Abstract
Postoperative pain is the main obstacle for safely rapid recovery of patients undergoing laparoscopic cholecystectomy (LC). In this study, we systemically evaluated the analgesic efficacy of intraperitoneal and incisional ropivacaine injected at the end of the LC. A total of 160 patients, scheduled for elective LC, were allocated into four groups. Group Sham received intraperitoneal and incisional normal saline (NS). Group IC received incisional ropivacaine and intraperitoneal NS. Group IP received incisional NS and intraperitoneal ropivacaine. Group ICP received intraperitoneal and incisional ropivacaine. At the end of the surgery, ropivacaine was injected into the surgical bed through the right subcostal port and infiltrated at the four ports. Dynamic pain by a visual analogue scale (VAS) and cumulative morphine consumption at 2 h, 6 h, 24 h, and 48 h postoperatively, as well as incidence of side-effects over 48 h after LC was recorded. Compared with those in group Sham, the time of post-anesthesia care unit (PACU) stay, dynamic VAS score (VAS-D) 2 h and 6 h postoperatively, cumulative morphine consumption 6 h and 24 h postoperatively, and incidence of nausea and vomiting 48 h after LC in group IC and ICP were less (P<0.05). Furthermore, intraperitoneal and incisional ropivacaine exerts more powerful analgesic effect than single usage with intraperitoneal or incisional ropivacaine (P<0.05). No patients exhibited signs of local anesthetic toxicity. In conclusion, intraperitoneal and incisional ropivacaine might facilitate PACU transfer and effectively and safely reduce pain intensity after LC.
Keywords: Intraperitoneal, incisional, laparoscopic cholecystectomy, postoperative pain, ropivacaine
Introduction
Since the first reported laparoscopic cholecystectomy (LC) was performed by a French surgeon, Phillipe Mouret, in 1987, LC has become the gold standard of treatment of enlarged gallbladder polyps or symptomatic cholelithiasis [1]. Though postoperative pain is much less severe than that induced by open cholecystectomy, it is still the most important independent predictor of patients’ recovery after LC. It may considerably affect the recovery process, delay the discharge from the hospital and necessitate the use of opioids [2]. Due to side effects such as postoperative nausea and vomiting (PONV), somnolence, constipation, and respiratory depression induced by opioids, the pain after LC is often treated without satisfaction [3]. Therefore, several alternative means of pain relief have been studied over time to improve patient recovery and limit the hospital stay.
Local anesthetics (LA) have been widely used for control of pain by various routes including port-site infiltration and intraperitoneal instillation. Although a number of recent randomized controlled trials reported a considerable reduction in postoperative pain after the use of LA [4-6], others have reported no benefits [7,8]. The reason for this discrepancy might be due to variance in the type, dose, and concentration of LA as well as timing and site of administration. Ropivacaine is known as a safer agent than bupivacaine in terms of its relative lower toxicity in cardiovascular and central nervous system (CNS). Its preincisional ports infiltration plus intraperitoneal infusion at the beginning of LC combined with normal saline (NS) infusion at the end of the procedure is a safe and valid method for reducing pain after LC [5]. However, application of LA before the surgery would not preempt the trauma induced by pneumoperitoneum. Because administration of LA at the end of the surgery offers a longer time delay to the need for analgesics as well as duration of surgery is variable, the usage of ropivacaine at the end of surgery might be more flexible and applicable.
Nowadays, we designed this randomized, double-blind, placebo-controlled, and single-center trial to systemically evaluate the analgesic efficacy of intraperitoneal and incisional ropivacaine injected at the end of the LC. Our hypothesis is that this analgesic method is a relatively promising approach to pain management and produces a good safety profile for postoperative patients.
Material and methods
Study population and study design
After getting approval from the Ethics Committee of the affiliated Hexian Memorial Hospital of Southern Medical University (IRB number: No. HMH-2014-11-2C) and written informed consents from the eligible patients, a total of 160 patients were enrolled in the present study. The inclusion and exclusion criteria for study participation are shown in Table 1.
Table 1.
Inclusion and exclusion criteria for study participation
| Inclusion criteria |
| Elective LC for enlarged gallbladder polyps or symptomatic cholelithiasis. |
| Adult patient (18-65 yr). |
| ASA physical status I/II. |
| Exclusion criteria |
| Acute pancreatitis or cholecystitis (<6 wk). |
| History of previous abdominal surgery. |
| Patients with comorbid diseases (i.e, diabetes mellitus, severe hepatic or renal impairment) or valvular heart disease using oral anticoagulant drugs. |
| Chronic pain treatment. |
| History of alcohol or drug addiction. |
| Extreme overweight (BMI > 35). |
| Allergy to the drugs used in the present study. |
| Cognitive impairment or communication problems. |
| Pregnancy or lactating. |
ASA, American Society of Anesthesiologist; BMI, body mass index; LC, laparoscopic cholecystectomy.
