Abstract
Objective: A meta-analysis was carried out to systematically evaluate the correlation between hepatitis B virus (hepatitis B virus, HBV) infection and risk of non-Hodgkin’s lymphoma (non-Hodgkin lymphoma, NHL). Methods: We searched Medline, EMBASE, PubMed, Cochrane Library, Chinese Biomedical Literature Database, Chinese Journal Full-text Database, Chinese scientific journals full text databases and collected information about HBV infection and risk of NHL associated case-control studies. Two reviewers extracted useful information which were included in the study independently, and Revman 5.2 was used for meta-analysis. Results: A total of 24 studies were included in this research. Meta-analysis showed that among all of the included studies the heterogeneity were existed (I2 = 76%, P<0.05). With random effects model the OR was 2.39 (95% CI, 1.93-3.96), indicating infection rate in NHL patients with HBV was higher than that in the control group. Subgroup analysis according to ethnicity suggested that HBV infection were associated with NHL risk both in Asian (OR = 2.46, 95% CI: 2.01, 3.00, P<0.001) and Caucasian (OR = 2.15, 95% CI: 1.37, 3.37, P<0.001) population. Conclusion: HBV infection may increase the risk of NHL, but it still need a large number of experiments and epidemiological studies to verify.
Keywords: Non-Hodgkin’s lymphoma, Hepatitis B virus, risk, meta-analysis
Introduction
Hepatitis B virus (hepatitis B virus, HBV) infection has become a major public health problem in the world [1]. There were about $ 350 million chronically infected patients worldwide [2]. There were about 62 million people each year died of liver disease that associated with HBV infection [3]. The hepatitis B infection situation in China is more severe [4]. Lymphoma accounted for the eighth in our common malignancies, which accounted for the fifth most common malignancy in the United States [5]. In recent years, its incidence is rising. Lymphoma were divided into Hodgkin’s lymphoma (Hodgkin lymphoma, HL) and non-Hodgkin’s lymphoma (non-Hodgkin lymphoma, NHL) two kinds by World Health Organization. Genetic, environmental and infectious factors together caused lymphoma [6]. NHL etiology is not fully understood. We have come to realize that different subtypes of NHL prognosis were different, and the causes may be different. HBV infection caused only not damage the liver, but also caused systemic reactions. There was a higher HBV DNA detection rate in peripheral blood mononuclear cells. NHL patients receiving chemotherapy may cause latent infection of HBV reactivation, and it can even lead to acute liver failure in severe cases. In recent years, a large number of studies concentrated on the correlation between incidence of HBV infection and lymphoma. However, the result is often inconsistent. In this study, we carried out the relevant case-control meta-analysis in order to provide a basis for clinical diagnosis and treatment decisions.
Materials and methods
Literature searching and screening
“hepatitis B virus, hepatitis B, hepatitis B”, and “lymphoma, lymphoma”, were set as the key words and literatures were retrieved from Medline, EMBASE, PubMed, Cochrane Library databases, Chinese Biomedical Literature Database, Chinese Journal Full-text databases and full-text databases Chinese scientific journals. At the same time we retrieved literatures from the world’s major academic institutions website ASCO, EMSO, NCCN, and Google, Medical Matrix and other searching engines were used to find the relevant literature on the Internet.
Literature inclusion and exclusion criteria
Inclusion criteria
literatures about the correlation between HBV infection and incidence of NHL case-control study and nested case-control study; NHL patients were confirmed by cytology or pathology; Hepatitis B virus infection was defined as a serum HBsAg positive; Languages were English and Chinese; Patient’s race, nationality, age and geographical were not limited; In literatures there were sufficient data to calculate the odds ratio (odd radio, OR) and 95% confidence intervals (confidence intervals, C1) to assess the infection difference in NHL group compared with the HBV infection in the control group.
