Abstract
Background: To investigate the antiapoptotic effect of Ukrain on intestinal lesion induced by mesenteric ischemia-reperfusion (I/R) injury. Methods: Male Sprague-Dawley rats were divided into three groups: laparotomy (L), I/R, and Ukrain and I/R (U + I/R). In the U + I/R group, Ukrain (7 mg/kg) was given by intraperitoneal at the beginning of the study. 1 h after ukrain application, ischemia was induced for 30 minutes, and reperfusion was subsequently allowed for 120 minutes in the I/R and U + I/R groups. Rats were sacrificed at the end of reperfusion and intestinal tissues were collected for biochemical and molecular examination. Intestinal tissues caspase 3 protein were assayed. Serum Bcl-xL and iNOS were measured. The expression level of caspase-3, Bcl-xL and iNOS in intestinal tissue of rats were detected by reverse transcription-polymerase chain reaction (RT-PCR). Results: Levels of serum iNOS and mRNA expression were increased in the I/R and decreased in the U + I/R group. In addition, levels of the proapoptotic gene caspase-3 protein and mRNA expression were increased in the I/R and decreased in the U + I/R group. Levels of the antiapoptotic gene Bcl-xL serum and mRNA expression were increased in the U + I/R group. Conclusions: Ukrain can reduce the ischemia-reperfusion injury in the intestinal tissue by inhibiting the cell apoptosis. The mechanism may be correlated with increased Bcl-xL mRNA expressions and decreased mRNA expressions of Caspase-3 and iNOS.
Keywords: Ukrain, intestinal ischemia reperfusion, apoptosis
Introduction
Mesenteric ischemia-reperfusion (I/R) injury is a common pathological process, which can occur as a treatment complication in many diseases [1]. Several conditions such as severe infection, trauma, shock and severe acute pancreatitis, abdominal aortic surgery, and small bowel transplantation can cause intestinal ischemia, intestinal barrier dysfunction and an increase of intestinal mucosal permeability [2]. I/R not only results in injury to the intestine but may also lead to sepsis, systemic inflammatory response syndrome, and even multiple organ dysfunction syndrome, owing to damage of the mucosal structure and barrier function [3]. I/R injury is multifactorial and involves impaired blood flow reconstitution, increased expression of adhesion molecules, neutrophil activation, cytokine/chemokine release by infiltrating leukocytes, oxidative stress, and endothelial cell apoptosis [4,5].
The anticancer drug Ukrain (NSC 631570), a semisynthetic compound derived from the extract of the plant Chelidonium majus L., has been shown to exert selective cytotoxic effects towards a variety of malignant cells including colon, brain, ovarian, melanoma and lymphoma without harmful side effects on healthy human cells [6].
Apoptosis, otherwise known as programmed cell death, is an essential natural process that eliminates damaged, unneeded or dangerous cells from the body [7]. In normal cells, apoptosis is caused by developmental cues and ce-llular stress or injury [8]. However, the effects of ukrain on rat I/R-induced intestinal apoptosis and apoptotic pathways are not yet completely understood are not fully known, although antiangiogenic and anticarcinogenic action on human endothelial cells and a proapoptotic action on lymphoma and glioblastoma cells have been reported in vitro. Ukrain has not been studied on intestinal apoptosis caused by ischemia-reperfusion injury in rats before. Therefore, the aims of this study were to determine whether ukrain ameliorates I/R-induced intestinal mucosal injury by suppressing cell apoptosis.
Methods
Animals
Sprague Dawley, 10-12-wk-old male rats (250-300 g), were purchased from Dumlupınar University Experimental Animal Center, Kütahya, Turkey. The rats were housed in a specific plastic cage and fed on laboratory chow and water. After a minimum of 7-day acclimation, therats were randomly allocated into three groups (n = 8 ineach group): (1) Laparotomy (L) group in which the rats were subjected to identical surgical procedure but no I/R injury), (2) Mesenteric ischemia-reperfusion injury (I/R) group, (3) Ukrain and I/R (U + I/R) in which therats were administered ukrain (Nowicky Pharmaceuticals, Margaretenstrasse 7, 1040 Vienna, Austria) was given by intraperitoneal (7 mg/kg) 1 h prior to I/R [9]. This project was approved by the Dumlupınar University Ethics Committee of Animal Care and Usage, Kütahya, Turkey.
