
| 65.6±12.2 (placebo) | |||||||
| Konstam 2007 [20] | 4133 | 30 mg/d | 65.9±11.7 | ACEI/ARB, β-blockers, diuretics, aldosterone-blockers | LVEF≤40%, signs of volume expansion, NHYA classes III to IV | 9.9 months | All-cause mortality and cardiovascular death or hospitaliz- -ation heat failure |
| 65.6±12.0 (placebo) | |||||||
| Udelson 2007 [21] | 240 | 30 mg/d | 65.0±12.0 | ACEI/ARB, β-blockers, diuretics, aldosterone-blockers | LVEF≤30%, NHYA classes II to III | 1 year | Symptom changes |
| 63.0±12.0(placebo) | |||||||
| Matsuzaki 2011 [22] | 117 | 15 mg/d | 66.9±9.60 | Furosemide | Signs of volume overload, (edema, JVD, hepatomegaly, et al.) | NG | Body weigh changes |
| 30 mg/d | 66.4±12.5 | ||||||
| 45 mg/d | 62.6±12.5 | ||||||
| 67.8±9.6 (placebo) | |||||||
| Matsuzaki 2011 [23] | 124 | 15 mg/d | 71.3±10.6 | Loop diuretic/ thiazide diuretic/ Furosemide, aldosterone-blockers | Signs of volume overload, (edema, JVD, pulmonary congestion,) | 27 days | Body weigh changes |
| 71.0±10.9 (placebo) | |||||||
| Hauptamn 2013 [24] | 4133 | 30 mg/d | 64.5±13.8 | ACEI, β-blockers, diuretics | LVEF≤40%, signs of fluid overload | NG | Body weigh changes, Serum sodium changes |
| 63.6±13.7 (placebo) |
D=day; ACEI=Angiotensin-Converting Enzyme Inhibitors; ARB=Angiotensin receptor blocker; LVEF=left ventricular ejection fraction; JVD=jugular venous distention; NHYA=New York Heart Association classification; NG=Not Given.
Table 2.
Summary of the quality evaluation by Jadad scale of clinical trials of tolvaptan in patients with heart failure
| Trials | Random sequence generation | Allocation concealment | Double blinding | Description of withdrawals and drop-out | Score |
|---|---|---|---|---|---|
| Gheorghiade 2003 [17] | 2 | 2 | 1 | 1 | 7 |
| Gheorghiade 2004 [18] | 2 | 2 | 1 | 1 | 7 |
| Gheorghiade 2007 [19] | 2 | 2 | 1 | 1 | 7 |
| Konstam 2007 [20] | 2 | 1 | 1 | 1 | 6 |
| Udelson 2007 [21] | 2 | 2 | 1 | 1 | 7 |
| Matsuzaki 2011 [22] | 2 | 2 | 1 | 1 | 7 |
| Matsuzaki 2011 [23] | 2 | 2 | 1 | 1 | 7 |
| Hauptamn 2013 [24] | 2 | 1 | 1 | 1 | 6 |
Changes of body weight
There were significant decreases in the change of body weight in each dose of tolvaptan (15 mg: MD=-1.09, 95% CI=-1.50 to -0.68, P<0.001; 30 mg: MD=-0.82, 95% CI=-0.89 to -0.74, P<0.001; 45 mg: MD=-1.29, 95% CI=-1.52 to -1.05, P<0.001; Figure 2A). The overall effect among subgroups show a statistical significance (MD=-0.87, 95% CI=-0.94 to -0.80, P<0.001; Figure 2A), but there was no distinct dose dependence.
Figure 2.