Exclusion criteria
The descriptions only concentrated on infection rate of HBV in patients with NHL while there was no descriptive study in control group. The literatures only concentrated on HL patients or there was no distinguish between NHL and HL patients; there was no clear definition on HBV infection or the definition was any one of the five items in two pairs of semi-detection was positive in literatures.
Based on the above criteria, two investigators read literatures independently from title and abstract to full text and screened the literatures layer by layer. For example, they first read the title and summary of the obtained documents and excluded the literatures which obviously did not meet the inclusion criteria. Then they read the full text of the document which may meet the inclusion criteria to identify whether the literature truly meet the inclusion criteria. If there were controversies or differences in determining whether they meet the inclusion criteria, a third investigator was enrolled in to decide whether to include it. For repetitive published literatures, we selected the latest and most comprehensive reports.
Finally two researchers extracted information from the included literatures independently, including the first author’s name, publication date, race, region, the source of patients in the control group, age, sex, the number of non-HBV-infectious and HBV infectious in the control and case group. They filled the form, extracted the table, and did cross-check, and then finally reached consensus.
Statistical analysis
Heterogeneity between the studies were assessed and calculated by Q test. Finally we calculated the combined value of OR and 95% CI in the studies to assess the correlation between risk of NHL and HBV. When P was less than 0.05 in z test, the combined OR values showed statistical significance. When there was no heterogeneity between studies (P>0.1, I2<50%), fixed effects model was used for meta-analysis in each study. If there is heterogeneity (P<0.1, I2>50%), the random effects model was used, and subgroup analyzes were based on possible sources of heterogeneity. Funnel plot was used to assess publication bias. In Egger test when P<0.1, publication bias showed statistical significance. The two researchers input the data into Revman 5.2 and did meta-analysis independently and finally they achieved the consistent results.
Results
Literature search results and the feature of research
444 documents were obtained after initial screening; by reading titles, abstracts and full text, duplicate publication was excluded, and according to the inclusion criteria and exclusion criteria, through primary screening and secondary screening, ultimately 24 literatures [7-30] were included, the flow chart as shown in Figure 1. A total of 46, 455 NHL patients and 1, 680, 957 controls were included (Table 1).
Figure 1.
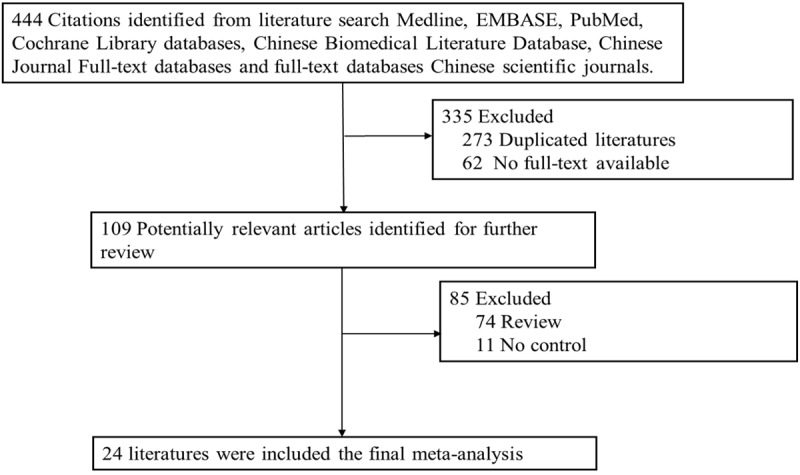
The flow chart of literature identified.
Table 1.
