Abstract
Introduction: Sexual dysfunction and vitamin D deficiency are highly prevalent in dialysis patients. Low levels of vitamin D have been linked to many diseases. To the best of our knowledge, the relationship between vitamin D and sexual dysfunction in dialysis patients has not been previously reported in the literature. Materials and methods: Cholecalciferol, 50,000 IU/week, was orally administered to 37 dialysis patients with vitamin D insufficiency for 3 months followed by dosage of 10,000 IU every other week for 3 months. The Arizona Sexual Experiences Scale (ASEX), Hospital Anxiety and Depression Scale and Pittsburgh Sleep Quality Index questionnaires were filled out by all patients at baseline and at the sixth month of the study. Results: Sexual dysfunction, poor sleep quality, anxiety and depression rates were 83.7%, 45.9%, 18.9% and 48.6%, respectively in all patients. ASEX total score was found to be positively correlated with age and was negatively correlated with serum 25(OH)D level and serum albumin level. After cholecalciferol treatment, 25(OH)D levels increased significantly, however no significant change was observed in any of the parameters. In multivariate linear regression analysis, age and 25(OH)D level were found to be independent predictors of ASEX total score. Conclusions: Vitamin D deficiency seems to contribute to sexual dysfunction in dialysis patients. However, it was observed in this study that; cholecalciferol replacement given to dialysis patients with vitamin D insufficiency did not result in any significant changes in sexual functions.
Keywords: Dialysis, sexual dysfunction, vitamin D
Introduction
Vitamin D deficiency and sexual dysfunction (SD) are highly prevalent in chronic kidney disease [1,2]. Sexual dysfunction is generally multifactorial and primarily organic in origin [3]. Among organic causes, it is known that vascular etiology is primarily responsible [4]. However it is not known whether there is an association between serum 25(OH)D levels and existence or frequency of sexual dysfunction in dialysis patients. In addition the effect of vitamin D supplementation on sexual dysfunction is not clear.
We aimed to evaluate the relationship between serum 25(OH)D levels, sexual dysfunction and changes in sexual functions after cholecalciferol replacement therapy.
Material and methods
Participants
This prospective single center study comprised of dialysis patients followed by Suleyman Demirel University Hospital, Dialysis Unit, Isparta, Turkey. Seventy patients who were 18 years or older, married, sexually active, capable of communication and on dialysis treatment for previous three months, were assessed for eligibility. Exclusion criteria were sarcoidosis, nephrolithiasis, chronic diarrhea and malabsorption history, corrected serum calcium ≥ 10.2 mg/dl, serum PTH < 150 pg/ml, being on dialysis treatment for less than 3 months and reluctance to attend the study. Twelve patients were excluded due to these criteria. Serum 25(OH)D levels of 58 patients were investigated. Vitamin D insufficiency was observed in 57 patients, however 21 patients did not give consent to participate in the study. Informed consent was obtained from 37 dialysis patients and the study was completed in six months. The study was approved by Local Medical Ethics Committee. Demographic data, primary renal disease, dialysis duration, coexisting diseases and medications were obtained from hospital records retrospectively.
Measurements
At baseline and at the end of sixth month, the following laboratory parameters were determined: 25(OH)D, serum calcium, serum phosphorus, intact parathyroid hormone (iPTH), albumin, uric acid and hemoglobin. Kt/V values were also calculated for all patients in terms of dialysis adequacy. All patients filled out the Arizona Sexual Experiences Scale (ASEX), Hospital Anxiety and Depression Scale (HADS) and Pittsburgh Sleep Quality Index (PSQI) questionnaires at baseline and at the end of sixth month under supervision of a psychiatrist.
Definition of vitamin D insufficiency and cholecalciferol usage
Having serum 25(OH)D level below 30 ng/ml was considered as vitamin D insufficiency and patients with this condition were recommended cholecalciferol treatment. While 37 patients adhered to therapy, 21 patients refused treatment. The treatment was adjusted as recommended by Garcia-Lopes et al. as 50,000 IU/week for three months and 10,000 IU/week for the following three months for patients whose serum 25(OH)D levels were below 15 ng/ml. and 10,000 IU/week for six months for patients with serum 25(OH)D levels of 15-30 ng/ml [5].