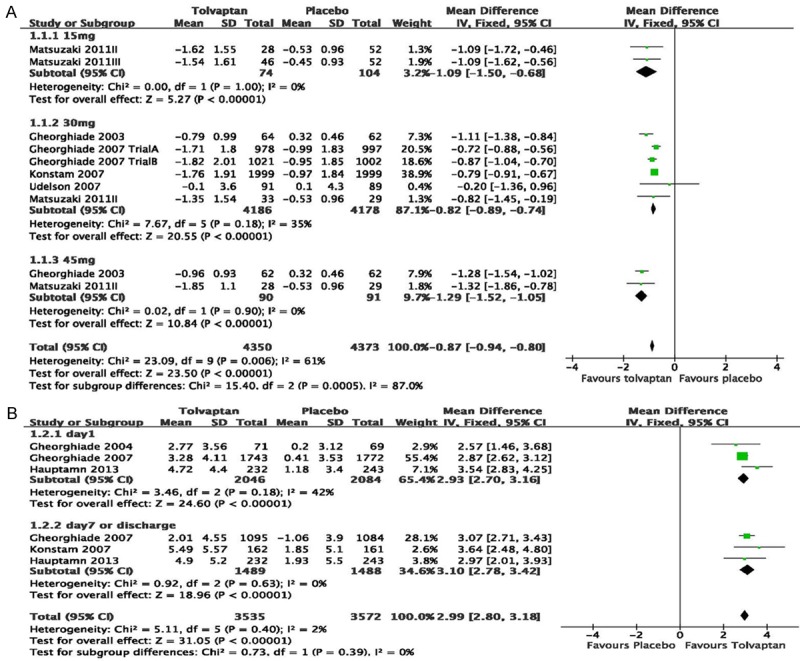
Pooled mean difference in the changes of body weight (A) and serum sodium (B) in the studies considering tolvaptan compared to placebo therapy in HF patients. There were significant decreases in the change of body weight in each dose of tolvaptan (15 mg: MD=-1.09, 95% CI=-1.50 to -0.68, P<0.001; 30 mg: MD=-0.82, 95% CI=-0.89 to -0.74, P<0.001; 45 mg: MD=-1.29, 95% CI=-1.52 to -1.05, P<0.001). The overall effect showed a statistical significance (MD=-0.87, 95% CI=-0.94 to -0.80, P<0.001), but there was no dose dependence. Significant increase was found between tolvaptan and placebo groups at day 1 (MD=2.93, 95% CI=2.70 to 3.16, P<0.001) and at day 7 or discharge (MD=3.10, 95% CI=2.78 to 3.42, P<0.001). The overall effect showed a statistical significance (MD=2.99, 95% CI=2.80 to 3.18, P<0.001).
Change of serum sodium
For the change of serum sodium, significant increase was found between tolvaptan and placebo groups at day 1 (MD=2.93, 95% CI=2.70 to 3.16, P<0.001; Figure 2B) and at day 7 or discharge (MD=3.10, 95% CI=2.78 to 3.42, P<0.001; Figure 2B). There were significant differences between the two subgroups (MD=2.99, 95% CI=2.80 to 3.18, P<0.001; Figure 2B).
Improvement of congestive symptoms
The improvement of congestive symptoms that we conducted a meta-analysis included dyspnea, edema, pulmonary rales, and pulmonary congestion. The results indicated a statistical significance in the improvement of dyspnea (RR=1.10, 95% CI=1.07 to 1.13, P<0.001; Figure 3A) and edema (RR=1.05, 95% CI=1.02 to 1.08, P<0.001; Figure 3B), whereas there were no significant differences in the improvement of pulmonary rales (RR=2.38, 95% CI=0.89 to 6.38,P=0.08; Figure 3C), pulmonary congestion (RR=1.02, 95% CI=0.71 to 1.45, P=0.93; Figure 3D).
Figure 3.
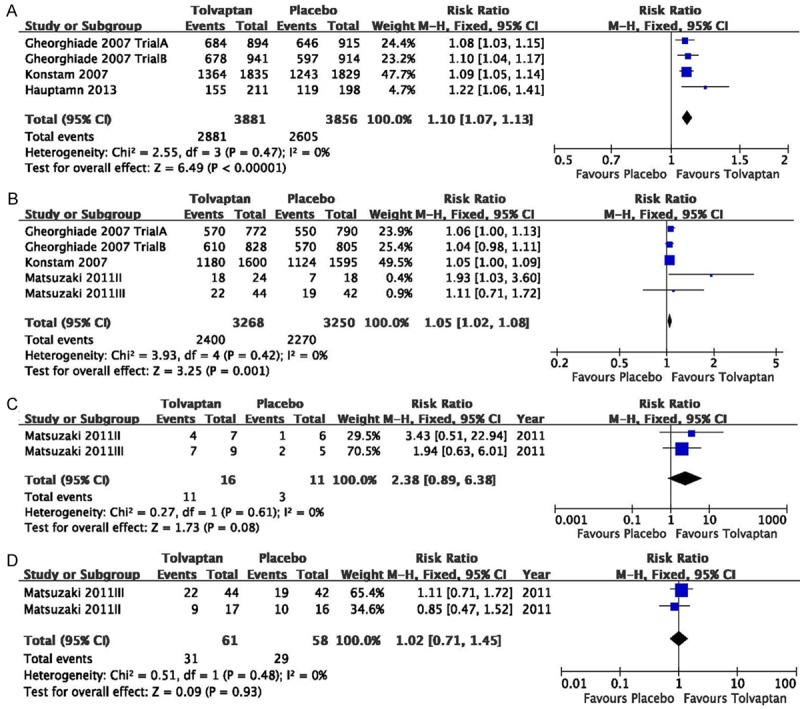
Pooled relative risk in the improvement of dyspnea (A), edema (B), pulmonary rales (C), pulmonary congestion (D) in the studies considering tolvaptan compared to placebo therapy in HF patients. The results indicated a statistical significance in the improvement of dyspnea (RR=1.10, 95% CI=1.07 to 1.13, P<0.001) and edema (RR=1.05, 95% CI=1.02 to 1.08, P<0.001), whereas there were no significant differences in the improvement of pulmonary rales (RR=2.38, 95% CI=0.89 to 6.38, P=0.08) and pulmonary congestion (RR=1.02, 95% CI=0.71 to 1.45, P=0.93).