On the day of the surgery, an investigator not involved with patients care confirmed patient eligibility and written consent. According to the different treatments at the end of LC just before the deflation of pneumoperitoneum, the eligible patients were randomly allocated 1:1:1:1 to four groups: group Sham received the bolus 10 ml of NS instillation intraperitoneally and incisional infiltration of NS 10 ml as well; group IC received intraperitoneal instillation of NS 10 ml and incisional infiltration of ropivacaine (0.75%, 10 ml, AstraZeneca AB, Sweden) 10 ml; group IP received intraperitoneal instillation of ropivacaine 10 ml and incisional infiltration of NS 10 ml later; and group ICP received 10 ml of intraperitoneal and incisional ropivacaine each. After gallbladder extraction, ropivacaine was intraperitoneally injected into the surgical bed using a feeding tube through the right subcostal port. After closure of the surgical wounds, the ropivacaine infiltration was conducted by the same surgeon at the four ports (epigastric port: 4 ml; umbilical and the two 5 mm right abdominal ports: 2 ml each).
Randomization and blinding
Randomization was based on reproducible computer-generated codes that were maintained in sequentially numbered opaque envelopes. The operating room (OR) nurse staff not involved in the study opened the sealed opaque envelope containing patient allocation and instructions for the solution preparation. One research staff blinded to the details of the study was scheduled to collect the postoperative data. The patient and surgeon were unaware of the given medications.
Surgical technique
According to the standard surgical protocol the LC was performed by the same surgical team. The patient was placed in the reverse Trendelenburg position (angled at nearly 30°) with the table tilted downward to the patient’s left side. A classical 4-trocar surgical technique that consisted of placement of a 12 mm port via the umbilical incision, a 10 mm port in the epigastric area, and two 5 mm ports on the right side of the abdomen was used for all patients. Pneumoperitoneum was created and maintained by insufflation with non-humidified and non-heated CO2 gas at 15 mmHg of the intra-abdominal pressure. Clipping and blunt transection were conducted until Calot’s triangle was exposed by electrocauterization. The gallbladder was dissected from the liver bed by a Hook bovie (Covidien, USA) and extracted through the epigastric port site. At the end of the surgery, CO2 was carefully evacuated by manual compression of the abdomen with open trocars.
Anesthesia protocol
The patients were fasted for 8 h and transferred to the preoperative room without premedication. Then a 20-G intravenous cannula was inserted into the right forearm and connected to a T-connector for drug administration. Upon arrival at the OR, monitoring was continuously accomplished by electrocardiography (ECG), non-invasive blood pressure (NIBP), pulse oxygen saturation (SpO2), and heart rate (HR). Lactated Ringer’s solution (6-8 ml·kg-1·h-1) was infused throughout the surgery. General anesthesia was induced with 6 µg/kg of fentanyl, 0.05 mg/kg of midazolam, and 2 mg/kg of propofol i.v. and orotracheal intubation was facilitated with cisatracurium (0.15 mg/kg, i.v.). Anesthesia was maintained with sevoflurane at 1.5-2.5% end-tidal concentration. Mechanical ventilation was controlled using a ventilator (Aestiva/5, Datex-Ohmeda, USA) and respiratory parameters were adjusted to keep the end-tidal CO2 at 35-45 mmHg. After tracheal intubation, an oesophageal temperature probe was placed. The OR temperature was set at 20°C and patients were kept warm using the forced warm-air device. At the end of the surgery, residual neuromuscular blockade was antagonized with neostigmine 0.03 mg/kg and atropine 0.02 mg/kg, and tracheal extubation was performed once clinical signs were observed and a TOF ratio of 0.9 was achieved.
All patients received 4 mg of ondansetron i.v. at the end of surgery for preventing from postoperative nausea and vomiting (PONV). To control the severity of postoperative pain, the patients complaining of pain in the post-anesthesia care unit (PACU) received a bolus of morphine 3 mg i.v. until the visual analogue scale (VAS) score was <30 mm. Then the postoperative analgesia was continued by the initiation of computerized patient-controlled intravenous analgesia (PCIA), morphine 1 mg bolus with a lockout time of 15 min. Patients were encouraged to ambulate as soon as possible and hospitalized for up to 72 h as part of our routine practice.
