Abstract
There are conflicting reports on the correlation between serum levels of ferritin with colorectal cancer. The purpose of the present study is to clarify the association between serum ferritin with colorectal cancer using a meta-analysis approach. We searched articles indexed in Pubmed published as of July 2015 that met our predefined criteria. Six eligible articles involving 927 subjects were identified. Overall, pooled analysis indicated that subjects with colorectal cancer had lower serum level of ferritin than the healthy controls (SMD=-1.569, 95% CI=[-2.718, -0.420], P= 0.007). Further subgroup analysis found lower serum level of ferritin among patients with colorectal cancer in eastern country (SMD=-1.956, 95% CI=[-3.750, -0.162], P=0.033), but not in western country (SMD=-1.285, 95% CI=[-2.778, 0.207], P=0.091). In conclusion, this meta-analysis supports a significant association between serum ferritin with colorectal cancer. However, the subgroup analysis found that there was significant effect modification of ferritin level by ethnic. Thus this finding needs further confirmation by trans-regional multicenter, long-term observation in a cohort design to obtain better understanding of causal relationships between serum ferrintin levels and colorectal cancer, through measuring ferritin at baseline to investigate whether the highest ferritin category versus lowest is associated with colorectal cancer risk.
Keywords: Ferritin, colorectal cancer, meta-analysis
Introduction
Colorectal cancer is the fourth most common cancer (after breast, lung, and prostate) and the second most common cause of cancer death (after lung) [1]. Iron is a necessary nutrient that has been associated with increased colorectal cancer risk [2,3]. Studies find that iron may be capable of mutagenic effects mediated through free-radical generation or tumor promotion through nutritional mechanisms [4,5]. Iron, as a transition metal, is possessed of loosely bound electrons that are capable of participating in lipid peroxidation reactions, which are thought to lead to DNA damage [6,7]. The single best indicator of iron stores is serum ferritin level [8,9]. Hyperferritinemia has often been reported in patients with liver and pancreatic cancer, and can be used as a tumor marker in these diseases [10,11]. However, the significance of ferritin as a tumor marker in gastrointestinal cancer is doubtful, because gastrointestinal cancer produces small amounts of ferritin, causes chronic anemia resulting from continuous bleeding, and occasionally infiltrates and destroys surrounding organs [1,12]. In colorectal cancer, serum ferritin has been reported to be either decreased or increased. Some clinical studies find that there is an inverse association between serum ferritin and colorectal cancer [1,13,14]. However, some studies suggest that there is no relationship between serum ferritin and colorectal cancer [15-17]. Although serum ferritin is plausibly linked to colorectal cancer, the inconsistency among the findings of previous studies precludes definitive recommendations at present.
Meta-analysis is a well established statistical tool that serves for integration of data from independent studies in order to formulate more general conclusions. The aim of this study was to assess the association between serum ferritin and colorectal cancer by conducting a meta-analysis of individual datasets from all eligible studies published to date.
Methods
Search strategy
We searched all English written articles indexed in Pubmed published up to July 2015. Literature searches were performed using medical subject heading (MeSH) or free text words. The searching keywords were: (“serum ferritin” OR ferritin) AND colorectal cancer. Reference lists of all eligible studies were screened to identify potentially eligible studies. Emails were sent to the authors of identified studies for additional information if necessary.
Selection criteria
Three authors (Zhe Feng, Ji-wei Chen, Bo Xu) conducted the search independently. Titles and abstracts were screened for subject relevance. Studies that could not be definitely excluded based on abstract information were also selected for full text screening. Two authors (Zhe Feng, Ji-Wei Chen) independently selected eligible studies for inclusion possibility. Where there was a disagreement for study inclusion, a discussion was held (with Bo Xu) to reach a consensus. The included studies should meet the following criteria: (1) human study; (2) studies focusing on the association between serum ferritin and colorectal cancer; (3) studies providing serum levels of ferritin for both subjects with colorectal cancer and healthy controls; (4) subjects with no other diseases and no drugs intake which might influence the serum levels of ferritin. Exclusion criteria included: (1) in vitro or laboratory study; (2) animal study; (3) review or case report; (4) studies not providing serum levels of ferritin for both subjects with colorectal cancer and healthy controls; (5) subjects with diseases/drugs which might influence the serum levels of ferritin; (6) sample size less than 10.