Adverse events
Pooled effect measure in the outcome of common adverse event, such as, dizziness, dry mouth, thirst (RR=1.08, 95% CI=0.99 to 1.18, P=0.08; Figure 4A) and serious adverse, such as, death, HF worsening, stroke (RR=0.96, 95% CI=0.88 to 1.04, P=0.29; Figure 4B) event both show no significant occurrence. A meta-analysis of the common adverse event, including dizziness, dry mouth, thirst, urinary frequency was performed. A significant occurrences were found in dry mouth (RR=5.89, 95% CI=3.41 to 10.17, P<0.001; Figure 4B), thirst (RR=6.79, 95% CI=5.24 to 8.81, P<0.001; Figure 4C), urinary frequency (RR=4.29, 95% CI=2.59 to 7.10, P<0.001; Figure 4D), but dizziness (RR=1.07, 95% CI=0.90 to 1.28, P=0.46; Figure 4A).
Figure 4.
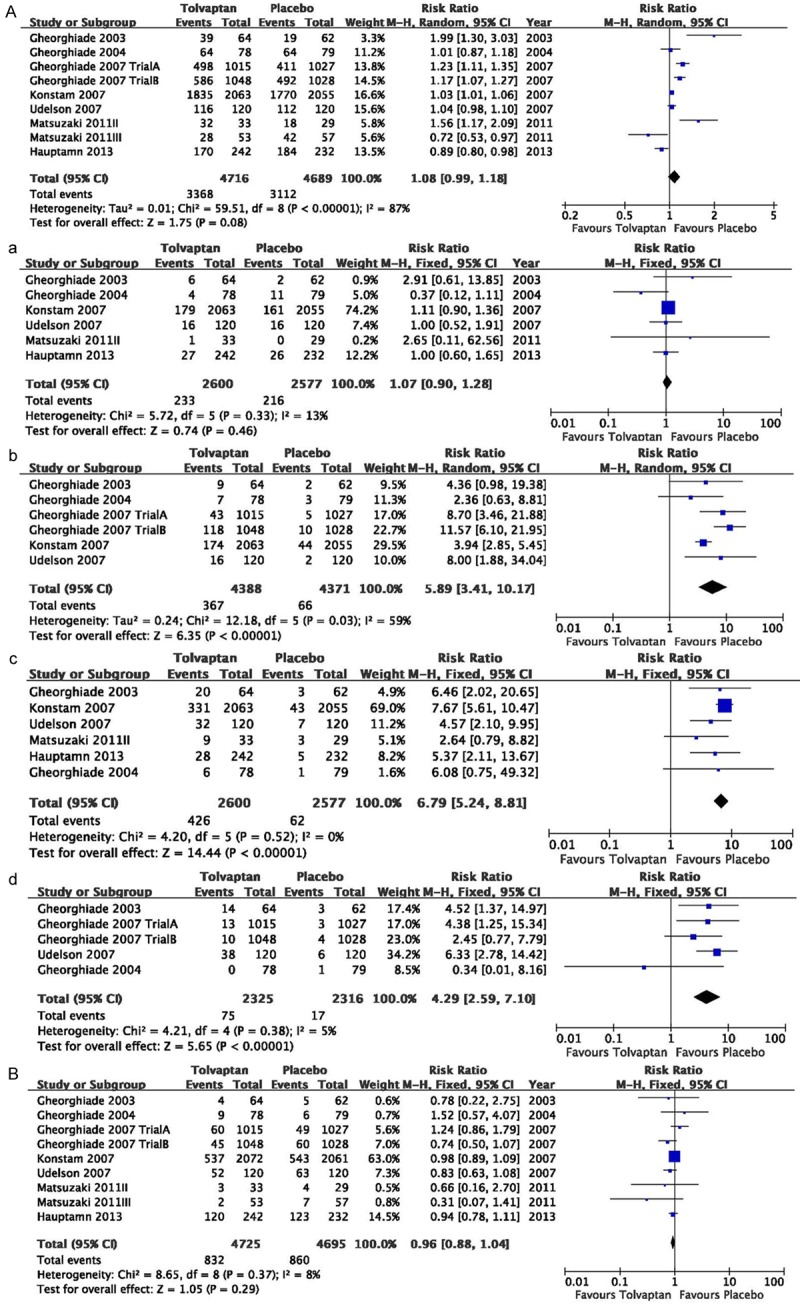
Pooled relative risk in the outcome of common adverse event (A), serious adverse event (B) in the studies considering tolvaptan compared to placebo therapy in HF patients. In the outcome of common adverse event (RR=1.08, 95% CI=0.99 to 1.18, P=0.08) and serious adverse (RR=0.96, 95% CI=0.88 to 1.04, P=0.29) event both show no significant occurrence. In the common