Abstract
Morus nigra has a long history of medicinal use in Chinese medicine, but the study on it is limited, the flavonoids are one of the main biological active substances. In this study, the Morus nigra flavonoids were extracted by ultrasonic and antioxidant activities both in vitro and in vivo were measured. The results showed that hydroxyl radicals clearance rate and superoxide radical anion clearance rate in vitro increased with the concentration of the total flavonoids in the range of 0-1.05 mg/mL and the maximum clearance rate was 80.33% and 87.69%, respectively. After mice were treated with flavonoids, the content of malonaldehyde (MDA) in serum and liver decreased; the activities of superoxide dismutase (SOD) in serum and liver, catalase (CAT) in liver and glutathione peroxidase (GSH-PX) in blood and liver increased; Langhans cells increased in spleen. These results revealed that the Morus nigra flavonoids possessed strong antioxidant activity.
Keywords: Morus nigra, total flavonoids, antioxidant activity
Introduction
Morus nigra, usually known as black mulberry, belongs to the genus Morus of the family Moraceae [1]. It is native to Iran and belongs to the cultivated and wild mulberry varieties. In China, it was planted in xinjiang since the 16th century and is the only black mulberry varieties [2]. Morus nigra is not only one of the ancient fruit trees, but it also has been treated as a folk medicine used as diuretic, laxative, antitussive, expectorant, sedative, anxiolytic, hypotensive, odontalgic, anthelmintic and emetic [3,4]. There are so many biological active substances in Morus nigra, including amylose, flavonoids and alkaloids, etc [5]. The flavonoids are one of the main biological active substances that exist widely in the plantage. Flavonoids often exhibit wide range of physiological activities, including antioxidant, antimicrobial, anti-inflammatory, anti-cancer, anti-tumor and anti-radiation properties [6-9], and some evidences suggest that the pharmacological effects of flavonoids are correlated with their antioxidant activities [10,11]. However, the study on the antioxidant activity of Morus nigra flavonoids was limited, therefore, the aim of this study was to determine the antioxidant activity of Morus nigra flavonoids. In vitro, the hydroxyl radical and superoxide radical anion clearance rates were determined by the colorimetric method; in vivo, the SOD, CAT, GSH-PX activites and MDA concentration of mice treated with Morus nigra flavonoids were measured [12]. Furthermore, this study can provide the theory basis for application of Morus nigra’ as nutrition, health protection and medicine in the future.
Materials and methods
Materials
Morus nigra was harvested from the orchard in Kuqa country in Aksu (xinjiang, PR China), during the fruit-bearing season (from July to August). Rutin from Chengdu Food and Drug Administration (chengdu, PR China). SOD, CAT, MDA and GSH-PX kits were purchased from Nanjing Jiancheng Bioengineering Institute (nanjing, PR China). Other chemicals were of analytical grade.
Animals
The experiment was carried out following the Regulations of Animal Experimentation of College of Veterinary Medicine, Sichuan Agricultural University, which is based on the Guidelines of the International Committee on Laboratory Animals. Kunming strain male and female mice (a closed strain coming from Kunming, Yunnan Province, PR China) were obtained from the Chengdu Dossy Experimental Animals Co., Ltd. (License No. SCXK (Sichuan) 2014-24), the average weight was 22-26 g. They were kept in the animal houses of Sichuan Agriculture University (Chengdu, China), and in well ventilated sterile polypropylene cages. Each cage contained 10 mice of the same sex. Animal rooms were maintained at a temperature of 22°C, a relative humidity of 40-70%, and a 12 h light/dark cycle. Experiments were started after acclimating the mice for one week. Animals were fed by a standard diet from Nuvital Nutrientes (Colombo/PR, Brazil) and water ad libitum.
Extraction of total flavonoids
The Morus nigra (dried, about 50 g) were ground to powder, and then refluxed with 500 mL petroleum ether twice at 50°C for 3 h to remove oil and grease in a Soxhlet extractor. The residues were extracted twice with 500 mL 95% alcohol (v/v) for 0.5 h by ultrasonic method reported by Joshi [13]. The solution was concentrated by a rotatory evaporator. Finally the solution was dried by oven to yield flavonoids extract.
Determination of total flavonoids content
The flavonoids extract, Morus nigra and rutin were dissolved in 60% alcohol to reach concentrations of 10, 10 and 0.1 mg/mL, respectively. The rutin was chosen as standard. Standard curve were constructed by measuring absorptivity of series of rutin diluent at 510 nm according to the method [14]. The content of total flavonoids was determined by the content of rutin in extract and raw material.