Data extraction and quality assessment
The following information was extracted from each included study: first author’s family name, year of publication, country, demography of subjects (age and number of patients), data on serum levels of ferritin, type of ferritin measurement. Two authors (Jian-hua Feng, Fei Shen) independently extracted data using a standard form.
Two authors (Wen-Song Cai, Jie Cao) assessed the quality independently. The qualities of all included studies were assessed using the Newcastle-Ottawa Scale (NOS). Using the tool, each study is judged on eight items, categorized into three groups: the selection of the study groups; the comparability of the groups; and the ascertainment of either the exposure or outcome of interest for case-control or cohort studies respectively. Stars awarded for each quality item serve as a quick visual assessment. Stars are awarded such that the highest quality studies are awarded up to nine stars. Studies were graded as good quality if they awarded 6 to 9 stars; fair if they awarded 3 to 5 stars; and poor if they awarded less than 3 stars.
Statistical analysis
The extracted data were used to perform meta-analysis to obtain the standardized mean difference (SMD) and 95% confidence intervals (CI). The SMDs were calculated using either fixed-effects models or, in the presence of heterogeneity, random-effects models. Hete-rogeneity between studies was tested through the Chi-square and I-square tests. If the I2 value was greater than 50% and the P value was less than 0.05, the meta-analysis was considered as homogeneous. Subgroup analyses involve splitting all the participant data into subgroups, often so as to make comparisons between them, which can be done as a means of investigating heterogeneous results. Subgroup analyses stratified by the geographical location, sample size and method for assessment were used to identify associations between serum levels of ferritin and other relevant study characteristics as possible sources of heterogeneity. Publication bias was defined as the publication or non-publication of studies depending on the direction and statistical significance of the results, and was measured using Begg’s test and visualization of funnel plot. The stability of the study was also detected by sensitivity analysis, through re-meta-analysis with one involved study excluded each time. All statistical analyses were performed with Stata version 11.0 (StataCorp, College Station, TX).
Results
Literature search
The literature search yielded a total of 131 primary articles. These articles were included for full-text assessment, of which 125 were excluded for one of the following reasons: (1) irrelevant to our topic (n=99), (2) non-original studies (reviews, etc.) (n=7), (3) non-human studies (n=12), (4) articles not providing serum level of ferritin for both subjects with colorectal cancer and healthy controls (n=7). Overall, 6 eligible articles with 7 case-control studies involving 927 subjects met the inclusion criteria for meta-analysis [1,13-17]. A flow diagram of the study selection process is presented in Figure 1.
Figure 1.
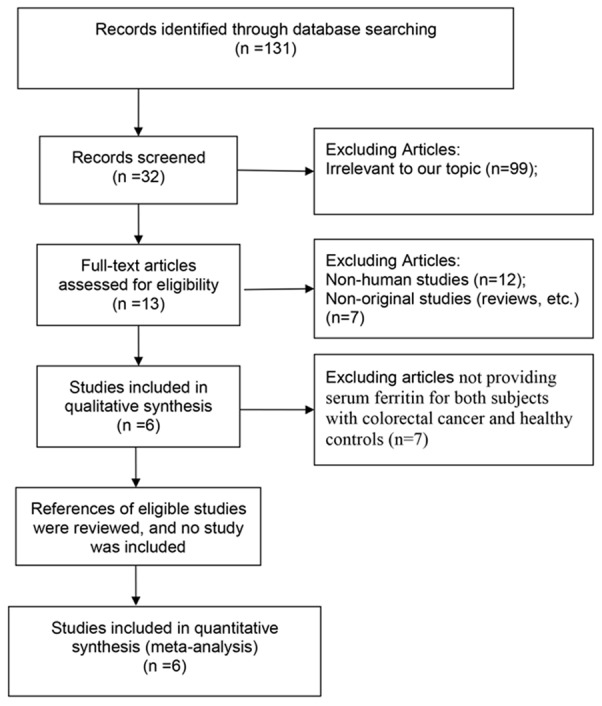
Flow diagram of screened and included papers.