Outcome measurements
Before the surgery patient was given an explanation of VAS and familiarized with use of the PCIA device. The collected data included patient age, gender, weight, American Society of Anesthesiologists (ASA) physical status, duration of surgery (from incision of skin to closure of the surgical wounds), temperature in the PACU, and duration of PACU stay. The primary end point is the dynamic pain intensity 2 h, 6 h, 24 h, and 48 h after the surgery. The intensity of dynamic pain was assessed using a 100-mm VAS (0 mm represents “no pain” and 100 mm represents “worst unbearable pain”) during deep breathing, coughing, or movement [9]. The secondary end points are cumulative morphine consumption 2 h, 6 h, 24 h, and 48 h after the LC and the proportion of patients with PONV over 48 h postoperatively. Meanwhile intra-operative arrhythmias or delayed awakening was noted to determine if the patients had signs of local anesthetic toxicity.
Statistical analysis
In our pilot study, we found that, compared with twenty patients receiving control usage with intraperitoneal and incisional NS 10 ml each, difference in pain score means 6 h after the LC of another twenty patients receiving same volume of intraperitoneal and incisional ropivacaine was 27 points. So we need to recruit 33 patients in each group to achieve 80% power and 0.05 significance level [10]. Assuming a compliance rate of 80%, we recruited 40 patients per group in the formal trial.
Because this study evaluated the effect of intraperitoneal and incisional ropivacaine on dynamic pain intensity after the LC, conversion to the open surgery was considered as protocol violation. These patients were excluded from data collection. For the safety analysis the excluded patients still received the same anesthesia and analgesia protocol as well as evaluations until their hospital discharge.
Data were analyzed using the statistical package SPSS 15.0 for windows (SPSS Inc, Chicago, USA). Continuous data (age, weight and duration of surgery, temperature after surgery, PACU stay, dynamic VAS scores, and cumulative morphine consumption) were presented as means (SD) and 95% confidence interval and analyzed with the two-way analysis of variance or the Friedman test as appropriate. Data regarding patient gender, ASA physical status, and number of patients with PONV were presented as frequency (percentage) and analyzed with the x2 test. Scheffe tests were used for post hoc multiple treatment comparisons. A P-value <0.05 was considered statistically significant.
Results
Demographic characteristics
Of the total of 174 patients assessed for eligibility in the study, we excluded 14 ones. Eleven patients did not meet inclusive criteria and 5 ones declined to participate. Then 160 patients were recruited into the study, with 152 ones included in the final data analysis. Three patients in the group Sham, one patient in the group IC, two patients in the group IP, and two patients in the group ICP were excluded due to the conversion to the open surgery (Figure 1).
Figure 1.
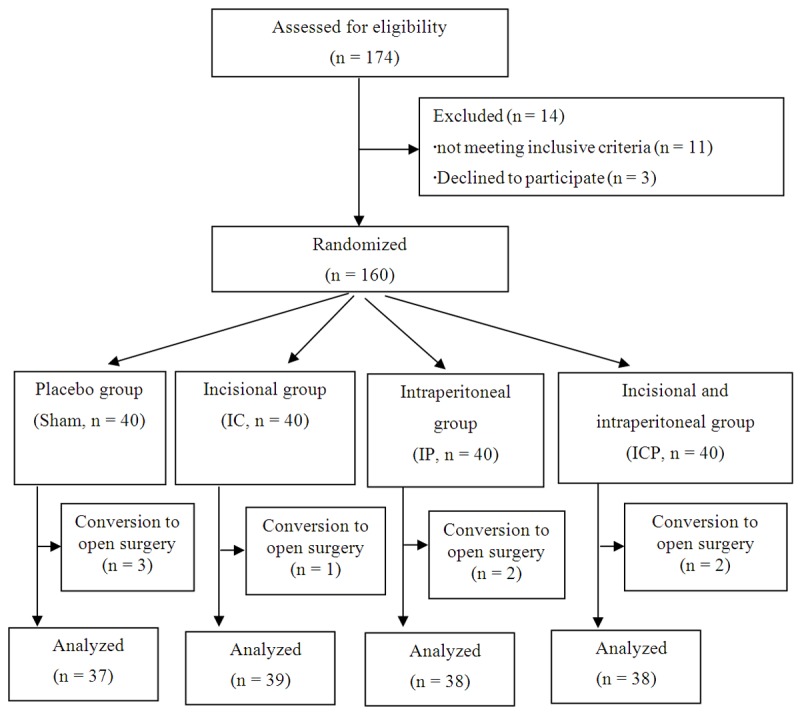
Flow diagram showing participants in this trial.
There were no significant differences among the four groups with regard to age, gender, weight, ASA physical status, duration of surgery, and temperature after surgery (Table 2). However, the time of PACU stay in group IC and ICP was less than that in group Sham (P<0.05: 30.4±12.6, 27.6±10.5 vs. 40.2±19.6).