Mesenteric ischemia-reperfusion (I/R) injury
The rats were anesthetized with an intraperitoneal injection of ketamin/xylazine HCl (75 mg/kg/10 mg/kg) and, throughout the surgery, the body temperature was maintained at 36°C-37°C with a homeothermic blanket. I/R injury model was performed as previously described [10]. The rats were placed in supine position and secured in a dissection tray. The abdominal region was shaved and cleaned with anti-septic solutions. The intestinal region was accessed by means of midline laparotomy. Superior mesenteric artery was lifted with care and occluded with a microvascular clamp, thus intestinal ischemia was created within 30 min. Ischemia was determined by the absence of pulses or pale color of the intestine. Then the abdomen was then closed. Following ischemia, the clamp was removed and a 120-min reperfusion was conducted. The return of the pulses and the pink color were assumed to be taken as the signs of intestinal reperfusion.
Collection of samples
Rats were sacrificed at the immediately after reperfusion, and serum and small intestine samples were collected. Blood was collected from the abdominal artery of rats for the determination of serum samples for Bcl-xL and iNOS analysis. Intestine tissue samples were then collected in liquid nitrogen for analysis of molecular biologic studies, and stored at -80°C until analysis.
Serum Bcl-xL and iNOS analysis
The serum levels of the Bcl-xL and iNOS were determined by using specific ELISA kits for rats according to the manufacturer’s instructions (Cusabio, China) by an ELISA microplate reader (Thermo Multiscan GO, 1510, Finland).
Tissue Caspase-3 analysis
Intestinal tissue samples were shred at 9500 rpm (4 × 10 sec at 4°C) by an homogenizer (WiseTis, HG-15A, Korea). Then, the upper clear supernatants were removed for tissue Cas-pase-3 analysis and it was measured using Colorimetric Assay Kit (Biovision, USA). The ELISA procedures were performed according to the manufacturer’s instructions. The results are expressed as pg/mg-protein.
Real-time polymerase chain reaction (RT-PCR) analysis
Total RNA was isolated from intestinal tissues by using GeneJET RNA Purification Kit (Thermo, Cat No: # K0732) in accordance with the manufacturer’s instructions, and 4 mg of total RNA was reversely transcribed into cDNA by using the EasyScript™ cDNA Synthesis Kit (abm) by following instructions. Real-time PCR amplifications were carried out by using the LightCycler 480 Real Time PCR System (Roche Diagnostics, Germany. PCR primers (Genscript, Piscataway, USA) for all analyzed genes are shown in Table 1. PCR was conducted at 95°C for 10 min, followed by 35 cycles at 95°C for 10 s, 57°C for 30 s and 72°C for 1 min. The amount of mRNA for each gene was normalized by β-actin, and the relative expressionlevels were calculated by using the 2-ΔΔCt method.
Table 1.
Primers used for real-time PCR analysis
| Gene | Sense strand sequence | Anti-sense strand sequence |
|---|---|---|
| Caspase 3 | GACTGCGGTATTGAGACAGA | CGAGTGAGGATGTGCATGAA |
| Bcl-xL | AGGCTGGCGATGAGTTTGAA | TGAAACGCTCCTGGCCTTTC |
| iNOS | TTGGAGCGAGTTGTGGATTGTTGTTC | GGTGAGGGCTTGCCTGAGTGAGC |
| β-actin | CTATCGGCAATGAGCGGTTCC | TGTGTTGGCATAGAGGTCTTTACG |
Immunohistochemical examinations
Tissue expression levels of Bax and Bcl-2 were examined with immunohistochemical method. Formaldehyde-fixed sections were embedded in paraffin for immunohistochemical stainings. Immunohistochemical stainings were performed using commercial kits (Abcam, Cambridge, MA, USA) according to the manufacturer’s instructions and examined under a light microscope (Olympus BX51, Tokyo, Japan). Stained cells were scored as semi-quantitative: no positive cells (score: 0), mild staining, <25% positive cells (score: 1), moderate staining, 25-50% positive cells (score: 2), and severe staining, >50% positive cells (score: 3).
Statistical analysis
Statistical analysis was done with SPSS (Statistical Package for Social Sciences, Chicago, IL, USA) 16.0 pocket program. All results were given as means ± standard error (SE). Comparisons among multiple groups were done with Kruskal-Wallis test, and between two groups were with Mann-Whitney U test. Values smaller than P≤0.05 were accepted as statistically significant.
Results
Levels of tissue Caspase 3 protein and mRNA
There were statistically significant differences in tissue caspase 3 protein and mRNA levels among the groups of L, I/R and U + I/R (P = 0.000 and P = 0.001). In the I/R group, tissue caspase 3 protein and mRNA expression levels were significantly increased when compared with the L and U + I/R groups (P<0.05), whereas the I/R-induced elevation in the tissue caspase 3 protein and mRNA expression was canceled out with administration of ukrain (P<0.05; Table 2 and Figure 1).
