Abstract
Background: Anterior cruciate ligament (ACL) rupture is the most common ligamentous injury for active adolescents and young adults each year. However, the precise etiologies of ACL injury are not fully understood. The present study was to investigate +104T/C polymorphism of growth differentiation factor 5 (GDF5) gene in patients with ACL rupture, and evaluate the effects of polymorphism on GDF5 mRNA levels in ligament of patients with ACL rupture in central China. Methods: A total of 286 Chinese patients with ACL rupture and 500healthy controls were enrolled in this study. The +104T/C polymorphism in GDF5 gene were genotyped by DNA sequencing. GDF5 mRNA expressions levels in ligament were determined by quantitative PCR. Results: The frequency of the TT genotype tended to be higher in ACL rupture group than in control group (62.6% vs. 48.0%, P< 0.001, OR = 1.81, 95% CI: 1.35-2.44). T allele of the GDF5 +104T/C polymorphism was more common in ACL rupture group than in control group (P< 0.001). Patients carrying TT genotype expressed lower levels of GDF5 mRNA than C carriers (P = 0.005) among ACL rupture. Conclusion: Our study indicated that GDF5 +104T/C polymorphism was associated with ACL rupture patients in central China. This is likely from decreased expressions of GDF5 mRNA. Further studies are necessary to explore the functional implication of the GDF5 +104T/C polymorphism in Chinese ACL rupture patients.
Keywords: Anterior cruciate ligament, rupture, growth differentiation factor 5, polymorphism, expression
Introduction
The anterior cruciate ligament (ACL) rupture is one of the most common knee injuries in sports and often results in joint effusion, muscle weakness, altered movement, and reduced functional performance [1,2]. Individuals who participate in these high risk sports, such as football, basketball, and skiing, are more likely to suffer from ACL rupture [3]. ACL rupture occurs mainly during non-contact events, and the long-term consequence of ACL injury may lead to the development of knee osteoarthritis [4]. Due to the outcome and high incidence of ACL rupture, it has always been an intense area of focus in the field of sports medicine [5,6].
Although intrinsic and extrinsic factors for ACL rupture have been identified, the exact etiology of this injury is not yet fully understood [7]. Many researchers have suggested that genetic elements should be considered as an intrinsic risk factor for ACL rupture [8]. Consistent with these reports, more and more studies have shown that single nucleotide polymorphisms (SNP) within the COL1A1, COL5A1, COL12A1 and MMP3 genes are associated with an increased risk of ACL rupture [8-12].
Growth differentiation factor 5 (GDF5), also known as cartilage-derived morphogenetic protein 1 (CDMP1) or bone morphogenetic protein 14 (BMP14), belongs to the transforming growth factor -beta (TGF-β) superfamily [13]. It has been extensively studied in literatures, and found to be involved in musculoskeletal processes, affecting endochondral ossification, synovial joint formation, tendon and ligament repair, and bone formation [13-17]. Especially, GDF5 is essential for the regulation of ligament homeostasis. In rabbits ACL fibroblasts, a higher level of GDF5 enhanced the expression of type I collagen (the predominant protein in ligaments), changed the stress fiber formation and cellular adhesion by modulating the distribution of integrin alpha2 [18]. Brachypodism mice exhibited developmental failure of the condyles and intra-articular ligament of the knee joints [14].
Several SNPs in GDF5 gene have beenidentified to be associated with sports injury related diseases such as osteoarthritis (OA), anterior cruciate ligament (ACL) and tendon rupture, and meniscus injury. However, some of these associations have not been confirmed, and there are even some contradictory data in different populations [19-25]. Interesting, GDF5 +104T/C polymorphism identified to be associated with OA in a meta-analysis of European and Asian cohorts was associated with ACL rupture and was a functional polymorphism [26]. The +104T/C polymorphism could alter the expression levels of GDF5 within a wide range of connective tissues [21,27,28]. Andrew et al. found that GDF5 +104T/C polymorphismwas predicted to affect transcription factor binding and it mighttherefore highlight a regulatory site that could be exploited to manipulate GDF5 expression and alleviate the detrimental effect mediated by the T-allele of rs143383 by using reporter constructs and electrophoretic mobility shift assays [29]. Although the association studies mentioned above suggested a role for the rs143383 variant and the predisposition to various phenotypes, the specific biological role of GDF5 in ligament is still not fully understood [25].