Table 2.
Demographic data of 152 patients who underwent laparoscopic cholecystectomy
| Characteristics | Group Sham (n = 37) | Group IC (n = 39) | Group IP (n = 38) | Group ICP (n = 38) | p-Value |
|---|---|---|---|---|---|
| Age (yr) | 46.4 (10.6) | 42.5 (13.4) | 41.2 (12.7) | 42.9 (14.2) | 0.7338 |
| Gender (M/F) | 21/26 | 18/31 | 20/28 | 23/25 | 0.5679 |
| Weight (kg) | 53.5 (13.1) | 57.4 (11.1) | 50.2 (14.6) | 54.3 (15.8) | 0.8312 |
| ASA (I/II) | 34/13 | 32/17 | 30/18 | 32/16 | 0.9052 |
| Duration of surgery (min) | 21.2 (7.6) | 23.9 (9.4) | 24.5 (10.6) | 25.1 (9.6) | 0.7724 |
| Temperature after surgery (°C) | 36.3 (0.2) | 36.2 (0.2) | 36.3 (0.1) | 36.2 (0.3) | 0.9824 |
| PACU stay (min) | 40.2 (19.6) | 30.4 (12.6)* | 37.2(15.3) | 27.6 (10.5)* | 0.0232 |
Sham, no intraperitoneal and incisional ropivacaine use; IC, no intraperitoneal but incisional ropivacaine use; IP, no incisional but intraperitoneal ropivacaine use; ICP, incisional and intraperitoneal ropivacaine use; M, male; F, female; ASA, American Society of Anesthesiologists; PACU, post-anesthesia care unit. Data are expressed as means (SD) or the number of patients.
P<0.05 when compared with group Sham.
Dynamic VAS score
With an increase in time following surgery, the dynamic pain in each group gradually declined (Table 3). At every evaluation 2 h and 6 h after the surgery, patients in the group Sham reported higher pain scores compared with those in the groups IC and ICP (P<0.05). Furthermore, intraperitoneal and incisional ropivacaine exerts more powerful analgesic effect 24 h after the LC. Meanwhile the dynamic pain scores in group ICP were also much less than those in groups IC and IP 2 h, 6 h, and 24 after the surgery (P<0.05). There were no differences among the four groups with regard to the dynamic pain 48 h after the surgery.
Table 3.
Dynamic VAS score (VAS-D) of 152 patients after laparoscopic cholecystectomy
| Group Sham (n = 37) | Group IC (n = 39) | Group IP (n = 38) | Group ICP (n = 38) | |
|---|---|---|---|---|
| VAS-D 2 h | 46 (24), 95% CI 38-60 | 27 (13)*,#, 95% CI 24-31 | 37 (18)#, 95% CI 28-49 | 12 (8)*, 95% CI 6-13 |
| VAS-D 6 h | 42 (18), 95% CI 33-50 | 22 (12)*,#, 95% CI 17-28 | 36 (13)#, 95% CI 27-40 | 10 (9)*, 95% CI 5-10 |
| VAS-D 24 h | 27 (13), 95% CI 19-31 | 23 (14)#, 95% CI 14-29 | 21 (12)#, 95% CI 14-28 | 10 (7)*, 95% CI 6-12 |
| VAS-D 48 h | 18 (12), 95% CI 14-26 | 18 (11), 95% CI 12-24 | 17 (11), 95% CI 12-24 | 15 (13), 95% CI 10-22 |
Sham, no intraperitoneal and incisional ropivacaine use; IC, no intraperitoneal but incisional ropivacaine use; IP, no incisional but intraperitoneal ropivacaine use; ICP, incisional and intraperitoneal ropivacaine use; VAS-D, Dynamic Visual Analgesia Scale. Data are expressed as means (SD) and 95% CI of VAS-D in mm on a 100-mm scale.
P<0.05 when compared with group Sham at the same time-point.
P<0.05 when compared with group ICP at the same time-point.
Cumulative morphine consumption
Cumulative morphine consumption is another reflection of pain intensity. As shown in Table 4, over the 48 h postoperative period, patients in group ICP consumed less dosage of morphine than those in the group Sham (P<0.05). The cumulative morphine consumption in group IC 6 h and 24 h after the surgery as well as that in group IP 24 h after the LC was also less than that in group Sham at the corresponding timepoints. At the evaluation 6 h and 24 h after the LC, intraperitoneal and incisional ropivacaine further produced a larger reduction in morphine consumption when compared with single usage of intraperitoneal ropivacaine (P<0.05).
Table 4.