Characteristics of the included studies
| First author | Publication Year | Country | Ethnicity | Number of case | Number of control | OR | 95% CI |
|---|---|---|---|---|---|---|---|
| Anderson et al. | 2008 | USA | Caucasion | 33940 | 122531 | 0.92 | 0.70-1.22 |
| Becker et al. | 2012 | Germany | Caucasion | 1518 | 1496 | 1.59 | 0.65-3.90 |
| Becker et al. | 2009 | European | Caucasion | 2362 | 2465 | 1.8 | 1.17-2.76 |
| Cocco et al. | 2008 | Italy | Caucasion | 164 | 334 | 1.71 | 0.80-3.65 |
| Cuculanu et al. | 1999 | Romania | Caucasion | 68 | 943 | 6.96 | 3.89-12.40 |
| EI-Saued | 2006 | Egypt | Caucasion | 29 | 36 | 2.59 | 0.22-30.12 |
| Franceschi et al. | 2011 | European | Caucasion | 739 | 2028 | 2.55 | 1.12-5.81 |
| Kang et al. | 2011 | South Korea | Asian | 2094 | 15562 | 4.3 | 1.59-14.52 |
| Kim et al. | 2002 | Italy | Caucasion | 222 | 883 | 2.26 | 1.39-3.67 |
| Kim et al. | 2011 | South Korea | Asian | 344 | 404 | 2.26 | 1.39-3.67 |
| Kuniyoshi et al. | 2001 | South Korea | Asian | 348 | 1513358 | 1.96 | 1.20-3.19 |
| Lim | 2007 | Singapore | Asian | 556 | 4698 | 2.68 | 1.97-3.65 |
| Luo et al. | 2011 | China | Asian | 316 | 316 | 2.71 | 1.72-4.27 |
| Luol et al. | 2010 | China | Asian | 1279 | 1340 | 1.5 | 1.21-1.87 |
| Lwata et al. | 2004 | Japan | Asian | 145 | 574 | 4.8 | 1.59-14.52 |
| Ma et al. | 2010 | China | Asian | 67 | 67 | 3.19 | 1.16-8.75 |
| Marcucci et al. | 2006 | Italy | Caucasion | 399 | 392 | 3.23 | 1.61-6.46 |
| Mehdi | 2006 | Saudi Arabia | Caucasion | 565 | 11118 | 3.76 | 2.85-4.95 |
| Park et al. | 2008 | South Korea | Asian | 235 | 235 | 1.86 | 1.02-3.37 |
| Qin et al. | 2007 | China | Asian | 109 | 128 | 4.14 | 2.21-7.75 |
| Sonmez | 2007 | Turkey | Caucasion | 109 | 551 | 0.69 | 0.24-1.99 |
| Wang et al. | 2007 | China | Asian | 586 | 1237 | 2.16 | 1.70-2.75 |
| Yan et al. | 2009 | China | Asian | 132 | 132 | 4.04 | 2.00-8.16 |
| Zhang et al. | 2010 | China | Asian | 129 | 129 | 2.83 | 1.37-5.83 |
Meta-analysis results
Meta-analysis showed that, HBV infection rate in NHL patients was higher than that in the control group, with statistical significance. Meta-analysis showed that among all of the included studies the heterogeneity were existed (I2 = 76%, P<0.05). With random effects model the OR was 2.39 (95% CI, 1.93-3.96), indicating infection rate in NHL patients with HBV was higher than that in the control group (Figure 2). Subgroup analysis according to ethnicity suggested that HBV infection were associated with NHL risk both in Asian (OR = 2.46, 95% CI: 2.01, 3.00, P<0.001, Figure 3) and Caucasian (OR = 2.15, 95% CI: 1.37, 3.37, P<0.001, Figure 4) population.
Figure 2.
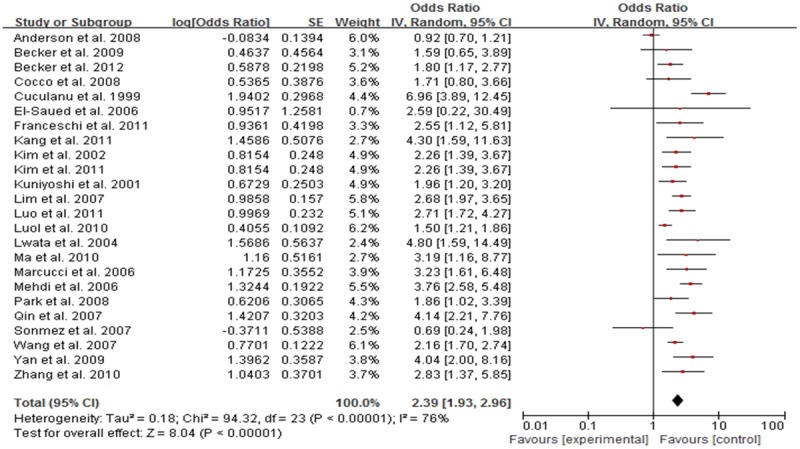
Forest plot of the relation between HBV infection and NHL risk in total population.
