Abstract
Aim: The epidermal growth factor receptor (EGFR) mutations and human epidermal growth factor receptor HER-2/neu (HER2) have been established roles in the signal transduction pathways leading to cell growth and differentiation. The present study focus on the significance of EGFR mutations combined with HER2 overexpression on survival outcomes in Non-small Cell Lung Cancer patients in Uygur population. Methods: A total of 111 consecutive Uygurods: A total of 111 consecutive Cell Lung Cancer under went lung Cell Lung biopsy or surgery at the Affiliated Tumor Hospital of Xin Jiang Medical University between March 2009 and January 2013 were included in this retrospective study. All the patients included had received gefitinib 250 mg once daily. The HER2 expression were evaluated by immunohistochemical staining with score of membranous staining being 0 = none, 1 = weak, 2 = 10-30% cells, 3≥30% cells stained, and Real-time PCR techniques were conducted to detect mutations of EGFR through 21 kinds of human EGFR gene mutation detection kits. A retrospective review of the medical records was analyzed to determine the correlation between the presence of EGFR mutations combined with HER2 overexpression and clinicopathological factors. Results: The overall rate of EGFR mutation was 10.81% (n = 12), which mainly involved exons 19 (83.33%, n = 10), 21 (16.67%, n = 2). The overall rate of HER2 overexpression was 21.62% (n = 24). EGFR mutation combined with HER2 overexpression analysis was performed in 111 patients, with an overall rate of 5.41% (n = 6). Median progression-free survival and overall survival were significantly longer in the EGFR mutations group than in the wild type group (PFS: 10.0±1.5 versus 3.8±1.4 months, P = 0.000; OS: 27.3±2.9 versus 19.1±4.7 months, P = 0.000). The ORR in patients with HER2 overexpression was 29.17%, and 13.80% in those patients with HER2 negative, but no significant difference (P = 0.121). The median PFS and OS in HER2 positive group showed no significant difference compared with HER2 negative group (PFS: 4.7±1.2 months versus 3.9±1.6 months, P = 0.085; OS: 20.5±2.4 versus 19.2±2.6 months, P = 0.094). As regarding to ORR, PFS and OS, EGFR mutations combined with HER2 overexpression patients showed no superior efficacy to gefitinib treatment compared with EGFR mutations combined with HER2 negative. Conclusion: In Uygur population, progression-free survivals were improved in Non-small Cell Lung Cancer with EGFR mutations. HER2 overexpression provided a poor prognostic factor in Non-small Cell Lung Cancer.
Keywords: Non-small cell lung cancer, epidermal growth factor receptor mutation, gefitinib, HER2 overexpression, Uygur population
Introduction
Nonsmall-cell lung cancer (NSCLC) is the leading cause of cancer death worldwide, with extremely poor prognosis, and the median survival rarely exceeds 10 months irrespective of conventional chemotherapy [1,2]. The identification of molecular-targeted agent leading to cell differentiation, migration, proliferation or survival could be effective in improving the median overall survival. As the first molecular-targeted agent for NSCLC, gefitinib targets the molecule as an inhibitor or tyrosine kinase of the epidermal growth factor receptor (EGFR-TKI) [3-5]. The phosphorylation of intracellular EGFR tyrosine kinase domain induced by ligand- EGFR complex drives tumor cell survival, proliferation and invasion [6-8]. The HER family includes three other members: EGFR (HER1/ERB1), HER3 (ERBB3) and HER4 (ERBB4), which structure contains three domains: an extracellular domain, homo/heterodimers formation and a transmembrane domain [9,10]. As the ligand binds to an extracellular domain, the signal, pass through the plasma membrane, then activates two key signaling pathways including the RAS/RAF/MAPK pathway and PI3K/Akt pathway which drives the tumor cell growth [11,12]. As a relatively new biomarker for NSCLC, the HER2 gene plays its central role in tumor growth, Brabender J et al. [13] highlighted EGFR and HER2-neu mRNA expression is correlated with survival in non-small cell lung cancer, representing an appealing prognostic and predictive factor in NSCLC. In membranous staining, HER2 overexpression was up to 20% of cases, the role of HER2 overexpression in lung cancer remains controversial although HER2 correlating with poor prognosis in breast and ovarian malignant tumors. Jian Ming Xu et al. have demonstrated that HER2 was established as poor prognostic and predictive factor for selecting Chinese patients sensitive to gefitinib treatment [14]. Therefore it appears that no convincing data available on clinical significance of HER2 protein overexpression as a prognosis factor in NSCLC.
In the present study, we performed a retrospective study in Uygur’s. NSCLC, aiming at identifying the role of EGFR gene mutations and HER2 overexpression as predictive factors for in selecting patients sensitive to gefitinib treatment.