Cumulative morphine consumption (mg) of 152 patients after laparoscopic cholecystectomy
| Group Sham (n = 37) | Group IC (n = 39) | Group IP (n = 38) | Group ICP (n = 38) | |
|---|---|---|---|---|
| 2 h | 9 (3), 95% CI 6-8 | 5 (4), 95% CI 2-5 | 6 (4), 95% CI 5-8 | 2 (3)*, 95% CI 2-3 |
| 6 h | 14 (9), 95% CI 15-19 | 6 (6)*, 95% CI 4-8 | 11 (7)#, 95% CI 9-14 | 4 (2)*, 95% CI 3-5 |
| 24 h | 23 (10), 95% CI 18-25 | 11 (8)*, 95% CI 9-15 | 12 (11)*, 95% CI 10-14 | 8 (8)*, 95% CI 6-9 |
| 48 h | 24 (14), 95% CI 20-28 | 17 (14), 95% CI 14-22 | 21 (11)#, 95% CI 17-24 | 12 (8)*, 95% CI 9-12 |
Sham, no intraperitoneal and incisional ropivacaine use; IC, no intraperitoneal but incisional ropivacaine use; IP, no incisional but intraperitoneal ropivacaine use; ICP, incisional and intraperitoneal ropivacaine use. Data are expressed as means (SD) and 95% CI for cumulative morphine consumption (mg) after the surgery.
P<0.05 when compared with group Sham at the same time-point.
P<0.05 when compared with group ICP at the same time-point.
Incidence of side effects
As shown in Table 5, the incidence of nausea and vomiting in group IC and ICP over 48 h postoperatively were much less than those in group Sham (P<0.05). No patients exhibited signs of local anesthetic toxicity during or after surgery.
Table 5.
Incidence of side effects of 152 patients 48 h after laparoscopic cholecystectomy
| Group Sham (n = 37) | Group I (n = 39) | Group N (n = 38) | Group IN (n = 38) | |
|---|---|---|---|---|
| Nausea | 21 (56.7) | 10 (25.6)* | 13 (34.2) | 6 (15.8)* |
| Vomiting | 14 (37.8) | 6 (15.3)* | 9 (23.6) | 5 (13.1)* |
| Signs of local anesthetic toxicity | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Sham, no intraperitoneal and incisional ropivacaine use; IC, no intraperitoneal but incisional ropivacaine use; IP, no incisional but intraperitoneal ropivacaine use; ICP, incisional and intraperitoneal ropivacaine use; Data are expressed as the number of patients (%).
P<0.05 when compared with group Sham.
Discussion
The main finding of this trial is that, compared with placebo and single usage of intraperitoneal or incisional ropivacaine, intraperitoneal and incisional ropivacaine (0.75%, 10 ml each) at the end of the LC significantly reduced the time of PACU stay, postoperative dynamic pain, cumulative morphine requirements, and incidence of PONV. In a previous study, combined usage with incisional ropivacaine (2 mg/ml, 20 ml) and its intraperitoneal infusion (2 mg/kg, 100 ml) ahead of the surgical procedure exerted additive effects on decreasing postoperative pain [11]. There is another similar report that local skin infiltration of bupivacaine and intraperitoneal lidocaine (2%, 10 ml) or bupivacaine (0.5%, 10 ml) after LC also were proven to lower the intensity of postoperative pain in a synergistic fashion [10]. Furthermore, intraperitoneal NS infusion at the end of the procedure has been proved to achieve postoperative pain reduction after LC [12]. To eliminate the influence of NS on the postoperative pain, we set up incisional and/or intraperitoneal NS treatment groups for control.
In this trial, the duration of operation was really very short. There are two reasons accounting for it. First, we excluded the patients with acute pancreatitis or cholecystitis (<6 wk) and the ones with history of previous abdominal surgery. So the surgeries were relative easily done. Second, it is really the very experienced team to perform laparoscopic cholecystectomy, because the surgeon has specialized in it for more than 10 years.
Ropivacaine is a long-acting LA that was developed after the emergence of bupivacaine-related severe toxicity. It is a pure left-isomer and has less toxic potential on the CNS and the circulatory system [13]. Though peak concentration of ropivacaine (0.75%, 300 mg) was 3.01-4.32 μg/ml when injected intraperitoneally at immediately after pneumoperitoneum and followed by at the end of the surgery, it did not induce any clinical evidence of toxicity [14]. The large dose of ropivacaine (300 mg) not only produced similar analgesia (when compared with 100 mg), but also led to large plasma concentrations. Considering the patients’ safety, the maximum dosage of ropivacaine used in the present trial was 150 mg, which is far below the maximum dose for infiltration anesthesia (200 mg) in an adult patient [15].