Determination of antioxidant activity in vitro
Determination of the hydroxyl radicals clearance rate
The hydroxyl radicals clearance rate was measured by the colorimetric method with salicylic acid [15]. The reaction solution contained 1 mL FeSO4 (0.15 mol/L), 1 mL H2O2 (6 mmol/L), 1 mL sodium salicylate (2 mmol/L) and 1 mL Morus nigra flavonoids (each group had a variety of concentrations, including 0.15, 0.30, 0.45, 0.60, 0.75, 0.90 and 1.05 mg/mL). After the H2O2 (1 mL) was added, the reaction mixtures were then incubated at 37°C for 1 hour. The absorbance of the reaction mixtures at 580 nm was measured [4,16,17]. The hydroxyl radicals clearance rate was calculated according to the following equation.
clearance rate (%) = [A0 - (Ai - A0i)]/A0 × 100%
where A0 is the absorbance of the control reaction, which replaces samples by distilled water. Ai is the absorbance of the reaction mixture. A0i is the absorbance of the background value of the samples.
Determination of the superoxide radical anion clearance rate
The superoxide radical anion clearance rate was measured by the pyrogallol autoxidation method [18]. The solution contains Tris-HCl, sample and pyrogallol. The flavonoids solution of a variety of concentrations (0.15, 0.30, 0.45, 0.60, 0.75, 0.90 and 1.05 mg/mL) were tested. After 5 minutes, the reaction was terminated by adding H2O2 (1 mL). The absorbance of the reaction mixtures at 360 nm was measured [16]. The superoxide radical anion clearance rate in percent was calculated according to the following equation.
clearance rate (%) = (A0 - (Ai - Aj))/A0 × 100%
where A0 is the absorbance of the solution without samples. Ai is the absorbance of the reaction mixture. Aj is the absorbance of the solution without pyrogallol.
Determination of antioxidant activity in vivo
Treatment of animals
Four groups, each containing 10 females and 10 males, were orally administrated with flavonoids extract from Morus nigra at concentrations of 0 (blank group), 100 (low dose Group), 200 (middle dose Group), 400 (high dose Group) mg/kg body weight (b.w) for 30 consecutive days, respectively. The animals were monitored for clinical and behavioral symptoms, and the body weight was measured every three days. After 30 days, the blood sample were collected by excising the eyes after fasting for 12 h, then all animals were euthanized and subjected to a full necropsy. Liver, lung, kidney, spleen, heart and thymus were weighed immediately after dissection. Some tissues and organs were preserved in 4% paraformaldehyde, and others were refrigerated.
Preparation of samples and determination of index
The blood samples were dissolved in double-distilled water. The serum was gained by centrifuging. The 10% liver homogenta was gained with glass homogenate tube. The activity of SOD, CAT and GSH-PX and the content of MDA were determined according to the requirements of kits strictly. The tissue section was made according to the method reported by Zhao [19].
Statistical analysis
All data were expressed as means ± standard deviation and analysed by the SPSS statistical software. The statistical significance was compared between the control and experimental groups by one way analysis of variance (ANOVA) followed by the Student-Newman-Keuls test.
Results
Extraction of total flavonoids
The total flavonoids in Morus nigra were extracted by the alcohol solution in cooperation with ultrasonic, the results of extract quality and yield were shown in Table 1. The average extraction of total flavonoids was 29.47 g, which was calculated on the basis of rutin equivalents. The average yield of flavonoids was 58.94%.
Table 1.
The results of flavonoids yield in Morus nigra
| Group | Morus nigra (g) | Extraction (g) | Yield (%) | Average yield (%) |
|---|---|---|---|---|
| 1 | 50 | 29.65 | 59.30 | 58.94 |
| 2 | 50 | 29.30 | 58.60 | |
| 3 | 50 | 29.46 | 58.92 |
The content of total flavonoids
The content of total flavonoids was measured by spectrophotometry and the results were shown in Table 2. As shown in Table 2, the concentration of total flavonoids extract was 1.167 mg/mL, the content was 11.67%, which was higher than that in powders (0.105 mg/mL, 1.05%). It showed that the total flavonoids in Morus nigra were extracted effectively. The result is higher than the literature [20-22]. On the whole, the method of this experiment was easy, it had low cost and high efficiency, which laid a foundation for the further research.
Table 2.