Patients and methods
111 Uygur NSCLC patients in this study histologically confirmed and treated with gefitinib between March 2009 and January 2013 in the Affiliated Tumor Hospital of Xinjiang Medical University were retrospectively analyzed (Table 1). Tumor materials were histologically confirmed. All patients were pretreated with at least one line of platinum-based chemotherapy regimen before receiving gefitinib monotherapy at a daily dose of 250 mg, until an intolerable toxicity event such as grade 3 was observed during the treatment in which gefitinib was administered without any dose reductions until disease progression. The dose of gefitinib will be reduced by changing the everyday schedule to every 2 days schedule when grade 2 toxicity was observed.
Table 1.
Population Characteristics
| Characteristics | N = 111 No (%) | Statistics |
|---|---|---|
| Age (years) | ||
| Mean | 56.8±11.5 | |
| Range | 21-76 | |
| Gender | ||
| Male | 78 | 70.27% |
| Female | 33 | 29.73% |
| ECOG performance satus score | ||
| 0 | 8 | 7.21% |
| 1 | 62 | 55.86% |
| 2 | 41 | 36.94% |
| Histologic diagnosis | ||
| Adenocarcinoma | 45 | 40.54% |
| Squamous cell carcinoma | 45 | 40.54% |
| Others | 21 | 18.92% |
| Smoking status | ||
| Previous or current smoker | 63 | 56.76% |
| Never smoked | 48 | 43.24% |
| Clinical stage | ||
| IIIB | 45 | 40.54% |
| IV | 66 | 59.46% |
| Type of EGFR mutation | 12 (Adenocarcinoma) | |
| EXON 19 deletion | 9 | 75.00% |
| EXON 21 (L858R) | 3 | 25.00% |
| Others | 0 | 0 |
Note: Tumor stage based on tumor-node-metastasis classification advocated by International Union against Cancer. Abbreviations: EGFR, epidermal growth factor receptor; HER, human epidermal growth factor receptor; n, number.
Immunohistochemistry
All tumor section staining was performed as the manufacturer’s protocol. The HER2 immunohistochemical analysis kit was used (Zymed, USA). The immunostaining was then scored by two independent pathologists who were blinded to the clinical information. Tumor membranous staining intensity was scored using a four-grade scale: (0, 1+, 2+, or 3+), 0 = no staining, +1 = if less than 10% tumor cells had weak staining, +2 = if at least 10%tumor cells had moderate staining, and +3 = if at least 10% tumor cells had strong staining. Cases Grade of 0 or +1 was considered as negative, and +2 or +3 was considered as positive.
Real-time fluorescence quantitative PCR
ARMS (Amplification Refractory Mutation System) was used to detect EGFR mutations: genomic DNA extracted from five parafin sections or frozen tumor tissues was used for Real-time fluorescence quantitative PCR to detect EGFR mutations. Each PCR reaction contained 10-15 ng DNA, 5-10 pM forward and reverse primers in 25 uL reaction volume with cycling parameters: first cycle of 95°C for 2 min and 30 s, 15 cycles of 95°C for 25 s, 64°C for 20 s and 72°C for 20 s, 31 cycles of 93°C for 25 s, 60°C for 35 s and 72°C for 20 s, and one cycle of 60°C for 7 min followed by analysis of FEM and HEX signals determing EGFR mutations.
Treatment with gefitinib
According to the RECIST criteria, the response to gefitinib was analyzed at 4 weeks [15]. The treatment response: complete remission (CR), partial remission (PR), stable disease (SD) and progressive disease (PD) were confirmed no less than 4 weeks apart. Objective response rate (ORR) and median progression free time (PFS) were assessed.
Statistical analysis
The χ2 test was used to analyze EGFR mutations or HER2 overexpression and patient characteristics. The logistic regression model was used in multivariate analysis. Kaplan-Meier product-limit method was used to calculate median overall survival and PFS (Progression Free Survival). A multivariate analysis using the stepwise Cox regression model was carried out.
Results
EGFR mutations
EGFR mutations were examined in 111 Uygurlculate median PFS. s use adenocarcinoma cases 45 squamous cell carcinoma and 20 other cases, with an overall mutation rate of 10.81% (12/111), in which 9/111 (8.11%) mutions in exon 19, 3/111 (2.70%) mutations in exon 21 (Figure 2 and Table 1). No significant difference was observed in the analysis of the presence of EGFR mutations between different gender patients. EGFR mutations positivity was statistically associated (P = 0.000) with adenocarcinoma histology, while not influenced by mean age, smoking status, clinical stage and ECOG performance satus score (Table 2).
Figure 2.