Study characteristics and quality assessment
The characteristics of the included studies and the results of the quality assessment were listed in Table 1. The earliest study was published in 1994, and the latest in 2011. By geographic location, 6 articles with 7 case-control studies were conducted in 5 different countries (USA, Japan, Poland, Belgium, and Turkey). The number of subjects in each study ranged from 41 to 359. 2 articles with 3 case-control studies measured ferritin concentration by radioimmunoassay (RIA), 2 articles with 2 case-control studies measured ferritin concentration by total-reflection X-ray fluorescence (TRXRF), while 1 article with 1 case-control studies measured ferritin concentration by electrochemiluminescence immunoassay (ECLIA). The overall study quality averaged 6 stars on a scale of 0 to 9.
Table 1.
Characteristics of subjects in eligible studies
| Studies | Country | Measurement | Colorectal cancer | Healthy controls | Quality score | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Age (year) | N | Concentration (mean ± SD) | Age (year) | N | Concentration (mean±SD) | ||||
| Nelson 1994 | USA | RIA | 26~87 | 29 | 125±161 ng/ml | 26~87 | 159 | 170±183 ng/ml | 6 |
| Kishida 1994-1 | Japan | RIA | 62.0±10.8 | 23 | 48.8±72.8 ng/ml | 60.7±8.9 | 23 | 117.1±46.8 ng/ml | 5 |
| Kishida 1994-2 | Japan | RIA | 59.6±10.1 | 18 | 80.5±35 ng/ml | 60.7±8.9 | 23 | 117.1±46.8 ng/ml | 5 |
| Gackowski 2002 | Poland | TRXRF | 26~87 | 45 | 232.32±278.09 ng/ml | 44~90 | 51 | 204.93±207.21 ng/ml | 6 |
| Kucharzewski 2003 | Poland | TRXRF | 25~86 | 67 | 60.4±9.6 ng/ml | 25~45 | 50 | 102.6±6.4 ng/ml | 7 |
| Joosten 2008 | Belgium | NR | 83.7±5 | 55 | 114±161 ng/ml | 82±5.7 | 304 | 144±217 ng/ml | 6 |
| Gür 2011 | Turkey | ECLIA | NR | 40 | 54.78±63.22 ng/ml | NR | 40 | 287.86±55.6 ng/ml | 6 |
RIA, Radioimmunoassay. TRXRF, Total-reflection X-ray fluorescence. ECLIA, electrochemiluminescence immunoassay. NR, not reported.
Serum ferritin and colorectal cancer
The random-effects meta-analysis results indicated that patients with colorectal cancer had lower serum levels of ferritin than the healthy controls (SMD=-1.569, 95% CI=[-2.718, -0.420], P=0.007). The 7 sets of results showed a statistically significant amount of heterogeneity (I2=97.5%, P<0.001) (Figure 2).
Figure 2.
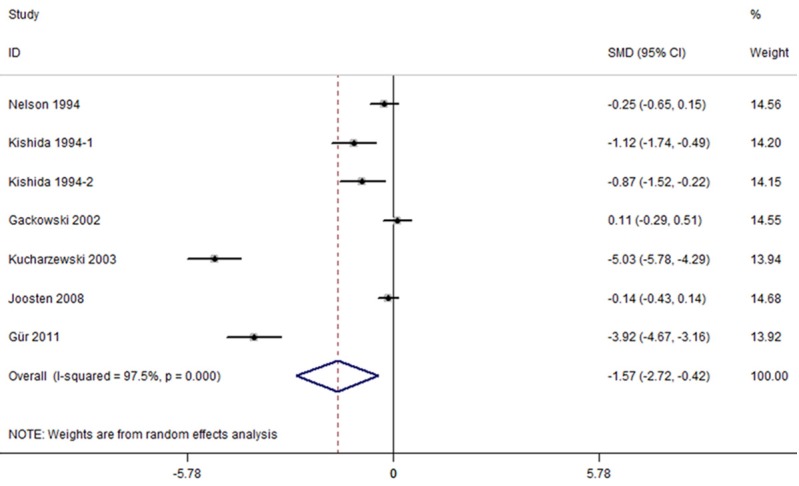
Forest plots of studies in serum ferritin for subjects with colorectal cancer versus healthy controls. The combined SMD and 95% confidence intervals (CIs) were calculated using the random-effects model.