adverse event of dizziness (a), dry mouth (b), thirst (c), urinary frequency (d), A significant occurrences were found in dry mouth (RR=5.89, 95% CI=3.41 to 10.17, P<0.001), thirst (RR=6.79, 95%CI=5.24 to 8.81, P<0.001), urinary frequency (RR=4.29, 95% CI=2.59 to 7.10, P<0.001), but dizziness (RR=1.07, 95% CI=0.90 to 1.28, P=0.46).
Publication bias
The Begg’s funnel plot was performed to evaluate the potential publication bias in the studies of clinical manifestations changes and outcomes considering tolvaptan compared to placebo therapy in HF patients. All the shape of funnel plots did not show any asymmetrical evidence and no significant publication bias (Figure 5).
Figure 5.
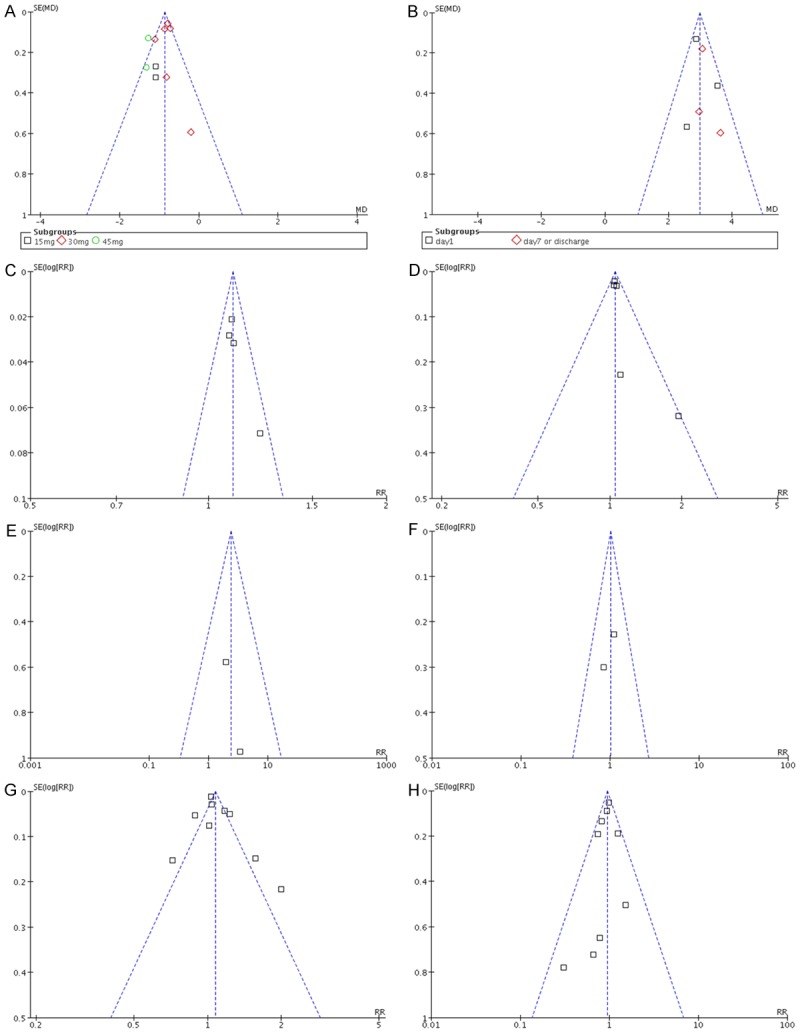
Begg’s funnel plots for publication bias test in the studies considering tolvaptan compared to placebo therapy in HF patients. A. The changes of body weight; B. The changes of serum sodium; C. The improvement of dyspnea; D. The improvement of edema; E. The improvement of pulmonary rales; F. The improvement of pulmonary congestion; G. The outcome of common adverse event; H. The outcome of serious adverse event.