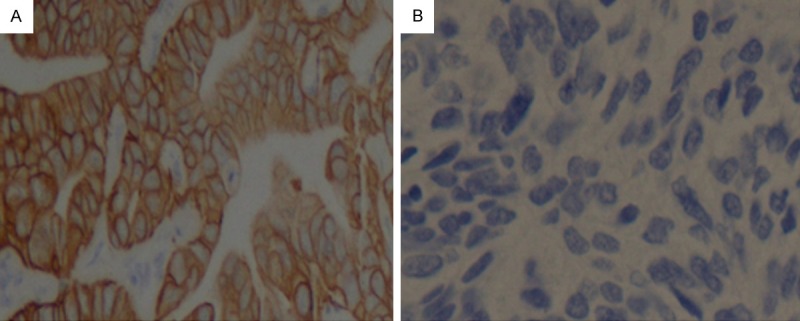
Representative image showing HER2 overexpression in NSCLC. Notes: (A) positive, (B) negative, respectively (original magnification ×400).
Table 2.
EGFR Mutation/HER2 Overexpression Status According to Demographics
| Variable/Categories | EGFR | HER2 | ||
|---|---|---|---|---|
|
|
|
|||
| Mutation N% | Wide-type N% | Positive N% | Negative N% | |
| Gender | ||||
| Male | 9/11.84 | 69/88.16 | 15/19.23 | 63/80.77 |
| Female | 3/9.09 | 30/90.9 | 9/27.27 | 24/72.72 |
| P | 0.497 | 0.243 | ||
| Age | ||||
| ≤65 | 6/7.41 | 75/92.59 | 15/19.23 | 63/80.77 |
| ≥65 | 6/20 | 24/80 | 9/27.27 | 24/72.72 |
| P | 0.065 | 0.243 | ||
| Ecog | ||||
| 0-1 | 5/7.14 | 65/92.86 | 9/15.79 | 48/84.21 |
| 2-3 | 7/17.07 | 34/82.93 | 15/27.78 | 39/72.22 |
| P | 0.065 | 0.065 | ||
| Histology | ||||
| Ade | 12/26.67 | 33/73.33 | 18/40 | 27/60 |
| No-Ade | 0/0 | 66/100 | 6/9.09 | 60/90.9 |
| P | 0.000 | 0.000 | ||
| Smoking History | ||||
| Never Smoker | 6/15.38 | 33/84.62 | 15/23.81 | 48/76.19 |
| Smoker | 6/8.33 | 66/91.67 | 9/18.75 | 39/81.25 |
| P | 0.204 | 0.344 | ||
| Stage | ||||
| IIIB | 4/8.88 | 41/91.11 | 9/15.79 | 48/84.21 |
| IV | 8/12.12 | 58/87.87 | 15/27.78 | 39/72.22 |
| P | 0.417 | 0.096 | ||
Note: ECOG Eastern Cooperative Oncology Group, Ade adenocarcinoma.
EGFR-mutation group showed superior efficacy (response rate) to gefitinib treatment compared with wild-type group (Table 3). Further survival analysis showed that PFS for EGFR-mutation group (10.0±1.5 months, 95% CI = 6.5-12.2 months, P<0.001) tended to be superior to that of wild type group (3.8±1.4 months, 95% CI = 3.1-4.7 months, Table 3 and Figure 3). Mean overall survival (27.3±2.9 months, 95% CI = 13.8-30.2 months, P<0.001) in EGFR mutant group was also significantly different from wild type (19.1±4.7 months, 95% CI = 8.8-21.9 months, Table 3 and Figure 3).
Table 3.
Tumor response and prognosis of EGFR mutation and HER2 overexpression to gefitinib
| Marker | N/% | OR (%) | Median PFS (months) | Median OS (months) |
|---|---|---|---|---|
| EGFR+ | 12/10.81 | 10/83.33 | 10.0±1.5 | 27.3±2.9 |
| EGFR- | 99/89.19 | 9/9.09 | 3.8±1.4 | 19.1±4.7 |
| P | 0.000 | 0.000 | 0.000 | |
| HER2+ | 24/21.62 | 7/29.17 | 4.7±1.2 | 20.5±2.4 |
| HER2- | 87/78.38 | 12/13.80 | 3.9±1.6 | 19.2±2.6 |
| P | 0.121 | 0.085 | 0.094 | |
| EGFR+/HER2+ | 6/5.41 | 5/83.33 | 10.3±1.3 | 28.1±3.1 |
| EGFR+/HER2- | 6/5.41 | 5/83.33 | 9.4±1.5 | 26.5±2.7 |
| P | 1.000 | 0.991 | 0.893 | |
| EGFR-/HER2+ | 18/16.22 | 2/11.11 | 4.5±1.3 | 20.2±5.3 |
| EGFR-/HER2- | 81/72.97 | 7/8.64 | 3.8±1.1 | 18.9±3.7 |
| P | 0.667 | 0.732 | 0.000 |
Note: OR Objective response; PFS Median progression free time; OS Overall survival.
Figure 3.