The results of flavonoids content
| Group | Absorptivity (A) | Concentration (mg/mL) | Content (%) | Average content (%) |
|---|---|---|---|---|
| The extract | 0.461 | 1.167 | 11.67 | 11.67 |
| 0.461 | 1.167 | 11.67 | ||
| 0.460 | 1.166 | 11.66 | ||
| The power | 0.057 | 0.105 | 1.05 | 1.04 |
| 0.056 | 0.102 | 1.02 | ||
| 0.057 | 0.105 | 1.05 |
The antioxidant activity in vitro
The hydroxyl radical clearance rate
The hydroxyl radical clearance rate of Morus nigra flavonoids in this test was measured by the spectrophotometry at 580 nm. The relationship between the hydroxyl radical clearance rate and the concentration of total flavonoids was shown in Figure 1. It was showed that the total flavonoids had a potent inhibitory effect on hydroxyl radicals, and the inhibitory effects increased with the concentration of flavonoids. When the concentration of the total flavonoids was 0.15 mg/mL, the average inhibition rate was 16.72%; when the concentration increased to 1.05 mg/mL, the average inhibition rate increased to 80.33%.
Figure 1.
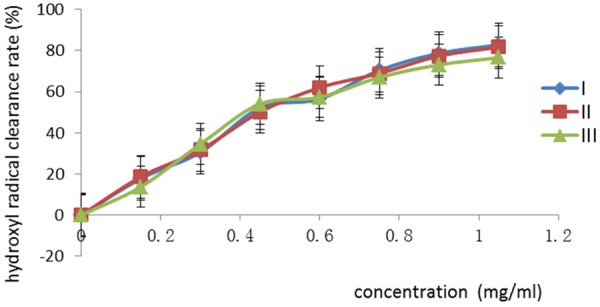
The relationship between the hydroxyl radical clearance rate and the concentration of total flavonoids. The concentration was in range of 0-1.05 mg/mL. The values are presented as a percentage.
The superoxide radical anion clearance rate
The superoxide radical anion clearance rate was measured by the spectrophotometry at 360 nm. The flavonoids provide reactive hydrogen for clearing the superoxide radical anion which plays the antioxidant effect. The flavonoids have the phenolic hydroxyl groups, which can provide reactive hydrogen, so the radicals reaction is stopped [25]. The results showed that the rate of pyorgallol autoxidation was inhibited effectively after the total flavonoids were added. The inhibitory effect increased with the concentration of samples increasing. The relationship between the superoxide radical anion clearance rate and the concentration of total flavonoids was shown in Figure 2. When the sample concentration was 0.15 mg/mL, the average clearance rate was only 36.38%; when the sample concentration was 1.05 mg/mL, the inhibition rate was 85.97%.
Figure 2.
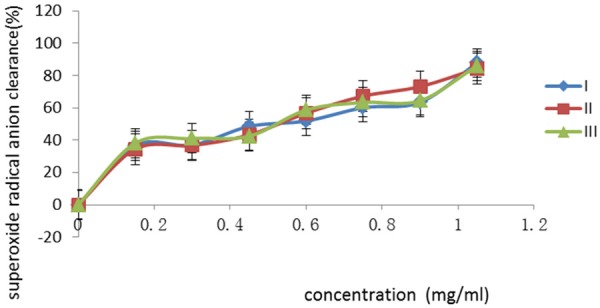
The relationship between the superoxide radical anion clearance rate and the concentration of total flavonoids. The concentration was in range of 0-1.05 mg/mL. The values are presented as a percentage.
The organ weight and coefficient
The results of organ weight and coefficient were shown in Tables 3 and 4. The organ coefficient in percent was calculated according to the following equation.
Table 3.
The results of organ weight of mice
| Group | High dose group | Middle dose group | Low dose group | Blank group |
|---|---|---|---|---|
| Heart (g) | 0.14±0.03 | 0.14±0.02 | 0.12±0.02 | 0.13±0.03 |
| Liver (g) | 0.93±0.18 | 0.83±0.09 | 0.83±0.17 | 0.93±0.20 |
| Spleen (g) | 0.11±0.02 | 0.07±0.02 | 0.06±0.04 | 0.06±0.03 |
| Lung (g) | 0.19±0.05 | 0.20±0.03 | 0.16±0.01 | 0.17±0.03 |
| Kidney (g) | 0.30±0.02 | 0.29±0.02 | 0.25±0.02 | 0.04±0.01 |
| Thymus (g) | 0.06±0.02 | 0.05±0.02 | 0.04±0.01 | 0.04±0.03 |
The values are presented as means ± standard deviation (10 mice/group). There was no significant difference in test groups and the blank (P>0.05).