Discussion
We conducted a systematic meta-analysis of RCTs evaluating the efficacy and safety of vasopressin antagonist tolvaptan compared with placebo in HF patients presenting with congestive symptoms. The meta-analysis demonstrated that tolvaptan had a beneficial effect in HF patients, in addition to standard therapy including diuretics, ACEI, ARB and β-blockers. There were significant decrease in the change of body weight, increase in the change of serum sodium, remission of part of congestion signs, without obvious significance in serious adverse event. All references included were based on design of The Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) [25], a prospective, randomized, multicenter, double-blind, placebo-controlled study, which relatively provided us concordant models.
AVP antagonist tolvaptan could decrease the expression of AQP channels by inhibiting the activation of V2 receptor on the renal collecting duct principal cells, which help removing excess fluid without increasing electrolyte excretion into urine [26]. Significance in the decrease of body weight and the increase of serum sodium demonstrated this point. We stratified the change of body weight three subgroups by means of dose, 15 mg, 30 mg and 45 mg, to analysis its sensibility. Heterogeneity of body weight change is probably coming from dose difference according to indexes I2 (15 mg: 0%, 30 mg: 35%, 45 mg: 0%, total: 61%). But there was no distinct dose dependent in the effect of draining off water, and then we chosen the 30 mg tolvaptan population to perform a meta-analysis in the change of serum sodium compare to placebo. The result that change of the index I2 of plasma sodium between two subgroups (day 1 and day 7 or discharge) was 2% indicated that tolvaptan generated a sustained and effective increase of plasma sodium levels without influence of time in HF patients with hyponatremia.
Congestive symptoms, such as dyspnea, edema, pulmonary rales and pulmonary congestion are the major cause of hospitalization and rehospitalization in HF patients [27]. The administration of tolvaptan could relive the congestion by the way of getting rid of excess fluid in patients hospitalized for heart failure. This meta-analysis of congestion improvement included dyspnea amelioration, edema disappearance, pulmonary rales vanishing, and pulmonary congestion relief. The results indicated that tolvaptan improved the symptoms associated with water overload, especially in dyspnea and edema in the studies considering tolvaptan compared to placebo therapy. But there was no significant difference in the improvement of pulmonary rales and pulmonary congestion. The time of observation, dose of tolvaptan and implementation of trials may be the influence of inconformity of congestion improvement. There was an apparently trend to get a remission of pulmonary rales and pulmonary congestion. Congestion may cause subendocardial ischemia or necrosis, ventricular shape and a series of ventricular remolding, even contribute to progression of heart failure, which associated with increasing of intravascular volume and left ventricular diastolic pressure [28]. The early application of tolvaptan is not only an effective measure to remit congestion, but also a way to delay the process of ventricular remolding and HF worsening.