Laboratory analysis
Serum biochemistry results were detected by Beckman Coulter AU 5800 (Miami, USA) autoanalyzer with spectrophotometric process. Complete blood counts were measured by Beckman Coulter Gen-S (Miami, USA) hematology analyzer. Serum intact PTH was assessed using chemiluminescence method by Immulite 2000 (Los Angeles, USA) analyzer. Serum 25(OH)D level was analyzed by ECLIA (Electro chemiluminescence immunologic test).
Statistical analysis
Statistical analysis was performed by SPSS software (Statistical Package for Social Sciences, version 18, Chicago, IL). Parametric continuous variables were presented as mean ± standard deviations (SD). Categorical variables were shown as frequency and percentages. We assessed the differences between baseline and 6th month values by using paired samples t test. Pearson or Spearman correlation test was performed to determine the relationships between continuous variables. Independent relationships of ASEX total score were examined with multivariate linear regression analysis. In the multivariate linear regression analysis, dependent variable was ASEX total score and independent variables; age, PTH, 25(OH)D, hemoglobin, albumin and Kt/V at the first model. Multivariate regression analysis was performed with backward method and significant variables in the final model are presented. All p-values were calculated as two-sided, and p-value < 0.05 was considered as significant with confidence intervals (CI) at the 95% level.
Results
Baseline clinical and laboratory findings of the 37 subjects (15 of them on hemodialysis, 22 of them on peritoneal dialysis) were demonstrated in Table 1. Mean serum 25(OH)D level of the patients was 7.5 ± 5.8 ng/ml. 67.6% (25/37) of the patients were on active vitamin D therapy. 73% (27/37) of the patients used erythropoietin. When all the patients’ basal data were assessed; sexual dysfunction was present in 86.5% (32/37) of them. Poor sleep quality was detected in 45.9% (17/37), anxiety disorder ratio was 18.9% (7/37) and depression was present in 48.6% (18/37). Sexual dysfunction prevalence was 73.3% in male patients while it was 95% in females (P = 0.001). While 81.8% of peritoneal dialysis patients had sexual dysfunction, this ratio was 93.3% for the hemodialysis patient subgroup (P = 0.031).
Table 1.
Baseline demographic and laboratory characteristics of the patients
| Characteristic | Mean ± SD | n (%) |
|---|---|---|
| Age, y | 52.7 ± 17.3 | |
| Gender | ||
| Male | 15 (40.5) | |
| Female | 22 (59.5) | |
| Type of dialysis | ||
| Hemodialysis | 15 (40.5) | |
| Peritoneal dialysis | 22 (59.5) | |
| Duration of dialysis, mo | 53.8 ± 53.6 | |
| 25(OH)D, ng/ml | 7.6 ± 5.9 | |
| Hemoglobin, g/dl | 11.5 ± 1.2 | |
| Albumin, g/dl | 3.6 ± 0.4 | |
| Calcium, mg/dl | 8.61 ± 1.05 | |
| Phosphor, mg/dl | 4.98 ± 1.14 | |
| Uric acid, mg/dl | 5.67 ± 1.21 | |
| Intact PTH, pg/ml | 573 ± 368 | |
| Kt/V | 1.97 ± 0.78 | |
| Use of calcitriol | 25 (67.6) | |
| Use of erythropoietin | 27 (73) | |
| DM | 7 (18.9) | |
| HT | 23 (62.2) | |
| CAD | 3 (8.1) |
Abbreviations: 25(OH)D, 25(OH) Vitamin D level; PTH, parathyroid hormone; Kt/V, adequacy of dialysis; DM, Diabetes Mellitus; HT, Hypertension; CAD, Coronary artery disease.
Cholecalciferol replacement was administered to 37 patients who accepted the treatment. Comparison of pre and post treatment data of participants were shown in Table 2. After 6 months of replacement therapy, serum 25(OH)D levels reached to intended levels (≥ 30 ng/ml) in 54.1% of the patients. In terms of serum 25(OH)D and serum albumin levels significant increases were observed in (P = 0.001 and P = 0.033, respectively). A significant decrease in hemoglobin levels was also remarkable (P = 0.001). No significant change in frequency of sexual dysfunction was observed (P > 0.05). No major changes in sexual dysfunction score and frequency was found in patients regardless of their vitamin D levels, whether they reached to target values or not (P > 0.05). At the sixth month visit it was observed that; depression, anxiety and sleep scores did not change significantly compared to baseline values, (P > 0.05, for all parameters). There was a significant decrease in hemoglobin level (from 11.5 ± 1.2 to 10.6 ± 1.6 g/dl, P = 0.001) along with a slightly but not significantly decrease in erythropoietin usage (from 73% to 56.8%, P = 0.07) in the comparison between baseline and sixth month assessments.