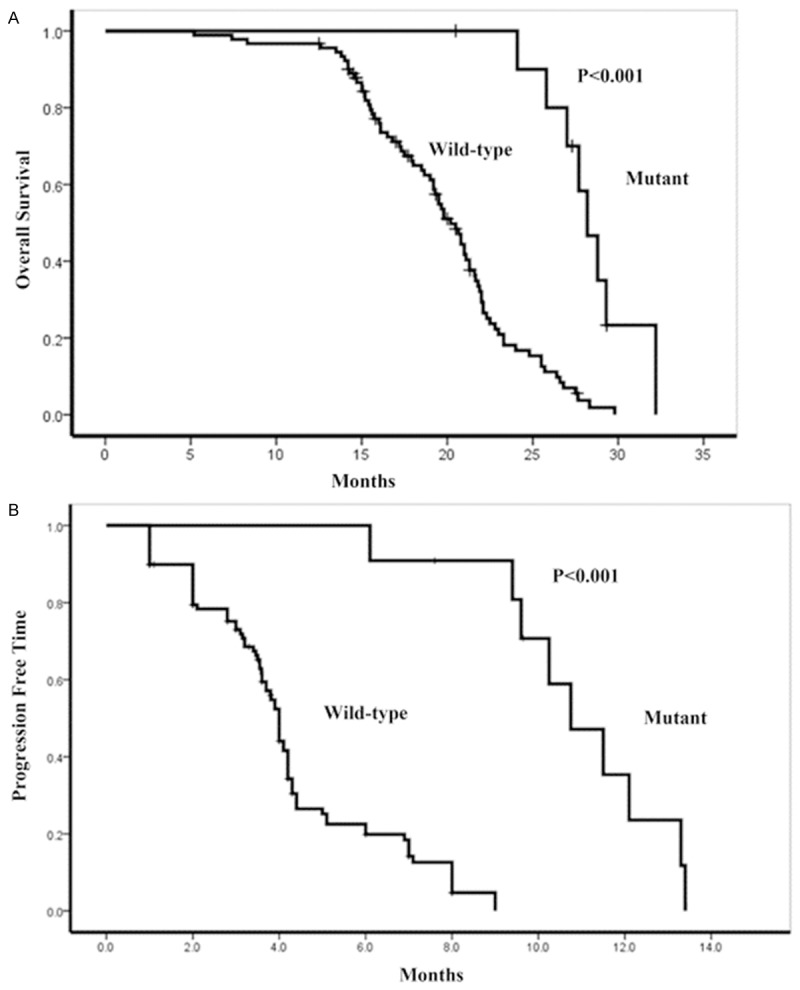
Effect on Kaplan-Meier curves for overall survival (A) and PFS (B) by EGFR mutation status.
HER2 overexpression
The HER2 overexpression status was determined in 111 cases, in which 21.62% (n = 24), were found to be positive (Figure 1 and Table 1). The HER2 overexpression was significantly associated (P = 0.000) with adenocarcinoma histology (40% in adenocarcinoma histology versus 9.09% in non-adenocarcinoma histology), while no association with gender, smoking status, clinical stage and ECOG performance satus score (Table 2).
Figure 1.
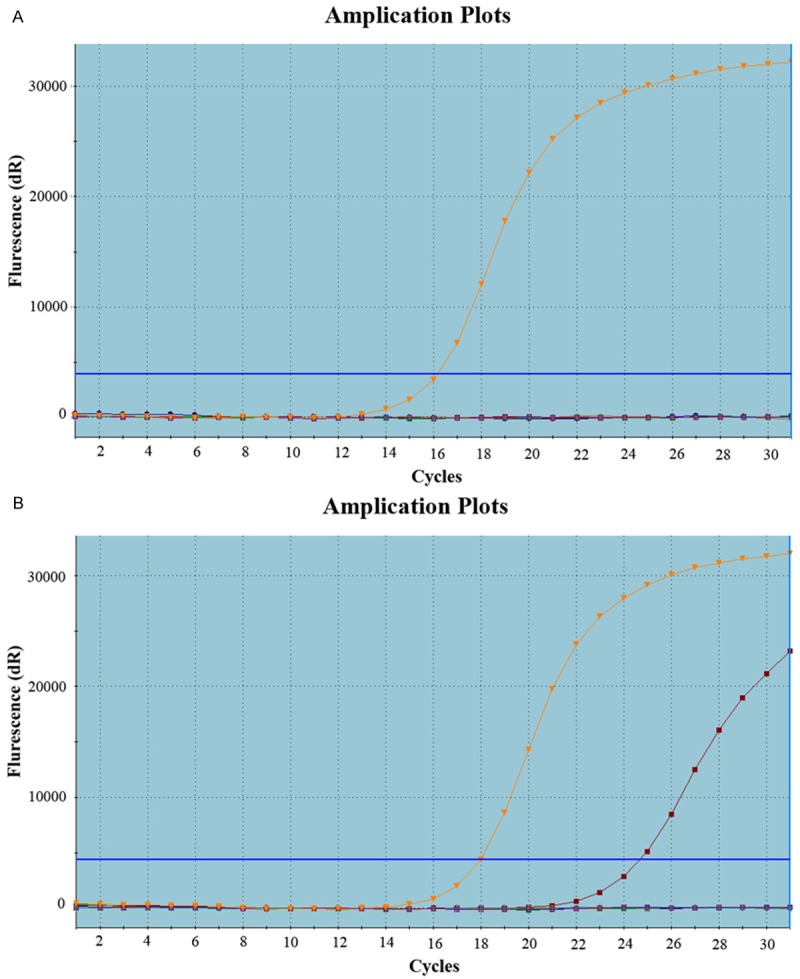
Representative image showing EGFR mutation in NSCLC, Real-time polymerase chain reaction: (A) negative, (B) positive respectively.