Figure 3.
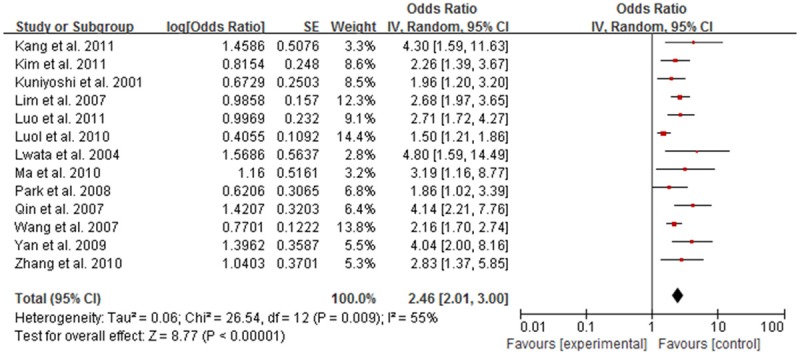
Forest plot of the relation between HBV infection and NHL risk in Asian population.
Figure 4.
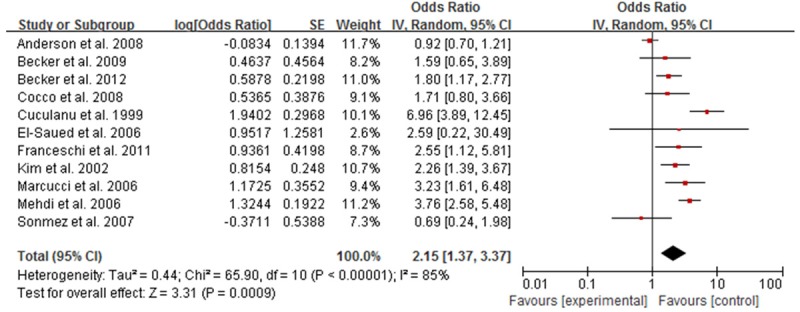
Forest plot of the relation between HBV infection and NHL risk in Caucasian population.
Publication bias of the included literature
Funnel plot was used to test the publication bias of all included studies. Funnel plot shape of all included studies prompted no obvious asymmetry (Figure 5), suggesting no obvious publication bias.
Figure 5.
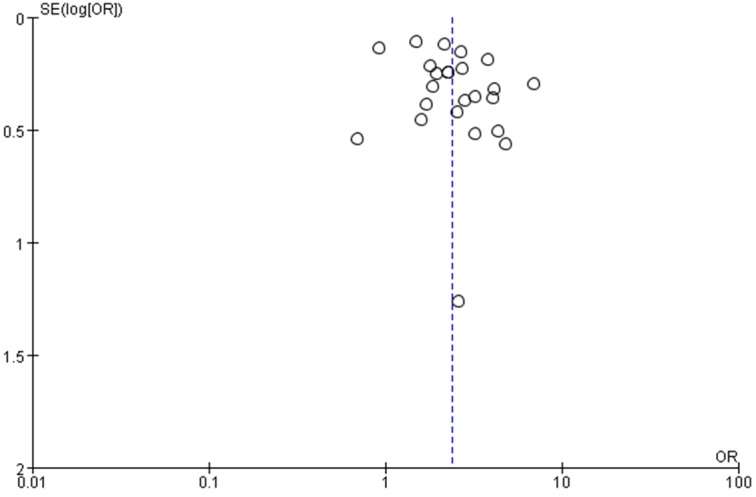
Funnel plot for publication bias tests.