To our knowledge, there have been few studies investigating the relationship between the functional SNP in GDF5 and ACL rupture. The purpose of this study is to investigate the possible association role of SNP rs143383 in GDF5 gene and ACL rupture in Chinese Han population.
Methods
Subjects
A total of 286 Chinese patients of Han ethnicity, surgically diagnosed as primary noncontact ACL rupture, all of whom qualified for ligament reconstruction, were enrolled in this study at the Department of Orthopaedic Surgery, Zhongnan Hospital, Wuhan University. Demographic characteristics and clinical features of the patients with ACL rupture were shown in Table 1.
Table 1.
Baseline characteristics of the patients with ACL rupture and healthy controls
| Variable | ACL rupture | Healthy Controls |
|---|---|---|
|
| ||
| n = 286 (%) | n = 500 (%) | |
| Female | 103 (36%) | 170 (34%) |
| Age (yrs) [Mean ± SD] | 28.7 ± 6.6 | 26.2 ± 8.1 |
| BMI (kg/m2) [Mean ± SD] | 23.6 ± 5.4 | 22.9 ± 4.8 |
Note: BMI, body mass index.
A total of 500 healthy controlswere selected from the medical staff of Zhongnan Hospital, Wuhan University, as well as from healthy volunteers in the same geographic area of Wuhan city. Thecontrols were ethnically matched andhad no history of ligament or tendon injury.Informed consent was taken from each study subject. The study protocol was approved by the ethics committees of Zhongnan Hospital of Wuhan University.
DNA extraction and genotyping
A blood sample was obtained from each case andcontrol for DNA extraction. Genomic DNA was isolated from 5 ml EDTA anticoagulated venous blood according to the standard protocol of the QiAamp DNA Blood Midi kit (Qiagen, Hilden, Germany). The quality of DNA samples was checked by measuring the A260/280 ratio using a fluorescence spectrophotometer F-4500 (Hitachi, Tokyo, Japan). The ratio fell between 1.8 and 2.0 for all samples.
The +104T/C polymorphism (rs143383) in the GDF5 gene was genotyped and determined by DNA sequencing analysis. The region containing +104T/C polymorphism site was amplified using a single primer set: forward 5’-CAG CAT TAC GCC ATT CTT CC-3’ and reverse 5’-CGC TGA ATG ACA CCA AAG AGA-3’ [30]. The PCR was performed as follows: initial denaturing at 95°C for 5 min, followed by 35 cycles of 95°C for 30 s, 57°C for 30 s, and 72°C for 45 s, and final extension at 72°C for 5 min. The PCR products were purified using QIAquick PCR Purification kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions, and the purified PCR products were sequenced using an ABI BigDye Terminators v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) and run on an ABI 3100 automatic sequencer (Applied Biosystems, Foster City, CA, USA).
For quality control purposes, negative and positive controls were processed with each batch of samples and all experiments were repeated twice to ensure consistency.
Quantification of GDF5 mRNA
Ligament tissue was taken from 62 patients with ACL rupture undergoing arthroscopic-assisted ACL reconstruction after written informed consent. Total RNA was extracted from fresh ligament tissue of ACL rupture patients, with Trizol reagent according to the manufacturer’s instruction (Invitrogen, Carlsbad, CA, USA). A total of 1 μg of RNA was reverse-transcribed into cDNA using a First Strand cDNA Synthesis Kit (Fermentas, Burlington, ON, Canada).