Objective response, median PFS and median OS in HER2 overexpression positivity group tended to be superior to that of wild type group (OR: 29.17% versus 13.80%, P = 0.121; PFS: 4.7±1.2 versus 3.9±1.6 months, P = 0.085; OS: 20.5±2.4 versus 19.2±2.6 months, P = 0.094, Table 3 and Figure 3).
EGFR mutations combined with HER2 overexpression
EGFR mutations combined with HER2 overexpression patients showed no superior efficacy (objective response rate) to gefitinib treatment compared with EGFR mutations combined with HER2 negative, (the response rate was 83.33%, 83.33% respectively, P = 1.00, Table 3). For median PFS and OS of EGFR mutations combined with HER2 overexpression patients, no significant difference was found compared with EGFR mutations combined with HER2 negative (PFS: 10.3±1.3 versus 9.4±1.5 months, P = 0.991; OS: 28.1±3.1 versus 26.5±2.7, P = 0.893 Table 3 and Figure 3).
Multivariate analysis of clinical outcome
Univariate analysis was performed to identify which variables were significantly associated with clinical outcome. Those variables statistically significant in the univariate analysis including presence of EGFR mutations and adenocarcinoma histology were added to a multivariate model exploring the predictive factors. A step-wise logistic regression analysis revealed that presence of EGFR mutations (RR 15.7, 95% CI = 6.2-47.8) was the strongest prognostic factors for OR to gefitinib. According to the multivariate Cox regression model, presence of EGFR mutations (RR 5.1, P<0.0001) and adenocarcinoma histology (RR 4.8, P<0.001) were significant predictors of long PFS. Also, presence of EGFR mutations (RR 5.3, P<0.0001) and adenocarcinoma histology (RR 3.9, P<0.001) predicted long OS.
Discussion
In East Asian, North American, West European and East European populations, the frequency of EGFR gene mutations in NSCLC has been shown different [16-20]. In this study, it is the first time, to our knowledge, for assessment of the prevalence of EGFR gene mutations and HER2 overexpression in Uygur population.
In concordance with previous studies [21-24], the main factor affecting frequency of EGFR gene mutations in NSCLC is histological subtype (P = 0.000), but no smoking status (P = 0.204) and gender (P = 0.497).
In the Chinese population, EGFR gene mutations were detected in 30.2% of the samples [14], which mainly involved exons 19 (53.1%), 21 (21.9%), and 18 (18.8%). In our study, EGFR gene mutations involve exon 19 (83.33%) and exon 21 (16.67%). In conformity with previous studies in different ethnics, EGFR gene mutations in Uygur population occur more frequently in ADCs (26.67%).
The never smokers was found to have higher gene mutations frequency than former and current smokers [25,26]. In this study, we compared it in Uygur population; however, no significant difference was found which should be confirmed with more consistent number of patients.
In concordance with previously reported studies [4,26-30], we observed that EGFR-mutation group showed superior efficacy (response rate, 83.33%), long median PFS and (PFS: 10.0±1.5 months, OS: 27.3±2.9 months) to gefitinib treatment. Overall, our study confirmed that the presence of EGFR mutations is a valuable prognosis factor in selecting patients to benefit from gefitinib treatment in NSCLC, especially those with histological subtype of adenocarcinoma.
The HER2 gene, known as human EGFR2 or ERBB2 or NEU, belonging to the ERBB family, was established as a relatively new biomarker for NSCLC, representing an appealing target for anti-cancer strategies [31-33]. The other ERBB family members such as EGFR (HER1/ERB1), HER3 (ERBB3) and HER4 (ERBB4) have their ligands including EGF, epiregulin, betacellulin, TGF and neuregulins targeted to potentiate their activity, but no known ligands proved for HER2.