Discussion
At present, there are a large number of reports on the correlation between pathogen infection and the incidence of lymphoma, such as Epstein-Barr virus and Hodgkin’s lymphoma, Burkitt’s lymphoma and primary central nervous system lymphoma, human T-cell leukemia virus type 1 (HTLV-1) and T lymphocytic leukemia, and Helicobacter pylori and gastric lymphoma [31-35]. HBV is a hepatotropic virus, and it also has a pro-lymphocyte characteristic. In the case of undetectable HBV antigen, expression of HBV DNA and HBV antigen is often detectable in peripheral mononuclear cells and lymphoid tissues. HBV infection can stimulate the expression, production and release of hematopoietic tumor growth factor; long-term chronic stimulation results in clonal expansion of lymphocytes. In addition, HBV DNA can also be integrated into the lymphocyte cell genome, thereby activating Bc1.2 oncogene or leading to its translocation.
The study screened and obtained the literature meeting the requirements of quality through comprehensive literature collection, and strict inclusion and exclusion criteria. Meta-analysis results suggest that, HBV infection rate in NHL patients was about 2.39 times of that in the control group. Because there is a higher prevalence of HBV infection in patients with NHL, the results of this study is important for clinical treatment. It has been reported that when NHL patients receiving immunosuppressive chemotherapy, there was a risk of HBV reactivation; the patient may appear asymptomatic elevated ALT, fatigue, nausea, vomiting, jaundice and other manifestations of viral hepatitis; severe cases may even appear peritoneal effusion, coagulation abnormalities, hepatic encephalopathy and other signs of liver failure. HBV reactivation leads to the interruption of anti-tumor therapy, seriously affecting its therapeutic effect and increasing mortality. Therefore, it needs careful measurement of HBV infection for NHL patients before chemotherapy; Lamivudine may play a preventive role when infected patients receiving chemotherapy.
This article included a larger number of patients and involved a wider scope, providing a strong support for the reliance of results. But there are still some limitations. Firstly, the included studies had statistical heterogeneity; although there was no statistical heterogeneity among nested case-control studies after subgroup analysis, most of the literature did not match the age and gender, and liver diseases were not classified; NHL typing was also not performed, and the merger of HCV infection was not clear; at the same time, control populations included healthy populations and other cancer patient populations; these factors can affect the reliability of the results. Secondly, there is not enough data to assess the effect of environmental factors on the correlation between HBV and NHL risk. Finally, although no significant publication bias had been found by statistical test, the results of this paper were still possibly false positive because of some unpublished negative results. Given the limitations of literature included in this study may affect the authenticity of the conclusions of this study to a certain extent, we look forward to conducting a large sample study based on the case-control studies with more rigorous design and higher quality to further confirm the results.
The results of the study showed that there was a higher infection rate of HBV in patients with NHL; before chemotherapy, the use of lamivudine can prevent viral reactivation and liver failure in patients with HBV infection during chemotherapy; while whether HBV infection was one of the reasons for NHL incidence needs further research to confirm.
Disclosure of conflict of interest
None.