Expression levels of GDF5 mRNA in ligament tissue were detected by quantitative PCR analysator with a Rotor-Gene 3000 system (Corbett research, Concorde, NSW, Australia) using the Quantitect SYBR Green PCR Kit (Takara, Shiga, Japan) following the manufacturer’s guidelines, employing the β-actin gene as a reference gene. The following primer pairs were used: GDF5 forward 5’-CTG TGA TTC CAG GAG TGC AG-3’, and reverse 5’-ATC CTC TTC ATT GAC TCT GCC-3’ [31], β-actin forward 5’-CGA GAT CGT GCG GGA CAT-3’ and reverse 5’-CAG GAA GGA GGG CTG GAA C-3’ [32]. The quantitative PCR reaction was carried out in a 25 µl volume containing 2 × SYBR® Premix Ex Taq™ (Takara, Shiga, Japan) 12.5 µl, 0.2 µM of each primer, and 1 µl cDNA. PCR conditions were as follows: an initial denaturation at 95°C for 10 min, followed by 45 cycles of 95°C for 15 s, 56°C for 15 s, and 72°C for 15 s, and final extension at 72°C for 10 min. Gene expressions of GDF5 in all samples were analyzed by applying the 2-ΔΔCt relative quantification method [33].
Statistical analysis
The statistical analysis was done by SPSS 18.0 software (SPSS Inc., Chicago, USA). The comparisons of the genotype and allelic frequencies were performed using the chi-square (χ2) test with Yates’ correction or Fisher’s exact test. Odds ratios (OR) and 95% confidence intervals (CI) were calculated for the disease in carriers of the specific alleles. Bonferroni multiple corrections were used for the corrected P (Pc) by multiplying the P value by the number of the statistical tests in order to avoid the α1 error. Continuous variables were reported as means ± standard deviation, and statistical comparisons were performed with the two-tailed, Student’s t-test and one-way ANOVA analysis. A P value < 0.05 was considered to be significant.
The statistical power to detect genetic association study was calculated using the QUANTO software (http://hydra.usc.edu/gxe). At the 0.05 level of significance with the two-sided test for GDF5 +104T/C polymorphism, our study had 87% power to detect an effect with a relative risk of 2.0 in the group of ACL rupture patients and healthy controls.
Results
Distribution of GDF5 allele and genotype frequencies in patients with ACL rupture and in healthy controls
In all participants, genotype distributions were in Hardy-Weinberg equilibrium for the +104T/C (rs143383) polymorphisms (P = 0.190 in ACL rupture and 0.067 in controls). The sex ratio and age distribution did not differ between ACL rupture and controls (P = 0.568; P = 0.849, respectively).
The distribution of allele and genotype frequencies of +104T/C (rs143383) in GDF5 gene in patients with ACL rupture and in controls was shown in Table 2. The prevalence of the TT genotype tended to be higher in ACL rupture than in controls, reaching a significant difference (62.6% vs. 48.0%, P< 0.001, OR = 1.81, 95% CI: 1.35-2.44). Similarly, the frequency of the T allele was increased in ACL rupture cases compared to the controls (79.9% vs. 70.5%, P< 0.001, OR = 1.67, 95% CI: 1.30-2.13).
Table 2.
Distribution of GDF5+104T/C (rs143383) allele and genotype frequencies in patients with anterior cruciate ligament ruptureand healthy controls
| GDF5(rs143383) allele | GDF5(rs143383) genotype | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| N | T | C | TT | TC | CC | |
| All participants | ||||||
| Healthy Controls | 500 | 705 (70.5) | 295 (29.5) | 240 (48.0) | 225 (45.0) | 35 (7.0) |
| ACL group | 286 | 457 (79.9)* | 115 (20.1) | 179 (62.6)† | 99 (34.6) | 8 (2.8) |
| Female participants | ||||||
| Healthy Controls | 170 | 236 (69.4) | 104 (30.6) | 79 (46.5) | 78 (45.9) | 13 (7.6) |
| ACL group | 103 | 162 (78.6)‡ | 44 (21.4) | 62 (60.2)¶ | 38 (36.9) | 3 (2.9) |
| Male participants | ||||||
| Healthy Controls | 330 | 462 (70.0) | 198 (30.0) | 158 (47.9) | 146 (44.2) | 26 (7.9) |
| ACL group | 183 | 261 (71.3) | 105 (28.7) | 94 (51.4) | 73 (39.9) | 16 (8.7) |
Note: ACL vs. healthy controls:
P< 0.001, OR = 1.67, 95% CI: 1.30-2.13;
P< 0.001, OR = 1.81, 95% CI: 1.35-2.44;
P = 0.019, OR = 1.81, 95% CI: 1.08-2.43;
P = 0.028, OR = 1.74, 95% CI: 1.06-2.86.