Table 2.
Comparison of pre and post-treatment parameters of participants
| Parameter | Baseline (n=37) | 6 th month (n=37) | P |
|---|---|---|---|
| 25(OH)D, ng/ml | 7.6 ± 5.9 | 31.8 ± 16.5 | < 0.001 |
| Hemoglobin, g/dl | 11.5 ± 1.2 | 10.6 ± 1.6 | 0.001 |
| Albumin, g/dl | 3.6 ± 0.4 | 3.7 ± 0.4 | 0.033 |
| Calcium, mg/dl | 8.61 ± 1.05 | 8.76 ± 1.05 | 0.121 |
| Phosphorus, mg/dl | 4.98 ± 1.14 | 4.87 ± 1.29 | 0.571 |
| Uric acid, mg/dl | 5.67 ± 1.21 | 5.65 ± 1.06 | 0.764 |
| Intact PTH, pg/ml | 573 ± 368 | 505 ± 367 | 0.093 |
| Kt/V | 1.9 ± 0.8 | 2.1 ± 1.2 | 0.264 |
| ASEX total score | 20.8 ± 7.72 | 22.2 ± 7.10 | 0.091 |
| Depression score | 7.5 ± 3.60 | 8.2 ± 3.88 | 0.225 |
| Anxiety score | 7.5 ± 4.04 | 7.2 ± 3.78 | 0.774 |
| Sleep score | 5.5 ± 2.63 | 7.3 ± 3.70 | 0.013 |
| Sexual dysfunction, n (%) | 32 (86.5) | 34 (91.9) | 0.687* |
| Depression, n (%) | 18 (48.6) | 19 (51.3) | 1.000* |
| Anxiety, n (%) | 7 (18.9) | 6 (16.2) | 1.000* |
| Sleep disorder, n (%) | 17 (45.9) | 25 (67.5) | 0.092* |
| Use of calcitriol, n (%) | 25 (67.6) | 26 (70.2) | 1.000* |
| Use of erythropoietin, n (%) | 27 (73) | 21 (56.8) | 0.070* |
Abbreviations: P, levels of significance; 25(OH)D, 25(OH) Vitamin D level; Kt/V, adequacy of dialysis; ASEX, Arizona Sexual Experiences Scale.
These p values are provided from Mc-Nemar test.
ASEX total score was positively correlated with age (r = 0.372, P = 0.023) and was negatively correlated with serum albumin level (r = -0.357, P = 0.030) and 25(OH)D level (r = -0.392, P = 0.016) (Figure 1; Table 3).
Figure 1.
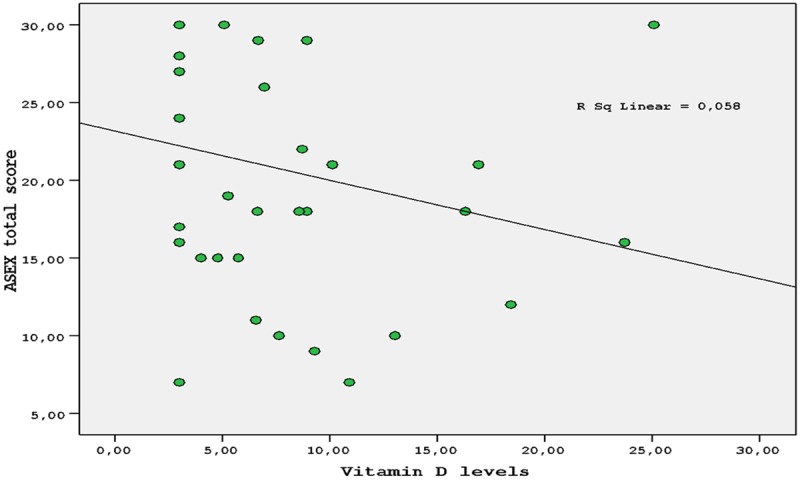
Correlation between Arizona Sexual Experiences Scale (ASEX) total score and 25 (OH) Vit D levels.
Table 3.