For safety, there was no significant difference in the trials considering tolvaptan compared to placebo therapy in HF patients both in common adverse event and serious adverse event. But we could see a trend to increase common adverse event and decrease serious adverse event in Figure 4. Among common adverse, dizziness, dry mouth, thirst and urinary frequency has been performed subgroups meta-analysis due to a significant heterogeneity (I2=87%). The occurrence of urinary frequency that was associated with the aquaretic effect presented an apparent significance in tolvaptan treatment compared to placebo. Then the augmented loss of fluid appeared to be compensated by a concomitant increase in feeling of dry mouth and thirst, resulting fluid intake [27]. These common adverse events were well tolerated in HF patient with tolvaptan. Tolvaptan appeared to generate increased urinary discharging without activation of the renin-angiotensin system and changes of renal function compared with conventional diuretic therapy [28,29]. This compound also has been manifested to increase renal blood flow, reduce renal vascular resistance, and ameliorate glomerular filtration rate in patients with heart failure [30]. These findings demonstrated that treatment with tolvaptan in HF patient is less associated with renal dysfunction than conventional diuretic therapy. There was a tendency for tolvaptan to get a lower rate of serious adverse event than placebo although no significance between the two groups. In other word, tolvaptan did not increase the occurrence of serious adverse event, such as, death, HF worsening, stroke.
Over all HF patients, hospitalization and mortality rates were 27% and 24% each year [3]. What were worse, among inpatients with HF, 42% died and 31% were residents within 1 year [3]. A recent study of mortality and readmission rates in acute decompensated heart failure discovered that 60 day mortality rate was 7.0% and 30 and 60 day readmission rates were 16.7% and 20.6% in general internal medicine [31]. A more effective and safety treatment should be used to remit these conditions. We believe that Tolvaptan may give a more balanced perspective in this population.
Potential limitations existed in the process of our meta-analysis. First, 8 RCTs trials contained two ethnic groups, American, and Japanese, which would affect the outcome. As we all know, different race may generate a diverse activation slightly in response to the same therapy. Second, follow-up of all references included were inconformity, leading to some discrepancies in the observation of adverse event. Third, the definition of serious adverse event was various in different trials. Nonetheless, our meta-analysis achieved a serious data significantly associate with sufficient population and accordant design.
Despite the limitation of this meta-analysis of RCTs, the findings of this study conformed that tolvaptan could decrease the body weight, increase the serum sodium, and improve part of congestion symptom without significant increase the adverse event in HF patient. This may provide an effective and safety therapy in HF patient. Future long-term clinical trials with larger sample sizes should be concentrated on understanding the exact mechanisms of tolvaptan treatment.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81170133, 81200088, 81470387) and Hubei Province’s Outstanding Medical Academic Leader program.
Disclosure of conflict of interest
None.
References
- 1.Nieminen MS, Böhm M, Cowie MR, Drexler H, Filippatos GS, Jondeau G, Hasin Y, Lopez-Sendon J, Mebazaa A, Metra M, Rhodes A, Swedberg K, Priori SG, Garcia MA, Blanc JJ, Budaj A, Cowie MR, Dean V, Deckers J, Burgos EF, Lekakis J, Lindahl B, Mazzotta G, Morais J, Oto A, Smiseth OA, Garcia MA, Dickstein K, Albuquerque A, Conthe P, Crespo-Leiro M, Ferrari R, Follath F, Gavazzi A, Janssens U, Komajda M, Morais J, Moreno R, Singer M, Singh S, Tendera M, Thygesen K. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:384–416. doi: 10.1093/eurheartj/ehi044. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 3.Foebel AD, Heckman GA, Ji K, Dubin JA, Turpie ID, Hussack P, McKelvie RS. Heart failurerelated mortality and hospitalization in the year following admission to a long-term care facility: the Geriatric Outcomes and Longitudinal Decline in Heart Failure (GOLD-HF) study. J Card Fail. 2013;19:468–477. doi: 10.1016/j.cardfail.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Izumi Y, Miura K, Iwao H. Therapeutic Potential of Vasopressin-Receptor Antagonists in Heart Failure. J Pharmacol Sci. 2014;124:1–6. doi: 10.1254/jphs.13r13cp. [DOI] [PubMed] [Google Scholar]