A significant association was found when the patients were divided into subgroups according to gender (Table 2). In female participants, the frequencies of T allele and TT genotype of +104T/C were significantly higher in ACL rupture than in controls (78.6% vs. 69.4%, P = 0.019, OR = 1.81, 95% CI: 1.08-2.43; 60.2% vs. 46.5%, P = 0.028, OR = 1.74, 95% CI: 1.06-2.86, respectively). In male participants, the distribution of +104T/C allele and genotype frequencies did not differ between ACL rupture and the healthy controls.
Association of +104T/C polymorphism with GDF5 mRNA expression levels in patients with ACL rupture
As illustrated in Figure 1, the +104T/C polymorphism seemed to affect GDF5 mRNA expression levels in patients with ACL rupture. The GDF5 mRNA expression levels were lower in TT genotype of +104T/C (n = 34) than those in subgroups of C carrier (n = 28, P = 0.005).
Figure 1.
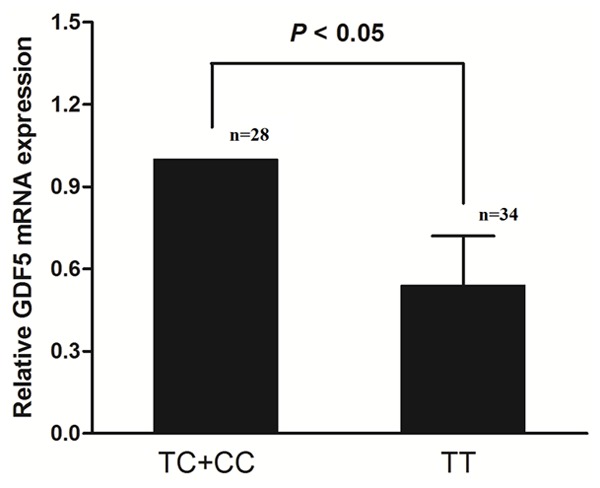
Relative expression levels of GDF5 mRNA in ligament tissue in patients with ACL rupture after stratification by +104T/C genotypes, utilizing arbitrary units. GDF5 mRNA expression levels were normalized to the expression in individuals for TT genotype in which GDF5 mRNA expression levels were set arbitrary as 1.0. Data are expressed as means ± SD.
Discussion
Although there has been an emerging interest in the role of GDF5 as the new target in the regulation of progress in sports injury related diseases, relatively limited information exists to date on the relationship between ACL rupture and GDF5 genetic polymorphisms. This study provided the evidence for the association between the +104T/C polymorphism in GDF5 gene and ACL rupture in the Han Chinese population in Central China. We found that the frequencies of T allele and TT genotype of +104T/C were significantly higher in ACL rupture group than in control group. Moreover, a significant association was found when the patients were further divided into subgroups according to gender. Our results suggested that in female participants the frequency of TT genotype of +104T/C were significantly higher in ACL rupture group than in control group, and GDF5 +104T/C polymorphism was likely to have some effect on disease prevalence in female subjects.