Correlation analysis of ASEX total scores and relevant factors (n = 37)
| Factors | r | P |
|---|---|---|
| 25(OH)D | -0.392 | 0.016 |
| Age | 0.372 | 0.023 |
| Sleep score | 0.058 | 0.739 |
| Anxiety score | 0.083 | 0.629 |
| Depression score | 0.165 | 0.337 |
| Hemoglobin | -0.200 | 0.236 |
| Albumin | -0.357 | 0.030 |
| Intact PTH | 0.033 | 0.847 |
| Kt/V | -0.089 | 0.611 |
Abbreviations: ASEX, The Arizona Sexual Experiences Scale; r, correlations coefficients; P, levels of significance; 25(OH)D, 25(OH) Vitamin D level; Kt/V, adequacy of dialysis.
In the multivariate linear regression analysis, dependent variable was ASEX total score and independent variables; age, PTH, 25(OH)D, hemoglobin, albumin and Kt/V at the first model. Multivariate regression analysis was performed with backward method and significant variables in the final model are presented. In multivariate regression analysis, age and 25(OH)D level were found to be independent predictors of ASEX total score (β = 0.324, P = 0.059, CI = -1.059 to -0.014 and β = -0.346, P = 0.045, CI = -0.006 to 0.299, respectively). Results of multivariate linear regression analysis for clinical and demographic parameters in relation to ASEX total scores (R2 of the model: 0.248) were presented in Table 4.
Table 4.
Results of multivariate linear regression analysis for clinical and demographic parameters in relation to ASEX total scores (R2 of the model: 0.248)
| Variable | β | P | 95% Confidence Interval |
|---|---|---|---|
| Age | 0.324 | 0.059 | -1.059 to -0.014 |
| 25(OH)D | -0.346 | 0.045 | -0.006 to 0.299 |
In the multivariate linear regression analysis, dependent variable was ASEX total score and independent variables; age, PTH, 25(OH)D, hemoglobin, albumin and Kt/V at the first model. Multivariate regression analysis was performed with backward method and significant variables in the final model are presented.
Discussion
Our study results revealed that sexual dysfunction is related to low serum 25(OH)D levels as well as old age and malnutrition. Sexual dysfunction was found to be more frequent in females than it was in males and also it was more frequent in hemodialysis patients than it was in the patients on peritoneal dialysis. Another remarkable result of our study is that no significant change was observed regarding sexual dysfunction in subjects after cholecalciferol replacement.
Studies have documented that 40-100% of CKD patients are vitamin D deficient or insufficient [6]. Low levels of vitamin D have been linked to many chronic conditions and diseases such as hypertension, endothelial dysfunction, cardiovascular diseases,diabetes mellitus, obesity, colorectal cancer, depression, multiple sclerosis, fibromyalgia, schizophrenia and Parkinson’s disease [7,8]. In our study 25(OH)D level was found to be an independent predictor of sexual dysfunction. This finding has been reported for the first time as far as we know.
Sexual dysfunction is a common problem in dialysis patients. In the literature, sexual dysfunction rate has been reported as 70% and 74% in two different studies [9,10]. In a study by Turk et al. erectile dysfunction rate was found to be similar between hemodialysis and peritoneal dialysis patients (71% for hemodialysis patients, 80% for peritoneal dialysis patients) [10]. In our study, the rate of sexual dysfunction was found to be 86.5%. Sexual dysfunction was seen more frequently in hemodialysis patients.
Sexual dysfunction is generally multifactorial [3]. It has been reported that people with sexual dysfunction are more anxious and depressive [11,12]. In the present study, most of the patients were depressed and their sleep quality was poor. In other words, sexual dysfunction got worse as depression and poor sleep quality grew. Because increasing sleep scores might have contributed to the condition, sexual functions did not change positively after cholecalciferol treatment.
In a study of Blumberg A et al. in 1980,treatment of the patients with secondary hyperparathyroidism with 2-4 months of 1.25(OH)2D3 did not enhance sexual dysfunction. In that study 25(OH)D levels were not investigated [13]. We observed that cholecalciferol replacement did not improve sexual dysfunction in dialysis patients with vitamin D insufficiency. We considered that this finding may have resulted from the fact that 25(OH)D levels did not reach to intended values in nearly half of the patients. In addition, probable changes in vascular structure due to uremia, decrease in hemoglobin levels, and increase in sleep quality scores might have also contributed to the unfavorable result. Therefore we assume that the probable positive effects of vitamin D replacement might have been overshadowed.
The limitations of our study are, not having a healthy control group, not having evaluated serum testosterone and estrogen levels of the patients and limited number of patients.