- 5.O’Connell JB, Alemayehu A. Hyponatremia, heart failure, and the role of tolvaptan. Postgrad Med. 2012;124:29–39. doi: 10.3810/pgm.2012.03.2534. [DOI] [PubMed] [Google Scholar]
- 6.Goldsmith SR, Gheorghiade M. Vasopressin antagonism in heart failure. J Am Coll Cardiol. 2005;46:1785–1791. doi: 10.1016/j.jacc.2005.02.095. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, Neyses L. Diuretic usage in heart failure: a continuing conundrum in 2005. Eur Heart J. 2005;26:644–649. doi: 10.1093/eurheartj/ehi176. [DOI] [PubMed] [Google Scholar]
- 8.Mpe MT, Klug EQ, Silwa KS, Hitzeroth J, Smith DA. Heart Failure Society of South Africa (HeFSSA) perspective on the European Society of Cardiology (ESC) 2012 chronic heart failure guideline. S Afr Med J. 2013;103:660–667. doi: 10.7196/samj.7319. [DOI] [PubMed] [Google Scholar]
- 9.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 10.Cleland JG, Coletta A, Witte K. Practical applications of intravenous diuretic therapy in decompensated heart failure. Am J Med. 2006;119:S26–S36. doi: 10.1016/j.amjmed.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Hirano T, Yamamura Y, Nakamura S, Onogawa T, Mori T. Effects of the V2-receptor antagonist OPC-41061 and the loop diuretic furosemide alone and in combination in rats. J Pharmacol Exp Ther. 2000;292:288–294. [PubMed] [Google Scholar]
- 12.Hobbs RE, Tang WH. Vasopressin receptor antagonists in heart failure. Recent Pat Cardiovasc Drug Discov. 2006;1:177–184. doi: 10.2174/157489006777442513. [DOI] [PubMed] [Google Scholar]
- 13.Gheorghiade M, Pang PS, Ambrosy AP, Lan G, Schmidt P, Filippatos G, Konstam M, Swedberg K, Cook T, Traver B, Maggioni A, Burnett J, Grinfeld L, Udelson J, Zannad F. A comprehensive, longitudinal description of the in-hospital and post-discharge clinical, laboratory, and neurohormonal course of patients with heart failure who die or are re-hospitalized within 90 days: analysis from the EVEREST trial. Heart Fail Rev. 2012;17:485–509. doi: 10.1007/s10741-011-9280-0. [DOI] [PubMed] [Google Scholar]
- 14.Imamura T, Kinugawa K, Minatsuki S, Muraoka H, Kato N, Inaba T, Maki H, Shiga T, Hatano M, Yao A, Kyo S, Komuro I. Urine osmolality estimated using urine urea nitrogen, sodium and creatinine can effectively predict response to tolvaptan in decompensated heart failure patients. Circ J. 2013;77:1208–1213. doi: 10.1253/circj.cj-12-1328. [DOI] [PubMed] [Google Scholar]
- 15.Kinugawa K, Sato N, Inomata T, Shimakawa T, Iwatake N, Mizuguchi K. Efficacy and safety of tolvaptan in heart failure patients with volume overload. Circ J. 2014;78:844–852. [PubMed] [Google Scholar]
- 16.Pang PS, Gheorghiade M, Dihu J, Swedberg K, Khan S, Maggioni AP, Grinfeld L, Zannad F, Burnett JC Jr, Ouyang J, Udelson JE, Konstam MA. Effects of tolvaptan on physician-assessed symptoms and signs in patients hospitalized with acute heart failure syndromes: analysis from the efficacy of vasopressin antagonism in heart failure outcome study with tolvaptan (EVEREST) trials. Am Heart J. 2011;161:1067–1072. doi: 10.1016/j.ahj.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 17.Gheorghiade M, Niazi I, Ouyang J, Czerwiec F, Kambayashi J, Zampino M, Orlandi C. Vasopressin V2-receptor blockade with tolvaptan in patients with chronic heart failure: results from a double-blind, randomized trial. Circulation. 2003;107:2690–2696. doi: 10.1161/01.CIR.0000070422.41439.04. [DOI] [PubMed] [Google Scholar]
- 18.Gheorghiade M, Gattis WA, O’Connor CM, Adams KF Jr, Elkayam U, Barbagelata A, Ghali JK, Benza RL, McGrew FA, Klapholz M, Ouyang J, Orlandi C. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. J Am Med Assoc. 2004;291:1963–1971. doi: 10.1001/jama.291.16.1963. [DOI] [PubMed] [Google Scholar]