The subgroup analysis showed that geographical location, method for assessment and sample size had an influence on the serum levels of ferritin in colorectal cancer and healthy controls. Further subgroup analysis stratified by geographical location indicated that subjects with colorectal cancer had lower serum level of ferritin than the healthy controls in eastern country (SMD=-1.956, 95% CI=[-3.750, -0.162], P=0.033), but not in western country (SMD=-1.285, 95% CI=[-2.778, 0.207], P=0.091) (Figure 3). The serum ferritin levels were lower in colorectal cancer than healthy controls measured by RIA and ECLIA (RIA: SMD=-0.700, 95% CI=[-1.262, -0.139], P=0.015; ECLIA: SMD=-3.915, 95% CI=[-4.670, -3.161], P<0.001), but not by TRXRF (SMD=-2.449, 95% CI=[-7.491, 2.592], P=0.341). The further subgroup analysis found lower serum ferritin levels in colorectal cancer than healthy controls with sample size less than 90 (SMD=-1.956, 95% CI=[-3.750, -0.162], P=0.033), but similar pattern was not found when sample size larger than 90 (SMD=-1.285, 95% CI=[-2.778, 0.207], P=0.091). Summary of further subgroup analysis is given in Table 3.
Figure 3.
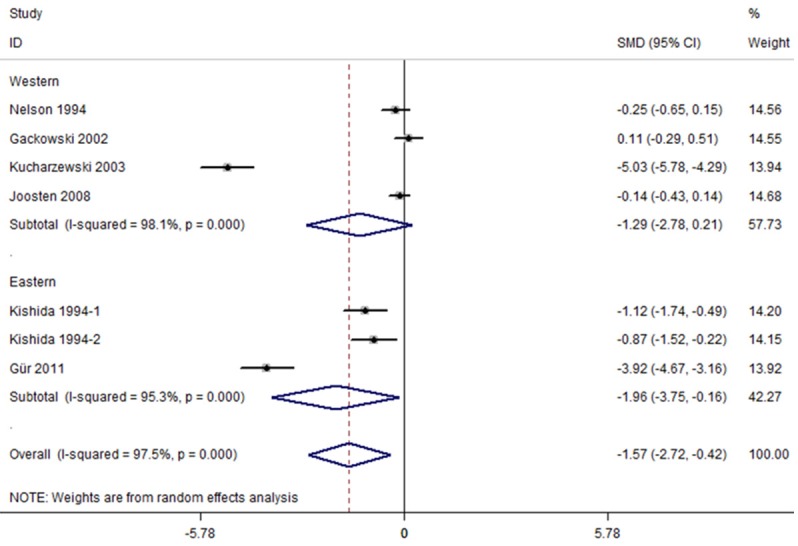
Subgroup analyses of studies in serum ferritin for subjects with colorectal cancer versus healthy controls.
Table 3.
Differences between studies by subgroup analysis
| Subgroups | Number of studies | SMD (95% CI) | Heterogeneity |
|---|---|---|---|
| Location | |||
| Eastern country | 3 | -1.956 (-3.750, -0.16) | 98.1% |
| Western country | 4 | -1.285 (-2.778, 0.207) | 95.3% |
| Sample size | |||
| ≥ 90 | 4 | -1.285 (-2.778, 0.207) | 98.1% |
| < 90 | 3 | -1.956 (-3.750, -0.162) | 95.3% |
| Method for assessment | |||
| RIA | 3 | -0.700 (-1.262, -0.139) | 68.1% |
| TRXRF | 2 | -2.449 (-7.491, 2.592) | 99.3% |
| ECLIA | 1 | -3.915 (-4.670, -3.161) | 0 |
RIA, Radioimmunoassay. TRXRF, Total-reflection X-ray fluorescence. ECLIA, electrochemiluminescence immunoassay.
Publication bias and sensitivity analysis
Publication bias was measured using Begg’s tests and visualization of funnel plots. There was no evidence of publication bias (Begg’s test: P=0.881) (Figure 4). Sensitivity analysis showed that excluding any one study from the pooled analysis did not vary the results substantially (Table 2).