Table 2.
Effect of Ukrain on tissue caspase 3, serum Bcl-xL and iNOS concentrations of Laparotomy (L), mesenteric ischemia-reperfusion injury (I/R) and ukrain + mesenteric ischemia-reperfusion injury (U + I/R) groups
| Groups | L (n = 8) | I/R (n = 8) | U + I/R (n = 8) | P |
|---|---|---|---|---|
| Caspase 3 (pg/mg-protein) | 0.73±0.08a,b | 2.30±0.09a,c | 1.21±0.15b,c | 0.000 |
| Bcl-xL (pg/ml) | 0.43±0.17a,b | 0.19±0.01a,c | 0.70±0.07b,c | 0.001 |
| iNOS (IU/ml) | 3.06±0.30a | 6.73±0.84a,b | 3.55±0.45b | 0.006 |
P: Shows the differences between all groups (Kruskal Wallis test).
In each line, the difference between the means with same letters are significant, P≤0.05 (Mann-Whitney U test);
In each line, the difference between the means with same letters are significant, P≤0.05 (Mann-Whitney U test);
In each line, the difference between the means with same letters are significant, P≤0.05 (Mann-Whitney U test).
Figure 1.
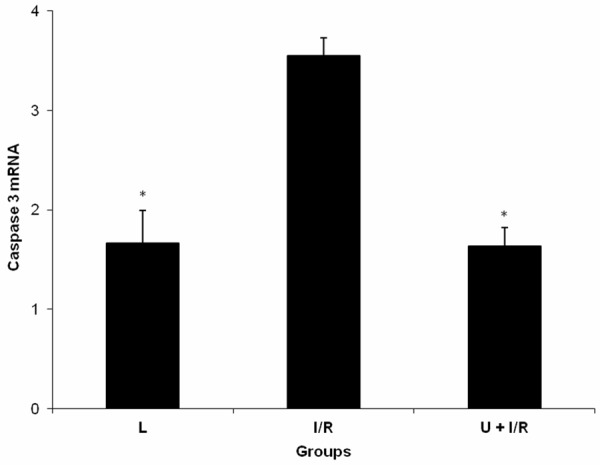
Expression of caspase 3 mRNA in intestinal tissue of laparotomy (L), mesenteric ischemia-reperfusion injury (I/R) and ukrain + mesenteric ischemia-reperfusion injury (U + I/R) groups. Data were expressed as mean ± standard error. *P≤0.05 vs I/R group.
Levels of serum Bcl-xL and tissue Bcl-xL mRNA
There were statistically significant differences in serum Bcl-xL and tissue Bcl-xL mRNA levels among the groups of L, I/R and U + I/R (P = 0.001 and P = 0.002). Serum Bcl-xL and tissue Bcl-xL mRNA levels were significantly reduced in I/R group compared with L and U + I/R groups (P = 0.001 and P = 0.001, respectively, Table 2 and Figure 2). On the other hand, the serum Bcl-xL and tissue Bcl-xL mRNA levels were significantly elevated in ukrain-treated I/R groups (P = 0.001).
Figure 2.
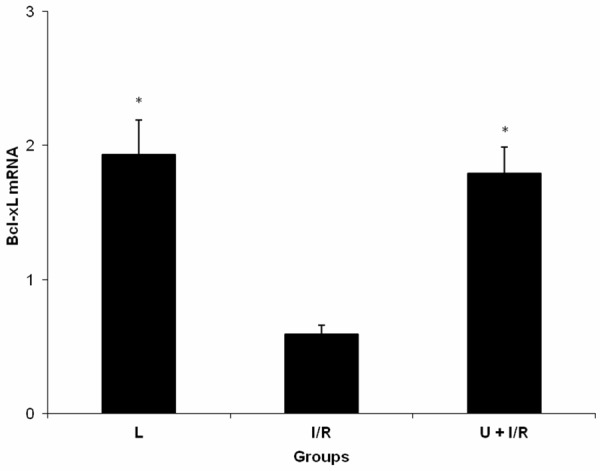
Expression of Bcl-xL mRNA in intestinal tissue of laparotomy (L), mesenteric ischemia-reperfusion injury (I/R) and ukrain + mesenteric ischemia-reperfusion injury (U + I/R) groups. Data were expressed as mean ± standard error. *P≤0.05 vs I/R group.