No other studies in the Chinese population regarding the +104T/C polymorphism in relation to ACL rupture have been published so far to compare our data. However, there were several studies showed similar results in connective tissue pathologies: it was worth mentioning that the GDF5 rs143383 TT genotype was previously associated with a 2-fold increase in risk of Achilles tendon ruptures and chronic tendinopathy [34]. The study in Asian populations and Japanese population indicated that the +104T/C polymorphism in GDF5 gene showed significant association with osteoarthritis [28]. Williams et al. found that the frequency of the TT genotype of +104T/C was significantly increased in patients with lumbar disc degeneration than in healthy controls [35]. Raleigh et al. showed that GDF5 rs143383 variant had no association with ACL rupture in Caucasians [25]. This difference might be explained by the limited power, as well as by ethnicity or gene - environment interactions present in Central China but not in Caucasians.
The current study found that individuals with ACL rupture who had TT genotype expressed lower levels of GDF5 mRNA compared with subjects who had C carriers (TC+CC). Our findings corroborated those of Egli et al. and Dodd et al. [13,29]. GDF5 rs143383 has recently been reported to be associated with OA susceptibility, with lower expression of the risk-associated T allele observed in vitro and in vivo. The in vivo studies were performed on cartilage tissue from OA patients. A luciferase reporter assay in the Asian study demonstrated a direct functional effect of the rs143383 on the expression of a reporter construct in a chondrogenic cell line, with the OA associated T allele showing reduced expression relative to the C allele [28]. Our findings suggested that a slight reduction in the expression of GDF5 in ACL tissue increased the individual’s risk of developing ACL rupture.
Some studies have shown that ACL rupture may be linked to the transmission of heritable factors [36,37]. Recently, the gene encoding GDF5 was shown to associate with Achilles tendon pathology (both ruptures and chronic tendinopathy) [34]. GDF-5 expression is an important regulator of ligament homeostasis, higher levels of GDF-5 stimulate the production of type I collagen (the predominant protein in ligaments) subunit genes in rabbit ACL by modulating integrin α2 activity [18]. So, the GDF5 gene has been proposed to affect ligament biology and as it associates with other musculoskeletal soft tissue injuries.
In conclusion, our study suggests that the T allele of +104T/C polymorphism in GDF5 gene were associated with patients with ACL rupture in the Han Chinese population in central China. Patients carrying TT genotype expressed lower levels of GDF5 mRNA than C carriersamong ACL rupture. Our results suggested that the T allele of +104T/C may be a risk factor for ACL rupture patients. This is likely from decreased expressions of GDF5 mRNA. We recognized that our current study had limitations of small sample size and lack of functional characterization of the T mutation at this position in the promoter activity and T cell activation. Therefore, further studies with a larger sample size in independent cohorts from the same ethnic group are necessary to determine the relation of the GDF5 +104T/C polymorphism with ACL patients, and to investigate the function of the GDF5 gene polymorphism.
Acknowledgements
This research was supported by the Natural Science Foundation of China (81201401, 81371940).
Disclosure of conflict of interest
None.
References