Consequently there is a relationship between low serum 25(OH)D levels and sexual dysfunction in dialysis patients. Cholecalciferol treatment alone, as we implemented, does not seem to be efficient in improving sexual functions. However randomized controlled trials with larger number of patients are required to enlighten this issue.
Disclosure of conflict of interest
None.
References
- 1.Slatopolsky E, Weerts C, Thielan J, Horst R, Harter H, Martin KJ. Marked suppression of secondary hyperparathyroidism by intravenous administration of 1,25-dihydroxy-cholecalciferol in uremic patients. J Clin Invest. 1984;74:2136–2143. doi: 10.1172/JCI111639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkelstein FO, Shirani S, Wuerth D, Finkelstein SH. Therapy Insight: sexual dysfunction in patients with chronic kidney disease. Nat Clin Pract Nephrol. 2007;3:200. doi: 10.1038/ncpneph0438. [DOI] [PubMed] [Google Scholar]
- 3.Anantharaman P, Schmidt RJ. Sexual function in chronic kidney disease. Adv Chronic Kidney Dis. 2007;14:119–125. doi: 10.1053/j.ackd.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Virag R, Bouilly P, Frydman D. Is impotence an arterial disorder? A study of arterial risk factors in 440 impotent men. Lancet. 1985;1:181–184. doi: 10.1016/s0140-6736(85)92023-9. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Lopes MG, Pillar R, Kamimura MA, Rocha LA, Canziani ME, Carvalho AB, Cuppari L. Cholecalciferol supplementation in chronic kidney disease: restoration of vitamin D status and impact on parathyroid hormone. Ann Nutr Metab. 2012;61:74–82. doi: 10.1159/000339618. [DOI] [PubMed] [Google Scholar]
- 6.Mehrotra R, Kermah D, Budoff M, Salusky IB, Mao SS, Gao YL, Takasu J, Adler S, Norris K. Hypovitaminosis D in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1144–1151. doi: 10.2215/CJN.05781207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, Murad MH, Kovacs CS. The Nonskeletal Effects of Vitamin D: An Endocrine Society Scientific Statement. Endocr Rev. 2012;33:456–492. doi: 10.1210/er.2012-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muszkat P, Camargo MB, Griz LH, Lazaretti-Castro M. Evidence-based non-skeletal actions of vitamin D. Arq Bras Endocrinol Metabol. 2010;54:110–117. doi: 10.1590/s0004-27302010000200005. [DOI] [PubMed] [Google Scholar]
- 9.Rosas SE, Joffe M, Franklin E, Strom BL, Kotzker W, Brensinger C, Grossman E, Glasser D, Feldman HI. Prevalence and determinants of erectile dysfunction in hemodialysis patients. Kidney Int. 2001;59:2259–2266. doi: 10.1046/j.1523-1755.2001.00742.x. [DOI] [PubMed] [Google Scholar]
- 10.Türk S, Karalezli G, Tonbul HZ, Yildiz M, Altintepe L, Yildiz A, Yeksan M. Erectile dysfunction and the effects of sildenafil treatment in patients on haemodialysis and continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant. 2001;16:1818–1822. doi: 10.1093/ndt/16.9.1818. [DOI] [PubMed] [Google Scholar]
- 11.Steele TE, Wuerth D, Finkelstein S, Juergensen D, Juergensen P, Kliger AS, Finkelstein FO. Sexual experience of the chronic peritoneal dialysis patient. J Am Soc Nephrol. 1996;7:1165–1168. doi: 10.1681/ASN.V781165. [DOI] [PubMed] [Google Scholar]
- 12.Toorians AW, Janssen E, Laan E, Gooren LJ, Giltay EJ, Oe PL, Donker AJ, Everaerd W. Chronic renal failure and sexual functioning: clinical status versus objectively assessed sexual response. Nephrol Dial Transplant. 1997;12:2654–2663. doi: 10.1093/ndt/12.12.2654. [DOI] [PubMed] [Google Scholar]
- 13.Blumberg A, Wildbolz A, Descoeudres C, Hennes U, Dambacher MA, Fischer JA, Weidmann P. Influence of 1,25 dihydroxycholecalciferol on sexual dysfunction and related endocrine parameters in patiens on maintenance hemodialysis. Clin Nephrol. 1980;13:208–214. [PubMed] [Google Scholar]