Levels of serum iNOS and tissue iNOS mRNA
There were statistically significant differences in serum iNOS and tissue iNOS mRNA levels among the groups of L, I/R and U + I/R (P = 0.006 and P = 0.008). The serum iNOS and tissue iNOS mRNA expression levels of I/R group was significantly higher than thatmeasured in the L and U + I/R groups (P<0.05; Table 2 and Figure 3). This elevated serum iNOS and tissue iNOS mRNA expression levels were significantly reduced by ukrain (P<0.05).
Figure 3.
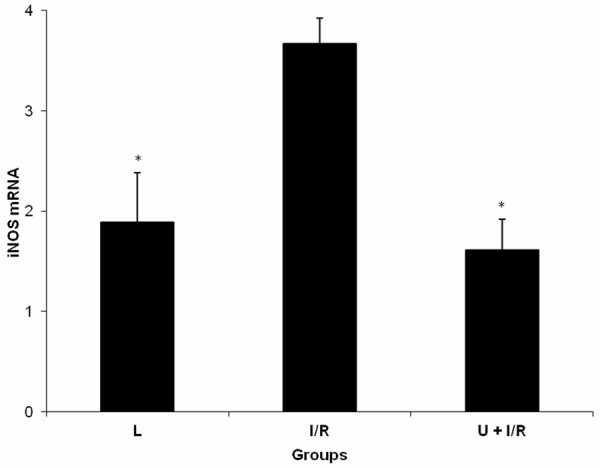
Expression of iNOS mRNA in intestinal tissue of laparotomy (L), mesenteric ischemia-reperfusion injury (I/R) and ukrain + mesenteric ischemia-reperfusion injury (U + I/R) groups. Data were expressed as mean ± standard error. *P≤0.05 vs I/R group.
Immunohistochemical examinations
There were statistically significant differences in the scored Bax and Bcl-2 values in intestinal tissue samples between assay groupsof L, I/R and U + I/R (P≤0.05; Table 3; Figures 4 and 5).
Table 3.
Comparisons of scored Bax and Bcl-2 values in intestinal tissue samples between Laparotomy (L), mesenteric ischemia-reperfusion injury (I/R) and ukrain + mesenteric ischemia-reperfusion injury (U + I/R) groups (mean ± SEM, n = 8)
| Scored values | L | I/R | U + I/R | P |
|---|---|---|---|---|
| Bax | 0.29±0.3a | 2.71±0.2a,b | 1.43±0.2a,b | <0.001 |
| Bcl-2 | 1.42±0.2a | 0.57±0.2a,b | 2.28±0.2a,b | <0.001 |
P: Shows the differences between all groups (Kruskal-Wallis test).
In each line, the differences between the means with same letters are significant, P≤0.05 (Mann-Whitney U test);
In each line, the differences between the means with same letters are significant, P≤0.05 (Mann-Whitney U test).
Abbreviations: SEM: Standart error of mean, Bax: Bcl-2-associated X protein, Bcl-2: B-cell lymphoma 2.
Figure 4.
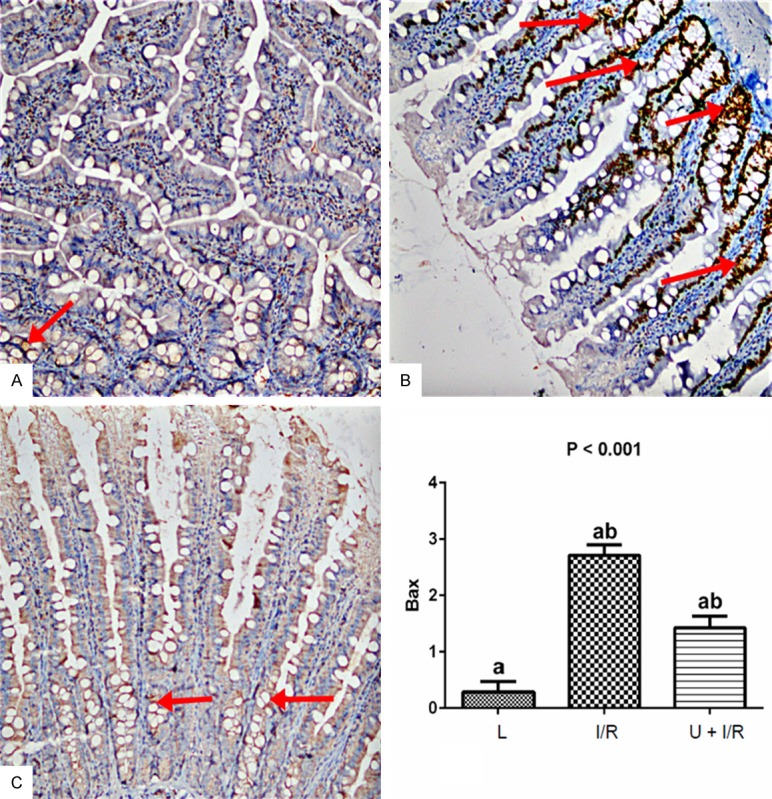
Examination of Bax expression in intestinal tissues in the assay groups. A. Bax staining (Bax x 200) showed that there was no change in control rats. B. Significant increase in the number of Bax positive cells were observed in IR group. C. The Ukrain significantly decreased the Bax positive cells. Bax expression was examined using immunohistochemical staining. Stained cells were scored as semiquantitative: no staining (score: 0), mild staining (score: 1), moderate staining (score: 2), and severe staining (score: 3). Results are expressed as the mean ± standard error of mean (SEM); n = 8. P: Shows the differences between all groups (Kruskal-Wallis test). a,b: In each line, the differences between the means with same letters are significant, P≤0.05 (Mann-Whitney U test).