Figure 4.
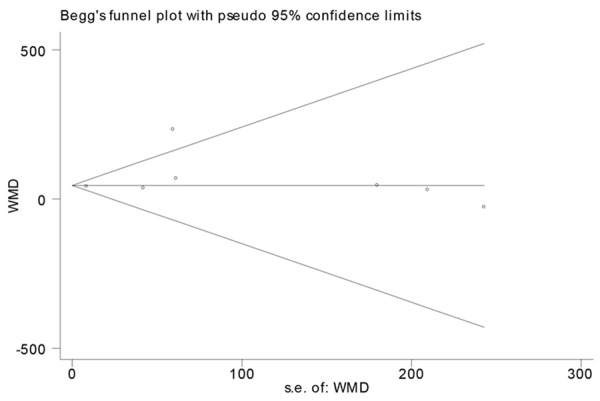
Funnel plots of studies in serum ferritin for subjects with colorectal cancer versus healthy controls.
Table 2.
The heterogeneity of the included studies through sensitivity analysis
| Excluded study | SMD (95% CI) | I2 (%) | P |
|---|---|---|---|
| Before excluding | -1.569 (-2.718, -0.420) | 97.5% | 0.007 |
| Nelson 1994 | -1.802 (-3.219, -0.385) | 97.8% | 0.013 |
| Kishida 1994-1 | -1.648 (-2.962, -0.335) | 97.9% | 0.014 |
| Kishida 1994-2 | -1.689 (-3.001, -0.377) | 97.9% | 0.012 |
| Gackowski 2002 | -1.861 (-3.233, -0.490) | 97.7% | 0.008 |
| Kucharzewski 2003 | -0.984 (-1.829, -0.138) | 95.0% | 0.023 |
| Joosten 2008 | -1.823 (-3.296, -0.351) | 97.7% | 0.015 |
| Gür 2011 | -1.181 (-2.256, -0.106) | 96.9% | 0.031 |
Discussion
Iron, as an element essential for mammalian life, is involved in oxygen transport [18]. It is known that iron takes part in the carcinogenic mechanism in three ways [19]. First, ferric ions are reduced by superoxide and reoxidized by peroxide to regenerate ferric ions and to yield hydroxyl radicals, which can alter normal cells by initiating autoxidation chain processes leading to cellular consequences include enhanced radiosensitivity, mutation, sister chromatid exchange, chromosomal aberrations, and oncogenesis. Second, iron can promote the growth of transformed cells by inhibiting host defenses. Iron-containing ferritin can suppress the tumoricidal activity of macrophages. Third, iron serves as an essential nutrient for unrestricted tumor cell multiplication. It has been speculated whether iron might be involved in the initiation or promotion of colonic disease, because of the high concentration of iron in the human colon [20]. The intimate mechanism by which iron induces carcinogenesis is still not understood, but evidence is accumulating indicating that iron toxicity is mediated by its capacity to produce free radicals [1,3].
In the present study, our systematic review of 927 participants from 7 case-control studies found that patients with colorectal cancer had lower serum levels of ferritin than the healthy controls. Campo et al. [21] found that colorectal cancer cells produced ferritin in their immunohistochemical study. Harju and Lindberg [22] noted that low serum ferritin concentrations, indicating empty iron stores, were common (40-50% of patients) in patients with colorectal cancer. Our observations are in favor of the interesting hypothesis, which suggests that most patients with advanced colorectal cancer, whether or not anemia is clinically evident, are considered to be in a state of iron deficiency because of continuous bleeding. The daily blood loss in the feces in a control subject has been reported to be less than 1 ml, which contains approx. 0.5 mg of iron; thus, a steady blood loss of as little as 3~4 ml/d (1.5-2.0 mg iron) can result in a negative iron balance [23].