- 1.McLean S, Mallett KF, Arruda EM. Deconstructing the Anterior Cruciate Ligament: What We Know and Don’t Know About Function, Material Properties, and Injury Mechanics. J Biomech Eng. 2015;137:020906. doi: 10.1115/1.4029278. [DOI] [PubMed] [Google Scholar]
- 2.Hewett TE, Di Stasi SL, Myer GD. Current concepts for injury prevention in athletes after anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41:216–224. doi: 10.1177/0363546512459638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dragoo JL, Braun HJ, Durham JL, Chen MR, Harris AH. Incidence and risk factors for injuries to the anterior cruciate ligament in National Collegiate Athletic Association football: data from the 2004-2005 through 2008-2009 National Collegiate Athletic Association Injury Surveillance System. Am J Sports Med. 2012;40:990–995. doi: 10.1177/0363546512442336. [DOI] [PubMed] [Google Scholar]
- 4.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 5.Griffin LY, Albohm MJ, Arendt EA, Bahr R, Beynnon BD, Demaio M, Dick RW, Engebretsen L, Garrett WE Jr, Hannafin JA, Hewett TE, Huston LJ, Ireland ML, Johnson RJ, Lephart S, Mandelbaum BR, Mann BJ, Marks PH, Marshall SW, Myklebust G, Noyes FR, Powers C, Shields C Jr, Shultz SJ, Silvers H, Slauterbeck J, Taylor DC, Teitz CC, Wojtys EM, Yu B. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am J Sports Med. 2006;34:1512–1532. doi: 10.1177/0363546506286866. [DOI] [PubMed] [Google Scholar]
- 6.Serpell BG, Scarvell JM, Ball NB, Smith PN. Mechanisms and risk factors for noncontact ACL injury in age mature athletes who engage in field or court sports: a summary of the literature since 1980. J Strength Cond Res. 2012;26:3160–3176. doi: 10.1519/JSC.0b013e318243fb5a. [DOI] [PubMed] [Google Scholar]
- 7.Posthumus M, Collins M, September AV, Schwellnus MP. The intrinsic risk factors for ACL ruptures: an evidence-based review. Phys Sportsmed. 2011;39:62–73. doi: 10.3810/psm.2011.02.1863. [DOI] [PubMed] [Google Scholar]
- 8.Malila S, Yuktanandana P, Saowaprut S, Jiamjarasrangsi W, Honsawek S. Association between matrix metalloproteinase-3 polymorphism and anterior cruciate ligament ruptures. Genet Mol Res. 2011;10:4158–4165. doi: 10.4238/2011.October.31.1. [DOI] [PubMed] [Google Scholar]
- 9.Collins M, Posthumus M, Schwellnus MP. The COL1A1 gene and acute soft tissue ruptures. Br J Sports Med. 2010;44:1063–1064. doi: 10.1136/bjsm.2008.056184. [DOI] [PubMed] [Google Scholar]
- 10.Pruna R, Artells R, Ribas J, Montoro B, Cos F, Munoz C, Rodas G, Maffulli N. Single nucleotide polymorphisms associated with non-contact soft tissue injuries in elite professional soccer players: influence on degree of injury and recovery time. BMC Musculoskelet Disord. 2013;14:221. doi: 10.1186/1471-2474-14-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Posthumus M, September AV, O’Cuinneagain D, van der Merwe W, Schwellnus MP, Collins M. The COL5A1 gene is associated with increased risk of anterior cruciate ligament ruptures in female participants. Am J Sports Med. 2009;37:2234–2240. doi: 10.1177/0363546509338266. [DOI] [PubMed] [Google Scholar]
- 12.Posthumus M, September AV, Keegan M, O’Cuinneagain D, Van der Merwe W, Schwellnus MP, Collins M. Genetic risk factors for anterior cruciate ligament ruptures: COL1A1 gene variant. Br J Sports Med. 2009;43:352–356. doi: 10.1136/bjsm.2008.056150. [DOI] [PubMed] [Google Scholar]