Figure 5.
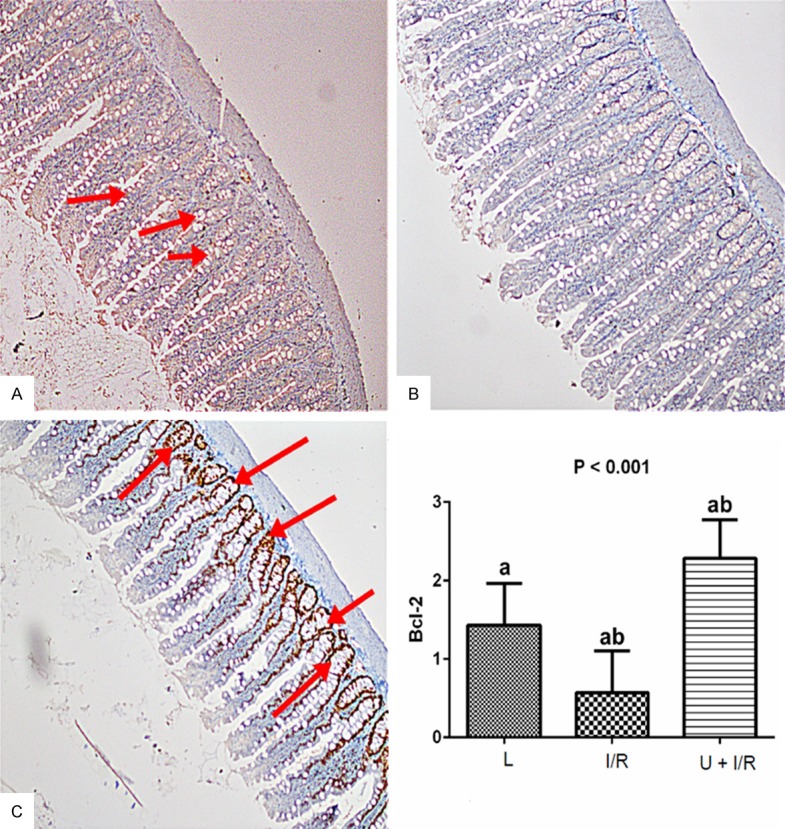
Examination of Bcl-2 expression in intestinal tissues in the assay groups. A. Bcl-2 staining (Bcl-2 x 100) showed that there was no change in L rats. B. Significant decrease in the number of Bcl-2 positive cells were observed in I/R group. C. The Ukrain significantly increased the Bcl-2 positive cells. Bcl-2 expression was examined using immunohistochemical staining. Stained cells were scored as semiquantitative: no staining (score: 0), mild staining (score: 1), moderate staining (score: 2), and severe staining (score: 3). Results are expressed as the mean ± standard error of mean (SEM); n = 8. P: Shows the differences between all groups (Kruskal-Wallis test). a,b: In each line, the differences between the means with same letters are significant, P≤0.05 (Mann-Whitney U test).
Discussion
The intestine is probably one of the most susceptible or-gans to I/R injury. I/R injury is a common consequence of acute mesenteric ischemia, resuscitation, hemorrhagic, traumatic or septic shock, thoracoabdominal aneurysm repair, severe burns, or some surgical procedures including small bowel transplantation and abdominal aortic surgery [11]. The underlying mechanisms of mesenteric I/R injury are complex. Many clinical and experimental research have demonstrated that oxidative stress, neutrophils, endothelial factors, cytokines, and apoptosis are involved in this process [12].