To the best of our knowledge, this is the first meta-analysis to estimate the association between serum levels of ferritin with colorectal cancer. We made sure to minimize the bias by means of study procedure. Not only did we search Pubmed to identify potential studies, but also we manually examined all reference lists from relevant studies. Sensitivity analysis showed that excluding any one study from the pooled analysis did not vary the results substantially. Publication bias was also absent, as determined by Begg’s test. However, the possible limitations of our study must be considered. First, there were only 927 subjects from 7 case-control studies included in the meta-analysis, which might weaken the quality of the results. Second, the heterogeneity could not be eliminated because of methodological diversities between studies, thus the conclusion should be conservative. Despite these limitations, it is still worth conducting the meta-analysis at this point of time. First, the inconsistency among the findings of previous studies for the association between ferritin with colorectal cancer precludes definitive recommendations, and our present meta-analysis draw a more clear and evidence-based conclusion on the association between serum ferritin with colorectal cancer. Second, our findings point out new directions for future research. The present study raised new question why there was no relationship between serum ferritin with colorectal cancer among western people. Thus, we suggest that a trans-regional multicenter, community-based and long-term observation in a cohort design should be performed to obtain better understanding of causal relationships between serum ferritin and colorectal cancer, through measuring ferritin at baseline to investigate whether the highest ferritin category versus lowest was associated with colorectal cancer risk.
Conclusion
In summary, this meta-analysis supports a significant association between serum ferritin and colorectal cancer. However, the subgroup analysis found that there was significant effect modification of ferritin level by ethnic. Thus this finding needs further confirmation by trans-regional multicenter, long-term observation in a cohort design to obtain better understanding of causal relationships between serum ferrintin levels and colorectal cancer, through measuring ferritin at baseline to investigate whether the highest ferritin category versus lowest is associated with colorectal cancer risk.
Acknowledgements
This work was supported by Guangdong Province Science and technology plan project (2014A020212033) and Guangzhou science and technology plan projects (201510010009). All authors designed the study together, performed the experiment together, analyzed the data and wrote the paper; all authors approved the final manuscript.
Disclosure of conflict of interest
None.
References
- 1.Kucharzewski M, Braziewicz J, Majewska U, Gozdz S. Iron concentrations in intestinal cancer tissue and in colon and rectum polyps. Biol Trace Elem Res. 2003;95:19–28. doi: 10.1385/BTER:95:1:19. [DOI] [PubMed] [Google Scholar]
- 2.Padmanabhan H, Brookes MJ, Iqbal T. Iron and colorectal cancer: evidence from in vitro and animal studies. Nutr Rev. 2015;73:308–317. doi: 10.1093/nutrit/nuu015. [DOI] [PubMed] [Google Scholar]
- 3.Ashmore JH, Rogers CJ, Kelleher SL, Lesko SM, Hartman TJ. Dietary Iron and Colorectal Cancer Risk: A Review of Human Population Studies. Crit Rev Food Sci Nutr. 2015 doi: 10.1080/10408398.2012.749208. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Jomova K, Valko M. Importance of iron chelation in free radical-induced oxidative stress and human disease. Curr Pharm Des. 2011;17:3460–3473. doi: 10.2174/138161211798072463. [DOI] [PubMed] [Google Scholar]