- 13.Egli RJ, Southam L, Wilkins JM, Lorenzen I, Pombo-Suarez M, Gonzalez A, Carr A, Chapman K, Loughlin J. Functional analysis of the osteoarthritis susceptibility-associated GDF5 regulatory polymorphism. Arthritis Rheum. 2009;60:2055–2064. doi: 10.1002/art.24616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harada M, Takahara M, Zhe P, Otsuji M, Iuchi Y, Takagi M, Ogino T. Developmental failure of the intra-articular ligaments in mice with absence of growth differentiation factor 5. Osteoarthritis Cartilage. 2007;15:468–474. doi: 10.1016/j.joca.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Hatakeyama Y, Tuan RS, Shum L. Distinct functions of BMP4 and GDF5 in the regulation of chondrogenesis. J Cell Biochem. 2004;91:1204–1217. doi: 10.1002/jcb.20019. [DOI] [PubMed] [Google Scholar]
- 16.Mikic B. Multiple effects of GDF-5 deficiency on skeletal tissues: implications for therapeutic bioengineering. Ann Biomed Eng. 2004;32:466–476. doi: 10.1023/b:abme.0000017549.57126.51. [DOI] [PubMed] [Google Scholar]
- 17.Edwards CJ, Francis-West PH. Bone morphogenetic proteins in the development and healing of synovial joints. Semin Arthritis Rheum. 2001;31:33–42. doi: 10.1053/sarh.2001.24875. [DOI] [PubMed] [Google Scholar]
- 18.Date H, Furumatsu T, Sakoma Y, Yoshida A, Hayashi Y, Abe N, Ozaki T. GDF-5/7 and bFGF activate integrin alpha2-mediated cellular migration in rabbit ligament fibroblasts. J Orthop Res. 2010;28:225–231. doi: 10.1002/jor.20981. [DOI] [PubMed] [Google Scholar]
- 19.Longo UG, Loppini M, Margiotti K, Salvatore G, Berton A, Khan WS, Maffulli N, Denaro V. Unravelling the genetic susceptibility to develop ligament and tendon injuries. Curr Stem Cell Res Ther. 2014;10:56–63. doi: 10.2174/1574888x09666140710112535. [DOI] [PubMed] [Google Scholar]
- 20.Ge W, Mu J, Huang C. The GDF5 SNP is associated with meniscus injury and function recovery in male Chinese soldiers. Int J Sports Med. 2014;35:625–628. doi: 10.1055/s-0033-1355417. [DOI] [PubMed] [Google Scholar]
- 21.Southam L, Rodriguez-Lopez J, Wilkins JM, Pombo-Suarez M, Snelling S, Gomez-Reino JJ, Chapman K, Gonzalez A, Loughlin J. An SNP in the 5’-UTR of GDF5 is associated with osteoarthritis susceptibility in Europeans and with in vivo differences in allelic expression in articular cartilage. Hum Mol Genet. 2007;16:2226–2232. doi: 10.1093/hmg/ddm174. [DOI] [PubMed] [Google Scholar]
- 22.Collins M, Raleigh SM. Genetic risk factors for musculoskeletal soft tissue injuries. Med Sport Sci. 2009;54:136–149. doi: 10.1159/000235701. [DOI] [PubMed] [Google Scholar]
- 23.Dai J, Shi D, Zhu P, Qin J, Ni H, Xu Y, Yao C, Zhu L, Zhu H, Zhao B, Wei J, Liu B, Ikegawa S, Jiang Q, Ding Y. Association of a single nucleotide polymorphism in growth differentiate factor 5 with congenital dysplasia of the hip: a casecontrol study. Arthritis Res Ther. 2008;10:R126. doi: 10.1186/ar2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaes RB, Rivadeneira F, Kerkhof JM, Hofman A, Pols HA, Uitterlinden AG, van Meurs JB. Genetic variation in the GDF5 region is associated with osteoarthritis, height, hip axis length and fracture risk: the Rotterdam study. Ann Rheum Dis. 2009;68:1754–1760. doi: 10.1136/ard.2008.099655. [DOI] [PubMed] [Google Scholar]
- 25.Raleigh SM, Posthumus M, O’Cuinneagain D, van der Merwe W, Collins M. The GDF5 gene and anterior cruciate ligament rupture. Int J Sports Med. 2013;34:364–367. doi: 10.1055/s-0032-1316361. [DOI] [PubMed] [Google Scholar]
- 26.Chapman K, Takahashi A, Meulenbelt I, Watson C, Rodriguez-Lopez J, Egli R, Tsezou A, Malizos KN, Kloppenburg M, Shi D, Southam L, van der Breggen R, Donn R, Qin J, Doherty M, Slagboom PE, Wallis G, Kamatani N, Jiang Q, Gonzalez A, Loughlin J, Ikegawa S. A metaanalysis of European and Asian cohorts reveals a global role of a functional SNP in the 5’UTR of GDF5 with osteoarthritis susceptibility. Hum Mol Genet. 2008;17:1497–1504. doi: 10.1093/hmg/ddn038. [DOI] [PubMed] [Google Scholar]