References
- 1.Rapti I, Hadziyannis S. Risk for hepatocellular carcinoma in the course of chronic hepatitis B virus infection and the protective effect of therapy with nucleos(t)ide analogues. World J Hepatol. 2015;7:1064–73. doi: 10.4254/wjh.v7.i8.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, Neitzel SM, Ward JW Centers for Disease Control and Prevention (CDC) Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1–20. [PubMed] [Google Scholar]
- 3.Persico E, De Renzo A, La Mura V, Bruno S, Masarone M, Torella R, Persico M. Occult hepatitis B virus infection in patients with non-Hodgkin lymphoma: The need for early diagnosis in anti-Hbv positive patients. Gut. 2007;56:1470–1471. doi: 10.1136/gut.2007.128777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang G, Han M, Chen F, Xu Y, Chen E, Wang X, Liu Y, Sun J, Hou J, Ning Q, Wang Z. Hepatitis B virus genotype B and mutations in basal core promoter and pre-core/core genes associated with acute-on-chronic liver failure: a multicenter cross-sectional study in China. Hepatol Int. 2014;8:508–516. doi: 10.1007/s12072-014-9554-4. [DOI] [PubMed] [Google Scholar]
- 5.Martin P, Lau DT, Nguyen MH, Janssen HL, Dieterich DT, Peters MG, Jacobson IM. A Treatment Algorithm for the Management of Chronic Hepatitis B Virus Infection in the United States: 2015 Update. Clin Gastroenterol Hepatol. 2015;13:2071–2087. e16. doi: 10.1016/j.cgh.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Laurent C, Do C, Gourraud PA, de Paiva GR, Valmary S, Brousset P. Prevalence of Common Non-Hodgkin Lymphomas and Subtypes of Hodgkin Lymphoma by Nodal Site of Involvement: A Systematic Retrospective Review of 938 Cases. Medicine (Baltimore) 2015;94:e987. doi: 10.1097/MD.0000000000000987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cucuianu A, Patiu M, Duma M, Basarab C, Soritau O, Bojan A, Vasilache A, Mates M, Petrov L. Hepatitis B and C virus infection in Romanian non-Hodgkin’s lymphoma patients. Br J Haematol. 1999;107:353–356. doi: 10.1046/j.1365-2141.1999.01692.x. [DOI] [PubMed] [Google Scholar]
- 8.Kuniyoshi M, Nakamuta M, Sakai H, Enjoji M, Kinukawa N, Kotoh K, Fukutomi M, Yokota M, Nishi H, Iwamoto H, Uike N, Nishimura J, Inaba S, Maeda Y, Nawata H, Muta K. Prevalence of hepatitis B or C virus infections in patients with non-Hodgkin’s lymphoma. J Gastroenterol Hepatol. 2001;16:215–219. doi: 10.1046/j.1440-1746.2001.02406.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Bang YJ, Park BJ, Yoo T, Kim CW, Kim TY, Heo DS, Lee HS, Kim NK. Hepatitis B virus infection and B-cell non-Hodgking lymphoma in a hepatitis B endemic area: A case-control study. Jpn J Cancer Res. 2002;93:471–477. doi: 10.1111/j.1349-7006.2002.tb01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lwata H, Matsuo K, Takeuchi K, Kishi Y, Murashige N, Kami M. High incidences of malignant lymphoma in patients infected with hepatitis B or hepatitis C virus. Haematologica. 2004;89:369–370. [PubMed] [Google Scholar]
- 11.E-Sayed GM, Mohamed WS, Nouh MA, Moneer MM, El-Mahallawy HA. Viral genomes and antigen detection of hepatitis B and C viruses in involved lymph nodes of egyptian non-Hodgkin’s lymphoma patients. Egypt J Immunol. 2006;13:105–114. [PubMed] [Google Scholar]