Following LC the postoperative pain at incision sites has the largest component (50%-70%), followed by the pneumoperitoneum (20%-30%) and cholecystectomy (10%-20%) [16]. Incisional pain is usually mild to moderate in intensity, maximal immediately postoperatively, subsiding with time, and dominant role during the first 48 h after LC [17]. For patients undergoing laparoscopic ventral/incisional hernia repair incisional bupivacaine (0.25%, plus epinephrine) immediately before suture placement reduced pain at the early postoperative stage [4]. Liu YY et al. also reported that, in patients undergoing LC, ropivacaine infusion (1.0%, 20 ml) at the port sites decreased postoperative pain immediately, reduced the meperidine use and shortened hospital stay [18]. Large volume ropivacaine at relative lower concentration injected into cholecystectomy wounds decreased wound pain and prolonged the time of the first request for postoperative analgesia [19]. In the present study, usage with less volume of ropivacaine also producing promising analgesic effect might account for its higher concentration (0.75% vs. 0.25%) and multiple usages.
Administration of intraperitoneal LA has been used by many surgeons as a method of postoperative pain relief. The evidence supporting intraperitoneal LA infusion as part of a multimodal analgesic regimen for reducing postoperative pain and analgesic consumption following LC is accumulating [11,20,21]. Increasing the dose of intraperitoneal LA infusion also seems important. The intraperitoneal instillation of ropivacaine 150 mg at the end of LC was more effective on postoperative pain relief than either 100 mg of bupivacaine or ropivacaine, whereas smaller doses of LA failed to demonstrate any beneficial efficacy on pain relief [22-24]. In the present study, intraperitoneal ropivacaine injected into the surgical bed after gallbladder extraction could not decrease the postoperative pain score, which suggested that identification of optimal intraperitoneal ropivacaine dosage needs to be further investigated. Meanwhile, mean morphine consumption in the 48 h postoperative period was 21 mg in intraperitoneal ropivacaine group, which was far from negligible. It demonstrates that, although intraperitoneal ropivacaine injection has some additive effect on postoperative pain, it remains a weak analgesic technique.
Intraperitoneal 5 mg/kg of 1.0% levoropivacaine in 200 ml of normal saline ahead of the surgery plus incisional 20 ml of 1.0% levoropivacaine at four port sites immediately after wound closure decreased the immediate postoperative pain and the duration of hospital stay [25]. But it is not the randomized and double-blinded trial. In the present study, we designed this randomized, double-blind, placebo-controlled trial in order to better evaluate the analgesic efficacy of intraperitoneal and incisional ropivacaine. Furthermore, we injected intraperitoneal and incisional ropivacaine at the end of the surgery without dilution. So it is convenient for the surgeon to conduct and helpful to decrease the duration of the surgery.
Postoperative analgesic requirement is an additional way to quantify the benefit of perioperative analgesia. In our study, intraperitoneal and incisional ropivacaine produced the largest reduction in cumulative morphine consumption. However, postoperative analgesic requirement was not the primary outcome measurement in our trial, so the trial was not powered to test for this difference. A larger trial focusing on the postoperative cumulative analgesic requirement may be important.
Pain and opioids both may induce PONV. For patients undergoing LC, a combination of incisional and intraperitoneal ropivacaine (286 mg, 66 ml) blockade reduced not only overall pain score but also the incidence of nausea at the early postoperative stage [17]. We got the similar results, which might attribute for the reduction in postoperative pain scores and dosage of cumulative morphine consumption.
There are some limitations relevant to our study. First, we did not compare the influence of the timing of ropivacaine treatment (preoperative vs. end of the surgery) on postoperative pain relief. Preincisional wound infiltration with ropivacaine (10 mg/ml, 20 ml) was reported to provide satisfactory postoperative analgesia and reduce the rescue analgesic requirements for patients undergoing laparoscopic procedures [24]. However, Sozbilen M et al. [26] found that administration of ropivacaine preoperatively and postoperatively for LC has similar effects on postoperative pain over a 24 h postoperative follow-up. Second, though shoulder pain is often mild in intensity, it may occur 35%-63% and sometimes lasts for 3 d [27,28]. It is a pity for us not to record this parameter. Third, earlier mobility is also an important parameter to indirectly reflect the recovery of patients. Finally, we did not record the duration of hospital stay and compare them among the four groups. It is really a key variable to measure here in health economic terms. If we had noted them, we might be better to overall judge the difference among the four treatment groups.
In conclusion, combined usage with intraperitoneal and incisional ropivacaine at the end of the LC might shorten the time of PACU stay and effectively reduce postoperative dynamic pain scores, cumulative analgesic consumption, and incidence of PONV. This analgesic method might be a better safety profile for postoperative patients undergoing LC.
Disclosure of conflict of interest
None.