The prognostic role of HER2 in non-small cell lung cancer remains controversial. The role of HER2 expression was investigated with discordant [12,32,34]. One study found that HER2-neu mRNA expression in non-small cell lung cancer correlated with survival [13]. In 2002, Hirsch et al. draw the conclusion that no significant difference in survival was observed among patients with positive (HercepTest 2+/3+) and negative (HercepTest 0/1+) tumors [35]. HER2 overexpression was analyzed as poor prognostic factor in lung cancer in the recent review and meta-analysis [36,37]. Similar findings have been observed in Uygur population, and we found that the HER2 overexpression positive status was 21.62%, which showed no superior efficacy, Median PFS and OS (response rate: 29.17%, Median PFS: 4.7±1.2 months, OS: 20.5±2.4 months) to gefitinib treatment compared with HER2 negative group (response rate: 13.80%, Median PFS: 3.9±1.6 months, OS: 19.2±2.6).
Also, EGFR mutations combined with HER2 overexpression patients showed no superior efficacy (response rate), PFS and OS to gefitinib treatment compared with EGFR mutations with HER2 negative Jian Ming Xu et al. [14] found that EGFR mutations combined with HER2 overexpression patients had a better outcome than patients with wild type EGFR regardless of HER2 and/or HER3 protein levels, which suggested EGFR mutations play a valuable role in predicting sensitivity to TKIs in Chinese NSCLC patients [14]. In concordance with previous data, in our study, EGFR mutations combined with HER2 overexpression patients showed no superior efficacy and long Median PFS to gefitinib treatment compared with EGFR mutations combined with HER2 negative. As a consequence of the limited number of patients in our study, efficient assays should be developed to confirm this.
In conclusion, in Uygur population, the prognostic role of EGFR mutations in lung cancer was confirmed in selecting patients sensitive to gefitinib treatment in NSCLC, especially those with histological subtype of adenocarcinoma. HER2 overexperession showed low incidence in Uygur population in this study, and poor prognosis factor in the evaluation of selecting patients sensitive to gefitinib treatment in NSCLC.
Acknowledgements
The protocol of this study was approved by the Research Ethics Committee of the Affiliated Tumor Hospital of Xinjiang Medical University, Urumqi, China. Written informed consent was obtained from all patients participated in this study.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Zhao J, Xiong J. Advances on Driver Oncogenes of Non-small Cell Lung Cancer. Zhongguo Fei Ai Za Zhi. 2015;18:42–47. doi: 10.3779/j.issn.1009-3419.2015.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sunaga N, Tomizawa Y, Yanagitani N, Iijima H, Kaira K, Shimizu K, Tanaka S, Suga T, Hisada T, Ishizuka T, Saito R, Dobashi K, Mori M. Phase II prospective study of the efficacy of gefitinib for the treatment of stage III/IV non-small cell lung cancer with EGFR mutations, irrespective of previous chemotherapy. Lung Cancer. 2007;56:383–389. doi: 10.1016/j.lungcan.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 4.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 5.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, Kosaka T, Holmes AJ, Rogers AM, Cappuzzo F, Mok T, Lee C, Johnson BE, Cantley LC, Janne PA. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 6.Baek JH, Sun JM, Min YJ, Cho EK, Cho BC, Kim JH, Ahn MJ, Park K. Efficacy of EGFR tyrosine kinase inhibitors in patients with EGFR-mutated non-small cell lung cancer except both exon 19 deletion and exon 21 L858R: A retrospective analysis in Korea. Lung Cancer. 2015;87:148–154. doi: 10.1016/j.lungcan.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Cai KC, Liu DG, Wang YY, Wu H, Huang ZY, Cai RJ, Wang HF, Xiong G, Zhang ZL. Gefitinib maintenance therapy in Chinese advanced-stage lung adenocarcinoma patients with EGFR mutations treated with prior chemotherapy. Neoplasma. 2015;62:302–307. doi: 10.4149/neo_2015_036. [DOI] [PubMed] [Google Scholar]
- 8.Cappuzzo F, Gregorc V, Rossi E, Cancellieri A, Magrini E, Paties CT, Ceresoli G, Lombardo L, Bartolini S, Calandri C, de Rosa M, Villa E, Crino L. Gefitinib in pretreated non-small-cell lung cancer (NSCLC): analysis of efficacy and correlation with HER2 and epidermal growth factor receptor expression in locally advanced or metastatic NSCLC. J. Clin. Oncol. 2003;21:2658–2663. doi: 10.1200/JCO.2003.01.039. [DOI] [PubMed] [Google Scholar]