Table 4.
The results of organ coefficient of mice
| Group | High dose group | Middle dose group | Low dose group | Blank group |
|---|---|---|---|---|
| Heart (%) | 0.61±0.14 | 0.61±0.05 | 0.60±0.09 | 0.60±0.03 |
| Liver (%) | 3.90±0.72 | 3.83±0.39 | 4.11±0.44 | 3.97±0.30 |
| Spleen (%) | 0.46±0.12 | 0.36±0.10 | 0.34±0.16 | 0.33±0.04 |
| Lung (%) | 0.79±0.21 | 0.90±0.14 | 0.68±0.09 | 0.67±0.07 |
| Kidney (%) | 1.27±0.08 | 1.33±0.03 | 1.17±0.16 | 1.17±0.25 |
| Thymus (%) | 0.25±0.05 | 0.24±0.04 | 0.23±0.01 | 0.23±0.10 |
The values are presented as means ± standard deviation (10 mice/group). There was no significant difference in test groups and the blank (P>0.05).
organ coefficient (%) = (organ weight/body weight) × 100%
The organ weight and coefficient of heart, liver, spleen, lung and kidney among all the experimental groups had no statistical differences (P>0.05). It suggested that there was no lesion in these tissues.
The antioxidant activity in vivo
The content of MDA and the activity of SOD in mice serum
The changes of the MDA concentration and SOD activity were showed in Table 5. The MDA concentrations in the test groups (high, middle and low dose groups) were significantly lower than the blank group (P<0.01). The SOD activity in the high dose group was higher than the blank group (P<0.01). The SOD activity in the middle and low dose group was significantly higher than the blank group (P<0.05). MDA is one of the main product of the lipid peroxidation, which is caused by free radicals [24]. The content of it can reflect the damage of the cells from the free radicals indirectly. SOD is an important antioxidant enzyme [25], it can clear the superoxide anion radical and protect cells from damage. The total flavonoids from Morus nigra significantly reduced MDA concentration and increased SOD activity.
Table 5.
Effect of total flavonoids on MDA and SOD of mice serum
| Group | MDA (nmol/mL) | SOD (U/mL) |
|---|---|---|
| High dose group | 5.19±0.29** | 134.74±10.76** |
| Middle dose group | 5.44±0.99** | 120.99±17.85* |
| Low dose group | 6.35±0.58** | 112.21±1.63* |
| Blank group | 8.46±0.84 | 94.4±4.01 |
The values are presented as means ± standard deviation (10 mice/group).
P<0.01 showed there was extremely significantly difference from blank.
P<0.05 showed there was significantly difference from blank.
The activity of the GSH-PX in mice blood
The changes of the GSH-PX activity were showed in Table 6. It was showed that the GSH-PX activity in blood increased with the concentration of the total flavonoids. The GSH-PX activity in the high and middle dose group was significantly higher than the blank group (P<0.01). The GSH-PX activity in the low dose group was significantly higher than the blank group (P<0.05). The GSH-PX is an important enzyme, which can catalytic reduce glutathione in reduction reaction of hydrogen peroxide specifically, protecting the cell membrane. The total flavonoids significantly improved GSH-PX activity.
Table 6.
Effects of total flavonoids on the GSH-PX activity in mice blood
| Group | GSH-PX (unit***) |
|---|---|
| High dose group | 437.14±41.14** |
| Middle dose group | 444.29±40.08** |
| Low dose group | 402.36±21.82* |
| Blank group | 311.43±16.16 |
The values are presented as means ± standard deviation (10 mice/group).
Showed every 1 mg protein responses per minute, excluding the effect of non enzymatic reaction, GSH concentration in the reaction system lower 1 µmol/L for one unit of enzyme activity.
P<0.01 showed there was extremely significantly difference from blank.
P<0.05 showed there was significantly difference from blank.