References
- 1.Soper NJ, Stockmann PT, Dunnegan DL, Ashley SW. Laparoscopic cholecystectomy. The new ‘gold standard’? Arch Surg. 1992;127:917–921. doi: 10.1001/archsurg.1992.01420080051008. [DOI] [PubMed] [Google Scholar]
- 2.Elhakim M, Amine H, Kamel S, Saad F. Effects of intraperitoneal lidocaine combined with intravenous or intraperitoneal tenoxicam on pain relief and bowel recovery after laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 2000;44:929–933. doi: 10.1034/j.1399-6576.2000.440806.x. [DOI] [PubMed] [Google Scholar]
- 3.Wheeler M, Oderda GM, Ashburn MA, Lipman AG. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain. 2002;3:159–180. doi: 10.1054/jpai.2002.123652. [DOI] [PubMed] [Google Scholar]
- 4.Bellows CF, Berger DH. Infiltration of suture sites with local anesthesia for management of pain following laparoscopic ventral hernia repairs: a prospective randomized trial. JSLS. 2006;10:345–350. [PMC free article] [PubMed] [Google Scholar]
- 5.Pappas-Gogos G, Tsimogiannis KE, Zikos N, Nikas K, Manataki A, Tsimoyiannis EC. Preincisional and intraperitoneal ropivacaine plus normal saline infusion for postoperative pain relief after laparoscopic cholecystectomy: a randomized double-blind controlled trial. Surg Endosc. 2008;22:2036–2045. doi: 10.1007/s00464-008-9762-x. [DOI] [PubMed] [Google Scholar]
- 6.Castillo-Garza G, Díaz-Elizondo JA, Cuello-García CA, Villegas-Cabello O. Irrigation with bupivacaine at the surgical bed for postoperative pain relief after laparoscopic cholecystectomy. JSLS. 2012;16:105–111. doi: 10.4293/108680812X13291597716221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheinin B, Kellokumpu I, Lindgren L, Haglund C, Rosenberg PH. Effect of intraperitoneal bupivacaine on pain after laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 1995;39:195–198. doi: 10.1111/j.1399-6576.1995.tb04042.x. [DOI] [PubMed] [Google Scholar]
- 8.Rademaker BM, Kalkman CJ, Odoom JA, de Wit L, Ringers J. Intraperitoneal local anaesthetics after laparoscopic cholecystectomy: effects on postoperative pain, metabolic responses and lung function. Br J Anaesth. 1994;72:263–266. doi: 10.1093/bja/72.3.263. [DOI] [PubMed] [Google Scholar]
- 9.Ingelmo PM, Bucciero M, Somaini M, Sahillioglu E, Garbagnati A, Charton A, Rossini V, Sacchi V, Scardilli M, Lometti A, Joshi GP, Fumagalli R, Diemunsch P. Intraperitoneal nebulization of ropivacaine for pain control after laparoscopic cholecystectomy: a doubleblind, randomized, placebo-controlled trial. Br J Anaesth. 2013;110:800–806. doi: 10.1093/bja/aes495. [DOI] [PubMed] [Google Scholar]
- 10.Khan MR, Raza R, Zafar SN, Shamim F, Raza SA, Pal KM, Zafar H, Alvi R, Chawla T, Azmi R. Intraperitoneal lignocaine (lidocaine) versus bupivacaine after laparoscopic cholecystectomy: results of a randomized controlled trial. J Surg Res. 2012;178:662–669. doi: 10.1016/j.jss.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Cha SM, Kang H, Baek CW, Jung YH, Koo GH, Kim BG, Choi YS, Cha SJ, Cha YJ. Peritrocal and intraperitoneal ropivacaine for laparoscopic cholecystectomy: a prospective, randomized, double-blind controlled trial. J Surg Res. 2012;175:251–258. doi: 10.1016/j.jss.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 12.Tsimoyiannis EC, Siakas P, Tassis A, Lekkas ET, Tzourou H, Kambili M. Intraperitoneal normal saline infusion for postoperative pain after laparoscopic cholecystectomy. World J Surg. 1998;22:824–828. doi: 10.1007/s002689900477. [DOI] [PubMed] [Google Scholar]
- 13.Scott DB, Lee A, Fagan D, Bowler GM, Bloomfield P, Lundh R. Acute toxicity of ropivacaine compared with that of bupivacaine. Anesth Analg. 1989;69:563–569. [PubMed] [Google Scholar]
- 14.Callesen T, Hjort D, Mogensen T, Schouenborg L, Nielsen D, Reventlid H, Kehlet H. Combined field block and i. p. instillation of ropivacaine for pain management after laparoscopic sterilization. Br J Anaesth. 1999;82:586–590. doi: 10.1093/bja/82.4.586. [DOI] [PubMed] [Google Scholar]