- 9.Wieduwilt MJ, Moasser MM. The epidermal growth factor receptor family: biology driving targeted therapeutics. Cell Mol Life Sci. 2008;65:1566–1584. doi: 10.1007/s00018-008-7440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgess AW. EGFR family: structure physiology signalling and therapeutic targets. Growth Factors. 2008;26:263–274. doi: 10.1080/08977190802312844. [DOI] [PubMed] [Google Scholar]
- 11.Landi L, Cappuzzo F. HER 2 and lung cancer. Expert Rev Anticancer Ther. 2013;13:10. doi: 10.1586/14737140.2013.846830. [DOI] [PubMed] [Google Scholar]
- 12.Takezawa K, Pirazzoli V, Arcila ME, Nebhan CA, Song X, de Stanchina E, Ohashi K, Janjigian YY, Spitzler PJ, Melnick MA, Riely GJ, Kris MG, Miller VA, Ladanyi M, Politi K, Pao W. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov. 2012;2:922–933. doi: 10.1158/2159-8290.CD-12-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brabender J, Danenberg KD, Metzger R, Schneider PM, Park J, Salonga D, Holscher AH, Danenberg PV. Epidermal growth factor receptor and HER2-neu mRNA expression in nonsmall cell lung cancer Is correlated with survival. Clin Cancer Res. 2001;7:1850–1855. [PubMed] [Google Scholar]
- 14.Xu JM, Han Y, Duan HQ, Gao EM, Zhang Y, Liu XQ, Zhang JS, Toschi L, Galetta D, Azzariti A, Paradiso A. EGFR mutations and HER2/3 protein expression and clinical outcome in Chinese advanced non-small cell lung cancer patients treated with gefitinib. J Cancer Res Clin Oncol. 2009;135:771–782. doi: 10.1007/s00432-008-0512-1. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Bae NC, Chae MH, Lee MH, Kim KM, Lee EB, Kim CH, Park TI, Han SB, Jheon S, Jung TH, Park JY. EGFR, ERBB2, and KRAS mutations in Korean non-small cell lung cancer patients. Cancer Genet Cytogenet. 2007;173:107–113. doi: 10.1016/j.cancergencyto.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Gao B, Sun Y, Zhang J, Ren Y, Fang R, Han X, Shen L, Liu XY, Pao W, Chen H, Ji H. Spectrum of LKB1, EGFR, and KRAS mutations in chinese lung adenocarcinomas. J Thorac Oncol. 2010;5:1130–1135. doi: 10.1097/JTO.0b013e3181e05016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchetti A, Martella C, Felicioni L, Barassi F, Salvatore S, Chella A, Camplese PP, Iarussi T, Mucilli F, Mezzetti A, Cuccurullo F, Sacco R, Buttitta F. EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J. Clin. Oncol. 2005;23:857–865. doi: 10.1200/JCO.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 19.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, Insa A, Massuti B, Gonzalez-Larriba JL, Paz-Ares L, Bover I, Garcia-Campelo R, Moreno MA, Catot S, Rolfo C, Reguart N, Palmero R, Sanchez JM, Bastus R, Mayo C, Bertran-Alamillo J, Molina MA, Sanchez JJ, Taron M. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 20.Vale CL, Burdett S, Fisher DJ, Navani N, Parmar MK, Copas AJ, Tierney JF. Should Tyrosine Kinase Inhibitors Be Considered for Advanced Non-Small-Cell Lung Cancer Patients With Wild Type EGFR? Two Systematic Reviews and Meta-Analyses of Randomized Trials. Clin Lung Cancer. 2014;16:173–182. e4. doi: 10.1016/j.cllc.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eck MJ, Yun CH. Structural and mechanistic underpinnings of the differential drug sensitivity of EGFR mutations in non-small cell lung cancer. Biochim Biophys Acta. 2010;1804:559–566. doi: 10.1016/j.bbapap.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He M, Capelletti M, Nafa K, Yun CH, Arcila ME, Miller VA, Ginsberg MS, Zhao B, Kris MG, Eck MJ, Janne PA, Ladanyi M, Oxnard GR. EGFR exon 19 insertions: a new family of sensitizing EGFR mutations in lung adenocarcinoma. Clin Cancer Res. 2012;18:1790–1797. doi: 10.1158/1078-0432.CCR-11-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorge SE, Kobayashi SS, Costa DB. Epidermal growth factor receptor (EGFR) mutations in lung cancer: preclinical and clinical data. Braz J Med Biol Res. 2014;47:929–939. doi: 10.1590/1414-431X20144099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yasuda H, Park E, Yun CH, Sng NJ, Lucena-Araujo AR, Yeo WL, Huberman MS, Cohen DW, Nakayama S, Ishioka K, Yamaguchi N, Hanna M, Oxnard GR, Lathan CS, Moran T, Sequist LV, Chaft JE, Riely GJ, Arcila ME, Soo RA, Meyerson M, Eck MJ, Kobayashi SS, Costa DB. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med. 2013;5:216ra177. doi: 10.1126/scitranslmed.3007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang SF, Liu HP, Li LH, Ku YC, Fu YN, Tsai HY, Chen YT, Lin YF, Chang WC, Kuo HP, Wu YC, Chen YR, Tsai SF. High frequency of epidermal growth factor receptor mutations with complex patterns in non-small cell lung cancers related to gefitinib responsiveness in Taiwan. Clin Cancer Res. 2004;10:8195–8203. doi: 10.1158/1078-0432.CCR-04-1245. [DOI] [PubMed] [Google Scholar]
- 26.Sakai Y, Yamasaki T, Kusakabe Y, Kasai D, Kotani Y, Nishimura Y, Itoh T. Large-cell neuroendocrine carcinoma of lung with epidermal growth factor receptor (EGFR) gene mutation and co-expression of adenocarcinoma markers: a case report and review of the literature. Multidiscip Respir Med. 2013;8:47. doi: 10.1186/2049-6958-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J, Lorimer I, Zhang T, Liu N, Daneshmand M, Marrano P, da Cunha Santos G, Lagarde A, Richardson F, Seymour L, Whitehead M, Ding K, Pater J, Shepherd FA. Erlotinib in lung cancer-molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 28.Douillard JY, Ostoros G, Cobo M, Ciuleanu T, McCormack R, Webster A, Milenkova T. Firstline gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase-IV, open-label, single-arm study. Br J Cancer. 2014;110:55–62. doi: 10.1038/bjc.2013.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knetki-Wroblewska M, Kowalski DM, Zajda K, Pluzanski A, Badurak P, Janowicz-Zebrowska A, Jaskiewicz P, Krzakowski M. Gefitinib in patients with advanced non-small-cell lung cancer. Pneumonol Alergol Pol. 2012;80:439–449. [PubMed] [Google Scholar]
- 30.Zhang XT, Li LY, Mu XL, Cui QC, Chang XY, Song W, Wang SL, Wang MZ, Zhong W, Zhang L. The EGFR mutation and its correlation with response of gefitinib in previously treated Chinese patients with advanced non-small-cell lung cancer. Ann Oncol. 2005;16:1334–1342. doi: 10.1093/annonc/mdi340. [DOI] [PubMed] [Google Scholar]
- 31.Cappuzzo F, Varella-Garcia M, Shigematsu H, Domenichini I, Bartolini S, Ceresoli GL, Rossi E, Ludovini V, Gregorc V, Toschi L, Franklin WA, Crino L, Gazdar AF, Bunn PA Jr, Hirsch FR. Increased HER2 gene copy number is associated with response to gefitinib therapy in epidermal growth factor receptor-positive non-smallcell lung cancer patients. J. Clin. Oncol. 2005;23:5007–5018. doi: 10.1200/JCO.2005.09.111. [DOI] [PubMed] [Google Scholar]
- 32.Hirata A, Hosoi F, Miyagawa M, Ueda S, Naito S, Fujii T, Kuwano M, Ono M. HER2 overexpression increases sensitivity to gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor, through inhibition of HER2/HER3 heterodimer formation in lung cancer cells. Cancer Res. 2005;65:4253–4260. doi: 10.1158/0008-5472.CAN-04-2748. [DOI] [PubMed] [Google Scholar]
- 33.Moasser MM, Basso A, Averbuch SD, Rosen N. The tyrosine kinase inhibitor ZD1839 (“Iressa”) inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer Res. 2001;61:7184–7188. [PubMed] [Google Scholar]
- 34.Takenaka M, Hanagiri T, Shinohara S, Kuwata T, Chikaishi Y, Oka S, Shigematsu Y, Nagata Y, Shimokawa H, Nakagawa M, Uramoto H, So T, Tanaka F. The Prognostic Significance of HER 2 Overextension in Non-small Cell Lung Cancer. Anticancer Res. 2011;31:4631–6. [PubMed] [Google Scholar]
- 35.Hirsch FR, Varella-Garcia M, Franklin WA, Veve R, Chen L, Helfrich B, Zeng C, Baron A, Bunn PA Jr. Evaluation of HER-2/neu gene amplification and protein expression in non-small cell lung carcinomas. Br J Cancer. 2002;86:1449–1456. doi: 10.1038/sj.bjc.6600286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, Shao X, Gao W, Bai J, Wang R, Huang P, Yin Y, Liu P, Shu Y. The role of human epidermal growth factor receptor 2 as a prognostic factor in lung cancer: a meta-analysis of published data. J Thorac Oncol. 2010;5:1922–1932. doi: 10.1097/jto.0b013e3181f26266. [DOI] [PubMed] [Google Scholar]
- 37.Thibault C, Khodari W, Lequoy M, Gligorov J, Belkacemi Y. HER2 status for prognosis and prediction of treatment efficacy in adenocarcinomas: a review. Crit Rev Oncol Hematol. 2013;88:123–133. doi: 10.1016/j.critrevonc.2013.03.003. [DOI] [PubMed] [Google Scholar]