The level of SOD, GSH-PX, CAT and MDA in mice liver
The levels of SOD, GSH-PX, CAT and MDA in mice liver were showed in Table 7. It was showed that the SOD, GSH-PX and CAT activities increased with the concentration of the total flavonoids, the MDA concentrations decreased with the increase of flavonoids concentrations. The SOD and GSH-PX activities in the high dose group significantly were higher than the blank group (P<0.01). The SOD and GSH-PX activities in the middle dose group were significantly higher than the blank group (P<0.05). The CAT activity in the high and middle dose groups was significantly higher than the blank group (P<0.05). The MDA concentrations in the test groups were significantly lower than the blank group (P<0.01). CAT is an important enzyme that decomposes the hydroxyl radical in vivo, which protects the body from damage from the free radical and plays the antioxidant activity. The total flavonoids from Morus nigra decreased the concentration of MDA and increased the activities of SOD, CAT and GSH-PX in mice liver.
Table 7.
Effect of total flavonoids on SOD, GSH-PX, CAT and MDA level in liver of mice
| Group | SOD (U/mgprot) | GSH-PX (unit***) | CAT (U/gprot) | MDA (nmol/mgprot) |
|---|---|---|---|---|
| High dose group | 302.93±23.16** | 444.29±41.14** | 398.17±21.91* | 5.37±0.58** |
| Middle dose group | 274.99±14.62* | 437.14±40.08* | 376.47±41.23* | 5.98±0.73** |
| Low dose group | 260.64±36.55 | 402.36±21.82 | 342.67±26.28 | 6.16±0.67** |
| Blank group | 224.21±16.09 | 368.27±39.74 | 310.03±5.00 | 9.06±0.69 |
The values are presented as means ± standard deviation (10 mice/group).
Showed every 4 µL blood at 37°C responses for 5 minutes, excluding the effect of non enzymatic reaction, GSH concentration in the reaction system lower 1 µl/L for one unit of enzyme activity.
P<0.01 showed there was extremely significantly difference from blank.
P<0.05 showed there was significantly difference from blank.
The histopathology study
In the heart, liver, spleen, lung and kidney, the cross-section showed no significant lesions between the test groups and the blank group. In the spleen of the test group, the cross-section showed the normal appearance of spleen, white pulp, red pulp and spleen trabecula (Figure 3A), the Langhans cells increased obviously after total flavonoids treatment (Figure 3B). The Langhans cell is an important immunocompetent cell, which plays an important role in the process of immunoregulation, immune surveillance and immune tolerance, and it can enhance the immunity of the body. The Langhans cells increasing in the spleen showed that the flavonoids can enhance immunity of body.
Figure 3.
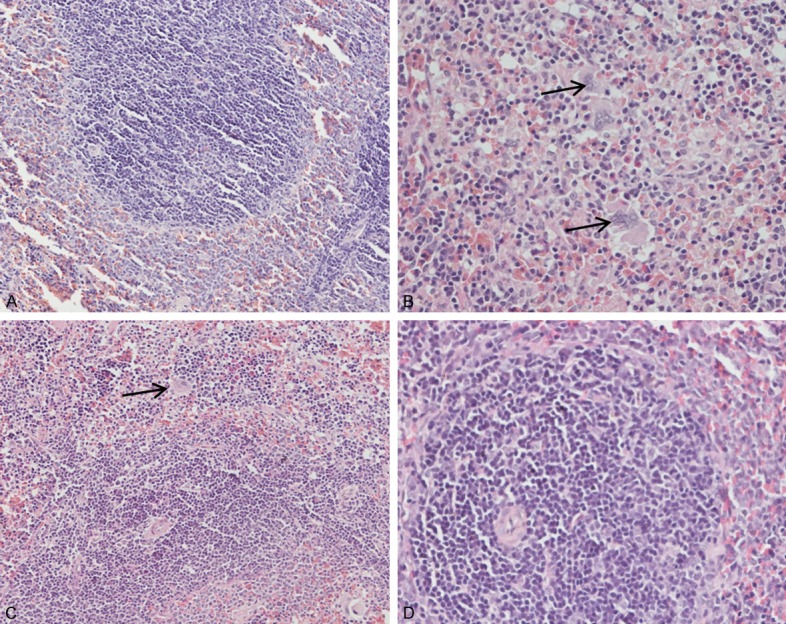
A. The spleen of mice in high dose group, white pulp, red pulp and spleen trabecula (HE, 200 ×). B. The spleen of mice in high dose group (HE, 400 ×). C. The spleen of mice in middle dose group (↑) (HE, 200 ×). D. The spleen of mice in control group (HE, 400 ×). And with the increase of concentration, there were more Langerhans cells increased (↑).