- 27.Rouault K, Scotet V, Autret S, Gaucher F, Dubrana F, Tanguy D, El Rassi CY, Fenoll B, Ferec C. Evidence of association between GDF5 polymorphisms and congenital dislocation of the hip in a Caucasian population. Osteoarthritis Cartilage. 2010;18:1144–1149. doi: 10.1016/j.joca.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 28.Miyamoto Y, Mabuchi A, Shi D, Kubo T, Takatori Y, Saito S, Fujioka M, Sudo A, Uchida A, Yamamoto S, Ozaki K, Takigawa M, Tanaka T, Nakamura Y, Jiang Q, Ikegawa S. A functional polymorphism in the 5’ UTR of GDF5 is associated with susceptibility to osteoarthritis. Nat Genet. 2007;39:529–533. doi: 10.1038/2005. [DOI] [PubMed] [Google Scholar]
- 29.Dodd AW, Syddall CM, Loughlin J. A rare variant in the osteoarthritis-associated locus GDF5 is functional and reveals a site that can be manipulated to modulate GDF5 expression. Eur J Hum Genet. 2013;21:517–521. doi: 10.1038/ejhg.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mu J, Ge W, Zuo X, Chen Y, Huang C. A SNP in the 5’UTR of GDF5 is associated with susceptibility to symptomatic lumbar disc herniation in the Chinese Han population. Eur Spine J. 2014;23:498–503. doi: 10.1007/s00586-013-3059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynard LN, Bui C, Syddall CM, Loughlin J. CpG methylation regulates allelic expression of GDF5 by modulating binding of SP1 and SP3 repressor proteins to the osteoarthritis susceptibility SNP rs143383. Hum Genet. 2014;133:1059–1073. doi: 10.1007/s00439-014-1447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen B, Qin J, Wang H, Magdalou J, Chen L. Effects of adenovirus-mediated bFGF, IL-1Ra and IGF-1 gene transfer on human osteoarthritic chondrocytes and osteoarthritis in rabbits. Exp Mol Med. 2010;42:684–695. doi: 10.3858/emm.2010.42.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Z, Brant SR, Li C, Shrestha UK, Jiang T, Zhou F, Jiang Y, Shi X, Zhao Y, Li J, Xia B. CTLA4 -1661A/G and 3’UTR long repeat polymorphisms are associated with ulcerative colitis and influence CTLA4 mRNA and protein expression. Genes Immun. 2010;11:573–583. doi: 10.1038/gene.2010.16. [DOI] [PubMed] [Google Scholar]
- 34.Posthumus M, Collins M, Cook J, Handley CJ, Ribbans WJ, Smith RK, Schwellnus MP, Raleigh SM. Components of the transforming growth factor-beta family and the pathogenesis of human Achilles tendon pathology-a genetic association study. Rheumatology (Oxford) 2010;49:2090–2097. doi: 10.1093/rheumatology/keq072. [DOI] [PubMed] [Google Scholar]
- 35.Williams FM, Popham M, Hart DJ, de Schepper E, Bierma-Zeinstra S, Hofman A, Uitterlinden AG, Arden NK, Cooper C, Spector TD, Valdes AM, van Meurs J. GDF5 single-nucleotide polymorphism rs143383 is associated with lumbar disc degeneration in Northern European women. Arthritis Rheum. 2011;63:708–712. doi: 10.1002/art.30169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flynn RK, Pedersen CL, Birmingham TB, Kirkley A, Jackowski D, Fowler PJ. The familial predisposition toward tearing the anterior cruciate ligament: a case control study. Am J Sports Med. 2005;33:23–28. doi: 10.1177/0363546504265678. [DOI] [PubMed] [Google Scholar]
- 37.Harner CD, Paulos LE, Greenwald AE, Rosenberg TD, Cooley VC. Detailed analysis of patients with bilateral anterior cruciate ligament injuries. Am J Sports Med. 1994;22:37–43. doi: 10.1177/036354659402200107. [DOI] [PubMed] [Google Scholar]