- 19.Gheorghiade M, Konstam MA, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. J Am Med Assoc. 2007;297:1332–1343. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 20.Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. J Am Med Assoc. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 21.Udelson JE, McGrew FA, Flores E, Ibrahim H, Katz S, Koshkarian G, O’Brien T, Kronenberg MW, Zimmer C, Orlandi C, Konstam MA. Multicenter, randomized, double-blind, placebo-controlled study on the effect of oral tolvaptan on left ventricular dilation and function in patients with heart failure and systolic dysfunction. J Am Coll Cardiol. 2007;49:2151–2159. doi: 10.1016/j.jacc.2007.01.091. [DOI] [PubMed] [Google Scholar]
- 22.Matsuzaki M, Hori M, Izumi T, Asanoi H, Tsutamoto T. Effects of tolvaptan on volume overload in Japanese patients with heart failure: results of a phase II, multicenter, randomized, double-blind, placebo-controlled, parallelgroup study. Cardiovasc Drugs Ther. 2011;25:S19–31. doi: 10.1007/s10557-011-6303-y. [DOI] [PubMed] [Google Scholar]
- 23.Matsuzaki M, Hori M, Izumi T, Fukunami M. Efficacy and safety of tolvaptan in heart failure patients with volume overload despite the standard treatment with conventional diuretics: a phase III, randomized, double-blind, placebo-controlled study (QUEST study) Cardiovasc Drugs Ther. 2011;25:S33–45. doi: 10.1007/s10557-011-6304-x. [DOI] [PubMed] [Google Scholar]
- 24.Hauptman PJ, Burnett J, Gheorghiade M, Grinfeld L, Konstam MA, Kostic D, Krasa HB, Maggioni A, Ouyang J, Swedberg K, Zannad F, Zimmer C, Udelson JE. Clinical course of patients with hyponatremia and decompensated systolic heart failure and the effect of vasopressin receptor antagonism with tolvaptan. J Card Fail. 2013;19:390–397. doi: 10.1016/j.cardfail.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Gheorghiade M, Orlandi C, Burnett JC, Demets D, Grinfeld L, Maggioni A, Swedberg K, Udelson JE, Zannad F, Zimmer C, Konstam MA. Rationale and design of the multicenter, randomized, double-blind, placebo-controlled study to evaluate the Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study With Tolvaptan (EVEREST) J Card Fail. 2005;11:260–269. doi: 10.1016/j.cardfail.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Sato N, Kajimoto K, Keida T, Mizuno M, Minami Y, Yumino D, Asai K, Murai K, Muanakata R, Aokage T, Sakata Y, Mizuno K, Takano T. TEND Investigators. Clinical features and outcome in hospitalized heart failure in Japan (from the ATTEND Registry) Circ J. 2013;77:944–951. doi: 10.1253/circj.cj-13-0187. [DOI] [PubMed] [Google Scholar]
- 27.Adamson PB, Magalski A, Braunschweig F, Böhm M, Reynolds D, Steinhaus D, Luby A, Linde C, Ryden L, Cremers B, Takle T, Bennett T. Ongoing right ventricular hemodynamics in heart failure: clinical value of measurements derived from an implantable monitoring system. J Am Coll Cardiol. 2003;41:565–571. doi: 10.1016/s0735-1097(02)02896-6. [DOI] [PubMed] [Google Scholar]
- 28.Miyazaki T, Fujiki H, Yamamura Y, Nakamura S, Mori T. Tolvaptan, an orally active vasopressin V(2)-receptor antagonist-pharmacology and clinical trials. Cardiovasc Drug Rev. 2007;25:1–13. doi: 10.1111/j.1527-3466.2007.00001.x. [DOI] [PubMed] [Google Scholar]
- 29.Matsue Y, Suzuki M, Seya M, Iwatsuka R, Mizukami A, Nagahori W, Ohno M, Matsumura A, Hashimoto Y. Tolvaptan reduces the risk of worsening renal function in patients with acute decompensated heart failure in high-risk population. J Cardiol. 2013;61:169–174. doi: 10.1016/j.jjcc.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 30.Burnett JC, Smith WB, Ouyang J, Zimmer CA, Orlandi C. Tolvaptan (OPC-41061), a V2 vasopressin receptor antagonist, protects against the decline in renal function observed with loop diuretic therapy [abstract] . J Card Fail. 2003;9:36. [Google Scholar]
- 31.Selim A M, Mazurek J A, Iqbal M, Wang D, Negassa A, Zolty R. Mortality and Readmission Rates in Patients Hospitalized for Acute Decompensated Heart Failure: A Comparison Between Cardiology and General-Medicine Service Outcomes in an Underserved Population. Clin Cardiol. 2015;38:131–8. doi: 10.1002/clc.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]