In studies, effect of ukrain on apoptosis in mesenteric I/R injury rats are yet to be reported. In the present study, we demonstrated for the first time that intraperitoneal injection of the protective drug ukrain in rats with mesenteric I/R injury. Apoptosis is a fundamental and complex biological process that enables an organism to kill and remove unwanted cells during animal development, normal homeostasis and disease [13,14].
Mesenteric I/R injury is a complicated process which associated various mechanisms as apoptosis. Apoptosis has been implicated in physiological and pathological conditions including mesenteric I/R and, plays a vital role in the initiation and progression of mesenteric I/R injury [15]. Caspase 3 plays a key role in the execution phase of apoptosis and is a common pathway associated with apoptosis signal transduction [16]. Zhang et al. [17] observed that activation of caspase-3, a marker of apoptosis, is usually marked in intestinal I/R. A study by Takeshita et al. [18] found an increase in the levels of expression of the proapoptotic gene caspase-3 in the intestinal I/R rats [18]. This study’s results are compatible with the aforementioned study. In the present study, the results demonstrated that mesenteric I/R attenuated the increase in caspase-3 protein and mRNA expression and decreased in the U + I/R group. In addition effects of intraperitoneal ukrain administration have not been studied on apoptosis in the mesenteric I/R rat models before. Therefore, there has not been any study in the literature between ukrain and apoptosis that can be compared with the results of this study. Previous studies have demonstrated that ukrain treatment led to the activation of effector caspase 3 expression as detected by positive Annexin-V staining in three breast cancer models tested in vitro [19]. Ukrain is a potent inducer of apoptosis and cell death in human Jurkat T lymphoma cells involving mitochondrial damage and caspase-activation [20]. In this study and previous studies on this subject, the dose and type of administration of ukrain differ leading to the differences in the results.
Bcl-xL is a transmembrane molecule in the mitochondria and acts as an apoptotic inhibitor. It is a member of the Bcl-2 family of proteins, and acts as a pro-survival protein by preventing the release of mitochondrial contents such as cytochrome c, which would lead to caspase activation [21]. Previous studies have shown that expression of the antiapoptotic gene Bcl-xL was decreased in the intestinal I/R [18,22]. In this study; mesenteric I/R attenuated the decrease in Bcl-xL serum and mRNA expression and increased in the U + I/R group. Habermehl et al. [20] demonstrated that over-expression of Bcl-2 or Bcl-xL partially reduced ukrain-induced apoptosis pointing to Bcl-2 controlled mitochondrial signalling events. Venkatesh et al. demonsrated that ukrain decreases the cell survival of androgen-independent prostate cancer cells and induces their apoptosis, increased the pro-apoptotic mRNA expression of Bad, Baxcaspase, decreased the anti-apoptotic protein Bcl-2 [23]. In this study, ukrain might inhibit apoptosis caused by mesenteric I/R injury in rats, although ukrain is induced apoptosis according to studies on human cancer cells.
iNOS produces large quantities of NO upon stimulation, such as by proinflammatory cytokines [24]. Induction of the high-output iNOS usually occurs in an oxidative environment [25]. The pathogenesis of intestinal I/R involves with the induction of iNOS. Naito et al. [26] observed that inflammation induced by mesenteric reperfusion in rats resulted in oxidative and nitrosative stress, characterized by the high production of NO, by the increased expression of iNOS and by an excess expression of lipidic peroxides [44]. According to Wu et al. [27] iNOS is related to increased apoptosis in the small-intestine mucosa of rats after I/R [27]. Margaritis et al. [28] reported that the inhibition of both the iNOS activity and the NO production could alleviate the I/R injury [28], supporting the role of iNOS as an important mediator of I/R injury in intestine. In this study; mesenteric I/R attenuated the increase in iNOS serum and mRNA expression compared with the L and U + I/R groups. In the study performed by Kiang et al. [29] the authors found that increased caspase-3 protein and activity are regulated via iNOS [29]. Thus, our results showing that mesenteric I/R injury is associated with both increased iNOS expression and caspase-3 activation. Conversely, iNOS serum and mRNA expression levels were decreased in the U + I/R group. In addition, in our previous study, we were demonstrated that treatment with ukrain reduced the degree of intestinal tissue injury on histopathologic examination [9].