- 12.Marcucci F, Mele A, Spada E, Candido A, Bianco E, Pulsoni A, Chionne P, Madonna E, Cotichini R, Barbui A, De Renzo A, Dore F, Iannitto E, Liso V, Martino B, Montanaro M, Pagano L, Musto P, Rapicetta M. High prevalence of hepatitis B virus infection in B-cell non-Hodgking lymphoma. Haematologica. 2006;91:554–557. [PubMed] [Google Scholar]
- 13.Mehdi SR, AAjlan AR. Hepatitis B & C virus infection in cases of non-Hodgkin’s lymphomain Saudi Arabia. Turk J Hematol. 2006;23:200–204. [PubMed] [Google Scholar]
- 14.Lim ST, Fei G, Quek R, Lim LC, Lee LH, Yap SP, Loong S, Tao M. The relationship of hepatitis B virus infection and non-Hodgkin’s lymphoma and its impact on clinical characteristics and prognosis. Eur J Haematol. 2007;79:132–137. doi: 10.1111/j.1600-0609.2007.00878.x. [DOI] [PubMed] [Google Scholar]
- 15.Martin P, Lau DT, Nguyen MH, Janssen HL, Dieterich DT, Peters MG, Jacobson IM. A Treatment Algorithm for the Management of Chronic Hepatitis B Virus Infection in the United States: 2015 Update. Clin Gastroenterol Hepatol. 2015;13:2071–2087. e16. doi: 10.1016/j.cgh.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Wang F, Xu RH, Hart B, Shi YX, Luo HY, Jiang WQ, Lin TY, Huang HQ, Xia ZJ, Guan ZZ. High incidence of hepatitis B virus infection in B-cell subtype non-Hodgkin lymphoma compared with other cancers. Cancer. 2007;109:1360–1364. doi: 10.1002/cncr.22549. [DOI] [PubMed] [Google Scholar]
- 17.Park SC, Jeong SH, Kim J, Kim YC, Choi KS, Cho JH, Lee M, Jung HH, Ki SS, Chang YH, Lee SS, Park YH, Lee KH. High prevalence of hepatitis B virus infection in patients with B-cell non-Hodgkin’s lymphoma in Korea. J Med Virol. 2008;80:960–966. doi: 10.1002/jmv.21168. [DOI] [PubMed] [Google Scholar]
- 18.Anderson LA, Pfeiffer R, Warren JL, Landgren O, Gadalla S, Berndt SI, Ricker W, Parsons R, Wheeler W, Engels EA. Hematopoietie malignancies associated with viral and alcoholic hepatitis. Cancer Epidemiol Biomarkers Prev. 2008;17:3069–3075. doi: 10.1158/1055-9965.EPI-08-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cocco P, Piras G, Monne M, Uras A, Gabbas A, Ennas MG, Palmas A, Murineddu M, Collu S, Melis M, Rais M, Todde P, Cabras MG, Angelucci E, Massarelli G, Nieters A. Risk of malignant lymphoma following viral hepatitis infection. Int J Hematol. 2008;87:474–483. doi: 10.1007/s12185-008-0086-3. [DOI] [PubMed] [Google Scholar]
- 20.Becker N, Fortuny J, Alvaro T, Nieters A, Maynadié M, Foretova L, Staines A, Brennan P, Boffetta P, Cocco PL, de Sanjose S. Medical history and risk of lymphoma: Results of a European case-control study (EPILYMPH) J Cancer Res Clin Oncol. 2009;135:1099–1107. doi: 10.1007/s00432-009-0551-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franceschi S, Lise M, Trepo C, Berthillon P, Chuang SC, Nieters A, Travis RC, Vermeulen R, Overvad K, Tjønneland A, Olsen A, Bergmann MM, Boeing H, Kaaks R, Becker N, Trichopoulou A, Lagiou P, Bamia C, Palli D, Sieri S, Panico S, Tumino R, Sacerdote C, Bueno-de-Mesquita B, Peeters PH, Rodríguez L, Barroso LL, Dorronsoro M, Sánchez MJ, Navarro C, Barricarte A, Regnér S, Borgquist S, Melin B, Hallmans G, Khaw KT, Wareham N, Rinaldi S, Hainaut P, Riboli E, Vineis P. Infection with hepatitis B and C viruses and risk of lymphoid malignancies in the European prospective investigation into cancer and nutrition (EPIC) Cancer Epidemiol Biomarkers Prev. 2011;20:208–214. doi: 10.1158/1055-9965.EPI-10-0889. [DOI] [PubMed] [Google Scholar]