- 5.Genaro-Mattos TC, Mauricio AQ, Rettori D, Alonso A, Hermes-Lima M. Antioxidant Activity of Caffeic Acid against Iron-Induced Free Radical Generation-A Chemical Approach. PLoS One. 2015;10:e0129963. doi: 10.1371/journal.pone.0129963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vijayavel K, Downs CA, Ostrander GK, Richmond RH. Oxidative DNA damage induced by iron chloride in the larvae of the lace coral Pocillopora damicornis. Comp Biochem Physiol C Toxicol Pharmacol. 2012;155:275–280. doi: 10.1016/j.cbpc.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Pra D, Bortoluzzi A, Muller LL, Hermes L, Horta JA, Maluf SW, Henriques JA, Fenech M, Franke SI. Iron intake, red cell indicators of iron status, and DNA damage in young subjects. Nutrition. 2011;27:293–297. doi: 10.1016/j.nut.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Guagnozzi D, Severi C, Ialongo P, Viscido A, Patrizi F, Testino G, Vannella L, Labriola R, Strom R, Caprilli R. Ferritin as a simple indicator of iron deficiency in anemic IBD patients. Inflamm Bowel Dis. 2006;12:150–151. doi: 10.1097/01.MIB.0000199223.27595.e3. [DOI] [PubMed] [Google Scholar]
- 9.Anttila R, Cook JD, Siimes MA. Body iron stores decrease in boys during pubertal development: the transferrin receptor-ferritin ratio as an indicator of iron status. Pediatr Res. 1997;41:224–228. doi: 10.1203/00006450-199702000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Z, Li C, Hu M, Li J, Liu R. Plasma ferritin levels, HFE polymorphisms, and risk of pancreatic cancer among Chinese Han population. Tumour Biol. 2014;35:7629–7633. doi: 10.1007/s13277-014-1978-x. [DOI] [PubMed] [Google Scholar]
- 11.Tang DJ, Lang YM, Feng YZ. Evaluation of combined assays of serum ferritin, alpha-1-antitrypsin and alpha-fetoprotein in liver cancer. Chin Med J (Engl) 1992;105:900–904. [PubMed] [Google Scholar]
- 12.Basso D, Fabris C, Del Favero G, Meggiato T, Panozzo MP, Vianello D, Plebani M, Naccarato R. Hepatic changes and serum ferritin in pancreatic cancer and other gastrointestinal diseases: the role of cholestasis. Ann Clin Biochem. 1991;28:34–38. doi: 10.1177/000456329102800105. [DOI] [PubMed] [Google Scholar]
- 13.Kishida T, Sato J, Fujimori S, Minami S, Yamakado S, Tamagawa Y, Taguchi F, Yoshida Y, Kobayashi M. Clinical significance of serum iron and ferritin in patients with colorectal cancer. J Gastroenterol. 1994;29:19–23. doi: 10.1007/BF01229068. [DOI] [PubMed] [Google Scholar]
- 14.Gur T, Demir H, Kotan MC. Tumor markers and biochemical parameters in colon cancer patients before and after chemotherapy. Asian Pac J Cancer Prev. 2011;12:3147–3150. [PubMed] [Google Scholar]
- 15.Nelson RL, Davis FG, Sutter E, Sobin LH, Kikendall JW, Bowen P. Body iron stores and risk of colonic neoplasia. J Natl Cancer Inst. 1994;86:455–460. doi: 10.1093/jnci/86.6.455. [DOI] [PubMed] [Google Scholar]
- 16.Gackowski D, Kruszewsk M, Banaszkiewicz Z, Jawien A, Olinski R. Lymphocyte labile iron pool, plasma iron, transferrin saturation and ferritin levels in colon cancer patients. Acta Biochim Pol. 2002;49:269–272. [PubMed] [Google Scholar]
- 17.Joosten E, Meeuwissen J, Vandewinckele H, Hiele M. Iron status and colorectal cancer in symptomatic elderly patients. Am J Med. 2008;121:1072–1077. doi: 10.1016/j.amjmed.2008.06.039. [DOI] [PubMed] [Google Scholar]
- 18.Carpenter C, Payne SM. Regulation of iron transport systems in Enterobacteriaceae in response to oxygen and iron availability. J Inorg Biochem. 2014;133:110–117. doi: 10.1016/j.jinorgbio.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinberg ED. Association of iron with colorectal cancer. Biometals. 1994;7:211–216. doi: 10.1007/BF00149550. [DOI] [PubMed] [Google Scholar]
- 20.Nelson RL. Iron and colorectal cancer risk: human studies. Nutr Rev. 2001;59:140–148. doi: 10.1111/j.1753-4887.2001.tb07002.x. [DOI] [PubMed] [Google Scholar]
- 21.Campo E, Palacin A, Benasco C, Condom E, Cardesa A. Ferritin immunohistochemical localization in normal and neoplastic colonic mucosa. Int J Biol Markers. 1987;2:177–183. doi: 10.1177/172460088700200308. [DOI] [PubMed] [Google Scholar]
- 22.Harju E, Lindberg H. Lack of iron stores in patients with diseases of the gastrointestinal tract. Surg Gynecol Obstet. 1985;161:362–366. [PubMed] [Google Scholar]
- 23.Li F, Kishida T, Kobayashi M. Serum iron and ferritin levels in patients with colorectal cancer in relation to the size, site, and disease stage of cancer. J Gastroenterol. 1999;34:195–199. doi: 10.1007/s005350050243. [DOI] [PubMed] [Google Scholar]