In conclusion, ukrain markedly inhibited the protein and mRNA expression of apoptotic factor as caspase 3, decreased iNOS serum and mRNA levels, and significantly increased Bcl-xL serum and mRNAexpression. The ukrain attenuates the I/R-induced intestinal injury by regulation of serum cytokine levels, and intestinal tissue Bcl-xL, iNOS and caspase-3 expressions. The Immunohistochemical examinations findings support the biochemical and moleculer findings. These results suggest that ukrain might have antiapoptotic effects and use as a therapeutic drug for the treatment of the mesenteric I/R injury.
Acknowledgements
The authors are greatful to Dumlupınar Research Center (DPU-ILTEM) for using the laboratory and equipments, to Didem Ocak and Arif Soylu for their help in experimental research.
Disclosure of conflict of interest
None.
References
- 1.Cerqueira NF, Hussni CA, Yoshida WB. Pathophysiology of mesenteric ischemia/reperfusion: a review. Acta Cir Bras. 2005;20:336–43. doi: 10.1590/s0102-86502005000400013. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad ST, Arjumand W, Nafees S, Seth A, Ali N, Rashid S, Sultana S. Hesperidin alleviates acetaminophen induced toxicity in Wistar rats by abrogation of oxidative stress, apoptosis and inflammation. Toxicol Lett. 2012;208:149–61. doi: 10.1016/j.toxlet.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Acosta S. Epidemiology of mesenteric vascular disease: clinical implications. Semin Vasc Surg. 2010;23:4–8. doi: 10.1053/j.semvascsurg.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Ritter T, Kupiec-Weglinski JW. Gene therapy for the prevention of ischemia/reperfusion injury in organ transplantation. Curr Gene Ther. 2005;5:101–9. doi: 10.2174/1566523052997451. [DOI] [PubMed] [Google Scholar]
- 5.Ritter T, Nosov M, Griffin MD. Gene therapy in transplantation: Toward clinical trials. Curr Opin Mol Ther. 2009;11:504–12. [PubMed] [Google Scholar]
- 6.Uglyanitsa KN, Nefyodov LI, Doroshenko YM. Ukrain: a novel antitumor drug. Drugs Exp Clinical Res. 2006;26:341–56. [PubMed] [Google Scholar]
- 7.Cory S, Huang DCS, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 8.Ashkenazi A, Herbst RS. To kill a tumor cell: the potential of proapoptotic receptor agonists. J Clin Invest. 2008;118:1979–1990. doi: 10.1172/JCI34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akcilar R, Akcilar A, Savran B, Ayada C, Koçak C, Koçak FE, Genç O. Effects of ukrain in rats with intestinal ischemia and reperfusion. J Surg Res. 2015;195:67–73. doi: 10.1016/j.jss.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 10.Arumugam TV, Shiels IA, Margolin SB, Taylor SM, Brown L. Pirfenidone attenuates ischaemıaereperfusion injury in the rat small intestine. Clin Exp Pharmacol Physiol. 2002;29:996. doi: 10.1046/j.1440-1681.2002.03766.x. [DOI] [PubMed] [Google Scholar]
- 11.Zou L, Sato N, Attuwaybi BO, Kone BC. Delayed administrationofalpha-melanocyte-stimulatinghormoneor combined therapy with BAY 11-7085 protects against gut ischemia-reperfusion injury. Shock. 2003;20:469. doi: 10.1097/01.shk.0000091205.08003.fd. [DOI] [PubMed] [Google Scholar]
- 12.Cerqueira NF, Hussni CA, Yoshida WB. Pathophysiology of mesenteric ischemia/reperfusion: a review. Acta Cir Bras. 2005;20:336. doi: 10.1590/s0102-86502005000400013. [DOI] [PubMed] [Google Scholar]
- 13.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 15.Avgerinos ED, Kostopanagiotou G, Costopanagiotou C, Kopanakis N, Andreadou I, Lekka M, Nakos G, Smyrniotis V. Intestinal preconditioning ameliorates ischemia-reperfusion induced acute lung injury in rats: an experimental study. J Surg Res. 2010;160:294–301. doi: 10.1016/j.jss.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Xiao F, Gao W, Wang X, Chen T. Amplification activation loop between caspase-8 and -9 dominates artemisinin-induced apoptosis of ASTC-a-1 cells. Apoptosis. 2012;17:600–611. doi: 10.1007/s10495-012-0706-5. [DOI] [PubMed] [Google Scholar]