- 22.Kang J, Cho JH, Suh CW, Lee DH, Oh HB, Sohn YH, Chi HS, Park CJ, Jang SS, Lee KH, Lee JH, Lee JH, Lee SW, Chung YH, Kim TH, Shin HR, Huh J. High prevalence of hepatitis B and hepatitis C virus infections in Korean patients with hematopoietic malignancies. Ann Hematol. 2011;90:159–164. doi: 10.1007/s00277-010-1055-5. [DOI] [PubMed] [Google Scholar]
- 23.Kim YM, Jeong SH, Kim JW, Lee SH, Hwang JH, Park YS, Kim N, Lee JS, Kim HY, Lee DH. Chronic hepatitis B, non-Hodgkin’s lymphoma, and effect of prophylactic antiviral therapy. J Clin Virol. 2011;51:241–245. doi: 10.1016/j.jcv.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Luo LH, Fan Y, Huang ZY. Correlation between HBV infection and NHL. Zhong Hua Zhong Liu Fang Zhi Za Zhi. 2010;17:1414–1417. [Google Scholar]
- 25.Yan XT, Zhang MZ, Chang Y. Analysis of the relationship between NHL and HBV infection. Yi Yao Lun Tai Za Zhi. 2009;30:24–25. [Google Scholar]
- 26.Zhang SL, Chen DP, Wang BQ. Analysis of the relationship between NHL and HBV infection. Xian Dai Zhong Liu Yi Xue. 2010;18:1005–1006. [Google Scholar]
- 27.Ma AF. Relationship between HBV infection in patients with NHL and Prognosis. Lin Chuang Yi Yao Shi Jian. 2011;20:173–175. [Google Scholar]
- 28.Qin XT, Lv Y, Chen XQ, Xu HP, Fan HJ. Relationship between HBV infection and NHL. Ai Zheng. 2007;26:294–297. [PubMed] [Google Scholar]
- 29.Luo CL, Zhao XH. Clinical analysis of NHL with hepatitis B virus infection. Zhong Guo Shi Yong Yi Yao. 2011;6:108–109. [Google Scholar]
- 30.Becker N, Schnitzler P, Boffetta P, Brennan P, Foretova L, Maynadié M, Nieters A, Staines A, Benavente Y, Cocco P, de Sanjose S. Hepatitis B virus infection and risk of lymphoma: Results of a serological analysis within the European case-control study Epilymph. J Cancer Res Clin Oncol. 2012;138:1993–2001. doi: 10.1007/s00432-012-1279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu L, Zhang HY, Yueng YH, Cheung KF, Luk JM, Wang FS, Lau GK. Intracellular levels of hepatitis B virus DNA and pregenomie RNA in peripheral blood mononuclear cells of chronically infected patients. J Viral Hepat. 2009;16:104–112. doi: 10.1111/j.1365-2893.2008.01054.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Wang H, Wang JH, Xia ZJ, Lu Y, Huang HQ, Jiang WQ, Zhang YJ. Post-treatment plasma EBV-DNA positivity predicts early relapse and poor prognosis for patients with extranodal NK/T cell lymphoma in the era of asparaginase. Oncotarget. 2015;6:30317–26. doi: 10.18632/oncotarget.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maeshima AM, Taniguchi H, Nomoto J, Makita S, Kitahara H, Fukuhara S, Munakata W, Suzuki T, Maruyama D, Kobayashi Y, Tobinai K. Clinicopathological features of classical Hodgkin lymphoma in patients ≥40 years old, with special reference to composite cases. Jpn J Clin Oncol. 2015;45:921–8. doi: 10.1093/jjco/hyv101. [DOI] [PubMed] [Google Scholar]
- 34.Lu TX, Liang JH, Miao Y, Fan L, Wang L, Qu XY, Cao L, Gong QX, Wang Z, Zhang ZH, Xu W, Li JY. Epstein-Barr virus positive diffuse large B-cell lymphoma predict poor outcome, regardless of the age. Sci Rep. 2015;5:12168. doi: 10.1038/srep12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAulay KA, Jarrett RF. Human leukocyte antigens and genetic susceptibility to lymphoma. Tissue Antigens. 2015;86:98–113. doi: 10.1111/tan.12604. [DOI] [PubMed] [Google Scholar]