Discussion
There are a number of methods of extracting total flavonoids, including organic solvent extraction, ultrasonic extraction and microwave assisted extraction. The solvent extraction method is simple and easy, but the extraction efficiency is low; the extraction costs a lot of time and produces many of impurities. The ultrasonic extraction is efficient, time-saving and energyefficient, but non-selective. The microwave assisted extraction has a good selectivity and high efficiency, and the extraction process is fast, but it only applies to the materials which have a good thermostability [26]. According to those factors, the ultrasonic extraction was used in this experiment. The average yield of total flavonoids was 58.94% (Table 1), so the method of extraction adopted in this experiment was feasible, where only a single organic solvent was used and was not need to be heated.
The results of antioxidant activity in vitro (Figures 1 and 2) revealed that when the concentration of the total flavonoids was in the range of 0.00-1.05 mg/mL, the higher the concentration of the total flavonoids was, the higher hydroxyl radical and superoxide anion radical clearance rates were. According to the Figure 1, the broken line was steep when concentration was in the range of 0.00-0.30 mg/mL, suggesting that the increase of antioxidant activity was significant. When concentration was in the range of 0.30-0.45 mg/mL, the slope of the broken line was maximum, suggesting that the increase of antioxidant activity was maximum. When concentration was in the range of 0.45-1.05 mg/mL, the broken line was flat, suggesting that the increase of antioxidant activity was slow, but the maximum hydroxyl radical clearance rate could still reach 80%.
According to the results shown in Figure 2, when the concentration of the total flavonoids was low, the superoxide anion radical clearance rate was low. With the concentration increasing, the clearance rate increased. When the concentration of the total flavonoids was in the range of 0.00~0.15 mg/mL, the development of the superoxide anion radical clearance rate was maximum. When concentration was in the range of 0.15~0.30 mg/mL, the increase of clearance rate slowed down, when the concentration increased, the increase of clearance rate was obvious, the maximum clearance rate was 87%.
Among the reactive oxygen species, the hydroxyl radical is the most reactive and induces severe damage to the adjacent biomolecules. It plays an important role during the peroxidation of unsaturated fatty acids [27]. The superoxide anion radical is a highly toxic species which is generated by numerous biological and photochemical reactions [28]. The superoxide anion radicals have been observed to kill cells, inactivate enzymes and degrade DNA, cells members and polysaccharide [29]. The antioxidation effect of flavonoids may be related to their hydroxyl radical and superoxide anion radical scavenging properties. So the hydroxyl radical and superoxide anion radical clearance rate are widely used as indexes in the evaluation and screening of antioxidants. The content of Morus nigra flavonoids had the extremely positive correlation with the two indexes, which showed that the total flavonoids in Morus nigra were one of the important antioxidant substances. The content of total flavonoids can be used to examine medicinal quality of Morus nigra.
Organ coefficient was used widely as one of indexes of hygiene toxicology, the increase of organ coefficient indicated organ lesions, including congestion, edema, hypertrophy, etc. while the decrease of organ coefficient indicated organs atrophy and other degenerative changes [30-33]. In this study, the results suggested that the organ coefficient of heart, liver, spleen, lung and kidney of the test groups had no statistical difference (P>0.05) compared with the blank group (Table 5). Moreover, the Figure 3 showed that there was no pathological change in tissues. These results suggested that the total flavonoids would not damage the tissues. The Morus nigra flavonoids are safe and effective antioxidants.
Studies have shown that the body has a lot of antioxidant enzymes, including SOD, CAT, GSGH-PX, etc. SOD is the only antioxidant enzyme that clears oxygen free radical directly [25]. GSH-PX can clear H2O2 and lipid peroxide and inhibit the production of free radical [32]. CAT is a specific enzyme that decomposes H2O2. The H2O2 is usually the precursor of free radicals [34]. MDA is one of the main products of lipid peroxidation caused by free radicals [35,36]. In this study, the results showed that the total flavonoids from Morus nigra decreased the content of MDA and increased the activities of SOD, CAT and GSH-PX in mice, suggesting that Morus nigra flavonoids have strong antioxidant activities.
Langhans cell is important in the immune system [35]. Langhans cells in spleen were increased obviously in the test groups (the high, middle and low dose groups) compared with the blank group. At the same time, the weight of spleen of test groups increased when compared with the blank group. These results showed that the total flavonoids could promote the growth of the spleen and boost immunity, which provided evidence for the antioxidant activity of Morus nigra flavonoids.