- 17.Zhang F, Tong L, Qiao H, Dong X, Qiao G, Jiang H, Sun X. Taurine attenuates multiple organ injury induced by intestinal ischemia reperfusion in rats. J Surg Res. 2008;149:101. doi: 10.1016/j.jss.2007.12.781. [DOI] [PubMed] [Google Scholar]
- 18.Takeshita M, Tani T, Harada S, Hayashi H, Itoh H, Tajima H, Ohnishi I, Takamura H, Fushida S, Kayahara M. Role of transcription factors in small intestinal ischemia-reperfusion injury and tolerance induced by ischemic preconditioning. Transplant Proc. 2010;42:3406–13. doi: 10.1016/j.transproceed.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 19.Mendoza J, Zamora R, Gallardo JC, Ceballos G, Aldana A, Espinosa M, Maldonado V, Melendez-Zajgla J. NF-kappaB does not influence the induction of apoptosis by Ukrain. Cancer Biol Ther. 2006;5:788–93. doi: 10.4161/cbt.5.7.2752. [DOI] [PubMed] [Google Scholar]
- 20.Habermehl D, Kammerer B, Handrick R, Eldh T, Gruber C, Cordes N, Daniel PT, Plasswilm L, Bamberg M, Belka C, Jendrossek V. Proapoptotic activity of Ukrain is based on Chelidonium majus L. alkaloids and mediated via a mitochondrial death pathway. BMC Cancer. 2006;17:6–14. doi: 10.1186/1471-2407-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boise LH, González-García M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nuñez G, Thompson CB. Bcl-x, bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 22.Mori S, Sawada T, Okada T, Kubota K. Erythropoietin and its derivative protect the intestine from severe ischemia/reperfusion injury in the rat. Surgery. 2008;143:556–65. doi: 10.1016/j.surg.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Venkatesh K, Govindaraj S, Ramachandran A, Kalimuthu S, Perumal E, Velayutham S, Jagadeesan A. Effect of ukrain on cell survival and apoptosis in the androgen-independent prostate cancer cell line PC-3. J Environ Pathol Toxicol Oncol. 2011;30:11–9. doi: 10.1615/jenvironpatholtoxicoloncol.v30.i1.20. [DOI] [PubMed] [Google Scholar]
- 24.Green SJ, Scheller LF, Marletta MA, Seguin MC, Klotz FW, Slayter M, Nelson BJ, Nacy CA. Nitric oxide: cytokine-regulation of nitric oxide in host resistance to intracellular pathogens. Immunol Lett. 1994;43:87–94. doi: 10.1016/0165-2478(94)00158-8. [DOI] [PubMed] [Google Scholar]
- 25.Mungrue IN, Husain M, Stewart DJ. The role of NOS in heart failure: lessons from murine genetic models. Heart Fail Rev. 2002;7:407–22. doi: 10.1023/a:1020762401408. [DOI] [PubMed] [Google Scholar]
- 26.Naito Y, Takagi T, Uchiyama K, Handa O, Tomatsuri N, Imamoto E, Kokura S, Ichikawa H, Yoshida N, Yoshikawa T. Suppression of intestinal ischemia-reperfusion injury by a specific peroxisome proliferator-activated receptorgamma ligand pioglitazone in rats. Redox Rep. 2002;7:294–99. doi: 10.1179/135100002125000983. [DOI] [PubMed] [Google Scholar]
- 27.Wu B, Iwakiri R, Tsunada S, Utsumi H, Kojima M, Fujise T, Ootani A, Fujimoto K. iNOS enhances rat intestinal apoptosis after ischemia-reperfusion. Free Radic Biol Med. 2002;33:649–58. doi: 10.1016/s0891-5849(02)00917-6. [DOI] [PubMed] [Google Scholar]
- 28.Margaritis EV, Yanni AE, Agrogiannis G, Liarakos N, Pantopoulou A, Vlachos I, Papachristodoulou A, Korkolopoulou P, Patsouris E, Kostakis M, Perrea DN, Kostakis A. Effects of oral administration of (L)-arginine, (L)-NAME and allopurinol on intestinal ischemia/reperfusion injury in rats. Life Sci. 2011;88:1070. doi: 10.1016/j.lfs.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Kiang JG, Bowman PD, Lu X, Li Y, Wu BW, Loh HH, Tsen KT, Tsokos GC. Geldanamycin inhibits hemorrhage-induced increases in caspase-3 activity: role of inducible nitric oxide synthase. J Appl Physiol. 2007;103:1045–1055. doi: 10.1152/japplphysiol.00100.2007. [DOI] [PubMed] [Google Scholar]