- 15.Betton D, Greib N, Schlotterbeck H, Joshi GP, Ubeaud-Sequier G, Diemunsch P. The pharmacokinetics of ropivacaine after intraperitoneal administration: instillation versus nebulization. Anesth Analg. 2010;111:1140–1145. doi: 10.1213/ANE.0b013e3181f3fb19. [DOI] [PubMed] [Google Scholar]
- 16.Bisgaard T, Kehlet H, Rosenberg J. Pain and convalescence after laparoscopic cholecystectomy. Eur J Surg. 2001;167:84–96. doi: 10.1080/110241501750070510. [DOI] [PubMed] [Google Scholar]
- 17.Bisgaard T, Klarskov B, Kristiansen VB, Callesen T, Schulze S, Kehlet H, Rosenberg J. Multi-regional local anesthetic infiltration during laparoscopic cholecystectomy in patients receiving prophylactic multi-modal analgesia: a randomized, double-blinded, placebo-controlled study. Anesth Analg. 1999;89:1017–1024. doi: 10.1097/00000539-199910000-00036. [DOI] [PubMed] [Google Scholar]
- 18.Liu YY, Yeh CN, Lee HL, Wang SY, Tsai CY, Lin CC, Chao TC, Yeh TS, Jan YY. Local anesthesia with ropivacaine for patients undergoing laparoscopic cholecystectomy. World J Gastroenterol. 2009;15:2376–2380. doi: 10.3748/wjg.15.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta A. Local anaesthesia for pain relief after laparoscopic cholecystectomy--a systematic review. Best Pract Res Clin Anaesthesiol. 2005;19:275–292. doi: 10.1016/j.bpa.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 21.Yang SY, Kang H, Choi GJ, Shin HY, Baek CW, Jung YH, Choi YS. Efficacy of intraperitoneal and intravenous lidocaine on pain relief after laparoscopic cholecystectomy. J Int Med Res. 2014;42:307–319. doi: 10.1177/0300060513505493. [DOI] [PubMed] [Google Scholar]
- 22.Kucuk C, Kadiogullari N, Canoler O, Savli S. A placebo-controlled comparison of bupivacaine and ropivacaine instillation for preventing postoperative pain after laparoscopic cholecystectomy. Surg Today. 2007;37:396–400. doi: 10.1007/s00595-006-3408-1. [DOI] [PubMed] [Google Scholar]
- 23.Joris J, Thiry E, Paris P, Weerts J, Lamy M. Pain after laparoscopic cholecystectomy: characteristics and effect of intraperitoneal bupivacaine. Anesth Analg. 1995;81:379–384. doi: 10.1097/00000539-199508000-00029. [DOI] [PubMed] [Google Scholar]
- 24.Pavlidis TE, Atmatzidis KS, Papaziogas BT, Makris JG, Lazaridis CN, Papaziogas TB. The effect of preincisional periportal infiltration with ropivacaine in pain relief after laparoscopic procedures: a prospective, randomized controlled trial. JSLS. 2003;7:305–310. [PMC free article] [PubMed] [Google Scholar]
- 25.Yeh CN, Tsai CY, Cheng CT, Wang SY, Liu YY, Chiang KC, Hsieh FJ, Lin CC, Jan YY, Chen MF. Pain relief from combined wound and intraperitoneal local anesthesia for patients who undergo laparoscopic cholecystectomy. BMC Surg. 2014;14:28. doi: 10.1186/1471-2482-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sozbilen M, Yeniay L, Unalp M, Makay O, Pirim A, Ulukaya S, Uyar M, Ersin S. Effects of ropivacaine on pain after laparoscopic cholecystectomy: a prospective, randomized study. Adv Ther. 2007;24:247–257. doi: 10.1007/BF02849892. [DOI] [PubMed] [Google Scholar]
- 27.Gupta A, Thörn SE, Axelsson K, Larsson LG, Agren G, Holmström B, Rawal N. Postoperative pain relief using intermittent injections of 0.5% ropivacaine through a catheter after laparoscopic cholecystectomy. Anesth Analg. 2002;95:450–456. doi: 10.1097/00000539-200208000-00040. [DOI] [PubMed] [Google Scholar]
- 28.Møiniche S, Jørgensen H, Wetterslev J, Dahl JB. Local anesthetic infiltration for postoperative pain relief after laparoscopy: a qualitative and quantitative systematic review of intraperitoneal, port-site infiltration and mesosalpinx block. Anesth Analg. 2000;90:899–912. doi: 10.1097/00000539-200004000-00024. [DOI] [PubMed] [Google Scholar]