Conclusion
In conclusion, the yield of total flavonoids was 58.94% and the content was 11.67%. The maximum hydroxyl radical clearance rate and superoxide anion radical clearance rate was 80.33% and 87.69%, respectively. The total flavonoids from Morus nigra decreased the content of MDA and increased the activities of SOD, CAT and GSH-PX in mice. No pathological changes were detected in mice treated with Morus nigra flavonoids. Langhans cells increased in spleen. The results of this study indicated that Morus nigra flavonoid is a safe and effective antioxidant.
Acknowledgements
This research was financially supported by the Program for Science and Technology Bureau of Yibin City (Grant No. 2012ZNY006, Grant No. 2014NY016); Supported by Key Lab of Aromatic Plant Resources Exploitation and Utilization in Sichuan Higher Education (Grant No. 2015XLZ004, Grant NO 2015XL005); The Project Supported by Scientific Research Fund of SiChuan Provincial Education Department (Grant No. 14TD0031); The National Natural Science Foundation of China (Grant No. 31372477); The Sichuan Youth Science and Technology Innovation Research Team for waterfowl disease prevention and control (Grant No. 2013TD0015); The program of Yibin University (Grant No. 2013QD06); The Sichuan International Cooperation Projects (2014HH0058).
Disclosure of conflict of interest
None.
References
- 1.Ercisli S, Orhan E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007;103:1380–1384. [Google Scholar]
- 2.Özgen M, Serçe S, Kaya C. Phytochemical and antioxidant properties of anthocyanin-rich Morus nigra and Morus rubra fruits. Scientia Horticulturae. 2009;119:275–279. [Google Scholar]
- 3.Kumar V, Chauhan S. Mulberry: life enhancer. J Med Plants Res. 2008;11:271–278. [Google Scholar]
- 4.Padilha MM, Vilela FC, Rocha CQ, Dias MJ, Soncini R, dos Santos MH, Alves-da-Silva G, Giusti-Paiva A. Antiinflammatory properties of Morus nigra leaves. Phytother Res. 2010;24:1496–1500. doi: 10.1002/ptr.3134. [DOI] [PubMed] [Google Scholar]
- 5.Pérez-Gregorio MR, Regueiro J, Alonso-González E, et al. Influence of alcoholic fermentation process on antioxidant activity and phenolic levels from mulberries (Morus nigra L. ) LWT-Food Science and Technology. 2011;44:1793–1801. [Google Scholar]
- 6.Butt MS, Nazir A, Sultan MT, Schroën K. Morus alba L. nature’s functional tonic. Trends in Food Science & Technology. 2008;19:505–512. [Google Scholar]
- 7.Zhang ZQ, Yang QX, Sun LH. Present situation of exploition and application of Mulberry. China Food Additives. 2009;4:65–68. [Google Scholar]
- 8.Wang XY, Zhang F, Halmurat UPUR, et al. Study on the Optimal Supercrjtica I CO2 Extraction of TotaI Flavonoids from Morus nigra Linn. Strait Pharmaceutical Journal. 2010;22:72–74. [Google Scholar]
- 9.Jiang H, Xu L, Liu JC, et al. Research Progress on Active Ingredients and Pharmacological Functions of Black Mulberry. Science of Sericlture. 2011;37:98–101. [Google Scholar]
- 10.Gryglewski RJ, Robak J. On the mechanism of antithrombotic action of flavonoids. Bioche Pharmacol. 1987;36:317–321. doi: 10.1016/0006-2952(87)90288-7. [DOI] [PubMed] [Google Scholar]
- 11.Gutteridge JM. Free radicals in disease processes: a compilation of cause and consequence. Free Radic Res Commun. 1993;19:141–158. doi: 10.3109/10715769309111598. [DOI] [PubMed] [Google Scholar]
- 12.Du J, He ZD, Jiang RW, Ye WC, Xu HX, But PP. Antiviral flavonoids from the root bark of Morus alba L. Phytochemistry. 2003;62:1235–1238. doi: 10.1016/s0031-9422(02)00753-7. [DOI] [PubMed] [Google Scholar]
- 13.Joshi R, Rana A, Gulati A. Studies on quality of orthodox teas made from anthocyanin-rich tea clones growing in Kangra valley, India. Food Chem. 2015;176:357–366. doi: 10.1016/j.foodchem.2014.12.067. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, Nie WJ. Nutritional composition and in vitro antioxidant capacity of black mulberry (morus nigra l. ) Fruits from Xinjiang Province. Food Sci. 2014;22:126–129. [Google Scholar]
- 15.Arfan M, Khan R, Rybarczyk A, Amarowicz R. Antioxidant activity of mulberry fruit extracts. Int J Mol Sci. 2012;13:2472–2480. doi: 10.3390/ijms13022472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang HY, Deng B, Jiang GB. Extraction of total flavanone form pleioblastus amarus laeves by ultrasonic and its antioxidation effect. Lishizhen Med Materia Med Res. 2009;20:1443–1445. [Google Scholar]
- 17.Li XY, Zhen RY, Wang H, et al. Study on flavonoids extraction process and antioxidation function in limonium bicolor. J Anhui Agricul Sci. 2010;38:8665–8666. [Google Scholar]
- 18.Xue SP, Zhang LW. Study on Scavenging of Mulberry leaf Flavonoids on Free Radicals. Journal of Shanxi Normal University (Natural Science Edition) 2009;23:1–8. [Google Scholar]
- 19.Zhao NJ. Tissue Section Technique. Chin J Veter Med. 2009;3:77–78. [Google Scholar]
- 20.Zhang F, Liu HB, Tian SG, et al. Ultrasonic extraction and content determination of morus nigra linn flavonoids and polysaccharides. Northwest Pharmaceutical Journal. 2008;23:282–283. [Google Scholar]
- 21.Maimaiti YM, Xu L, Wu C, et al. Xinjiang Mulberry flavonoids and polyphenols extraction and analysis of the Preliminary Report. North Sericulture. 2008;29:12–14. [Google Scholar]
- 22.Wei YD, Sun TT, Liu HH, et al. The Total Flavanone of Movus Aalba Extraction and the Identification by Ultrasonic. Lishizhen Medicine and Materia Medica Research. 2012;23:2811–2812. [Google Scholar]
- 23.Chang WG, Chen NF. Extraction and Analysis of the ChrysanthemumSauce Flavonoids. J Biol. 2005;22:35–37. [Google Scholar]
- 24.Zhang F, Jin J, Liu CF, et al. Research progress in antioxidant of fruit vinegar. China Brewing. 2008;17:8–11. [Google Scholar]
- 25.Gao LC, Chen WR. Free radicals and Aging. Biol Teach. 2001;1:8–10. [Google Scholar]
- 26.Hou CX, Yao HF, Quan C. Study on flavonoids extraction and purification process. Shanghai Chemical Industry. 2012;37:27–31. [Google Scholar]
- 27.Nice DJ, Robinson DS. Inhibition of lipid autoxidation by bovine superoxide dismutase. Food Chem. 1992;45:99–103. [Google Scholar]
- 28.Banerjee A, Dasgupta N, De B. In vitro study of antioxidant activity of Syzygium cumini fruit. Food Chem. 2005;90:727–733. [Google Scholar]
- 29.Fridovich I. The biology of oxygen radicals. Science. 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 30.Kristiansen E, Madsen C. Induction of protein droplet (α2μ-globulin) nephropathy in male rats after short-term dosage with 1, 8-cineole and l-limonene. Toxicol Lett. 1995;80:147–152. doi: 10.1016/0378-4274(95)03390-7. [DOI] [PubMed] [Google Scholar]
- 31.Wei JZ, Wu XG, Lin HH. A further observation on the serum biochemical values of chickens infected with Eimeriatenella or E. acervulina and the discussion on the pathological lesions. Chin J Prevent Veter Med. 2002;32:25–27. [Google Scholar]
- 32.Yuan BL. The significane and short coming of organ/body weight ratio used in drug safety evaluation. Chin J N Drugs. 2003;11:960–963. [Google Scholar]
- 33.Wang SL. Veterinary clinical diagnostics. Beijing: China Agriculture Press; 2006. [Google Scholar]
- 34.Wang CL, Guo F, Wang YL. Free radicals and Aging. J Med Univ. 2005;04:308–311. [Google Scholar]
- 35.Gungor N, Sengul M. Antioxidant activity, total phenolic content and selected physicochemical properties of white mulberry (Morus alba L. ) fruits. International J Food Propert. 2008;11:44–52. [Google Scholar]
- 36.Fu BY, Xia ZDM, MaojudaiX EMMD. Antioxidant and Antifati2ue Activities of Flavonoid from the Seeds of Foeniculum Vulgare Hotian. Journal of Xinjiang Normal University (Natural Sciences Edition) 2013;32:33–38. [Google Scholar]


