Abstract
Diffuse large B-cell lymphoma (DLBCL) is the most frequent type of non-Hodgkin’s lymphoma (NHL) in adults, and approximately 50% of cases of DLBCL occur in patients above the age of 60. Although RCHOP regimen was established as the standard therapy for DLBCL patients, there are still a large number of DLBCL patients who can’t bear the toxicity of doxorubicin, especially in elderly patients. Pegylated liposomal doxorubicin (PLD) offers a new strategy for elderly DLBCL patients. In our study, we reviewed 103 newly diagnosed patients with DLBCL aged between 60 years to 75 years old who were treated with RCHOP (62 cases) or DRCOP (41 cases) regimen. All the patients completed a mean follow-up period of 28 months (range, 2 to 48 months). There was no statistical difference of OS between the DRCOP (78.0%) and RCHOP (72.6%) groups (P = 0.787). And there were less grade 3-4 cardiotoxicity in patients treated with DRCOP (9.8%) than RCHOP regimen (27.4%, P = 0.029). Our findings in this study indicate that the DRCOP regimen offers similar oncologic efficacy when weighed against the standard RCHOP regimen in elderly DLBCL patients, and it might be a more secure treatment for elderly DLBCL patients who have additional risk factors for cardiac diseases.
Keywords: Pegylated liposomal doxorubicin, diffuse large B-Cell lymphoma, elderly, R-CHOP, cardiotoxicity
Introduction
Diffuse large B-cell lymphoma (DLBCL) is defined by the World Health Organization (WHO) Classification as a heterogeneous entity, encompassing morphologic and genetic variants with variable clinical presentations and outcomes and it is the most frequent type of non-Hodgkin’s lymphoma (NHL) in adults. In Western countries, DLBCL accounts for about 31% of NHL cases and the percentage in Asia is over 40% [1]. The incidence of DLBCL rises steadily with age so that approximately 50% of cases of DLBCL occur in patients above the age of 60 [2]. Since the life expectancy of the population is growing, clinicians are increasingly confronted with the clinical care of geriatric patients diagnosed with aggressive lymphoma such as DLBCL [3,4].
Cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP) regimen was once the classical front-line treatment for DLBCL, but the development of monoclonal antibodies has led to a distinguished improvement in the outcome of DLBCL treatments. Currently, patients with DLBCL are medicated with immunochemotherapy, usually Rituximab, plus CHOP (RCHOP) regimen. There were also several researches such as GELA LNH-98.5, RICOVER-60, Intergroup USA [5-7] demonstrated that Rituximab can benefit elderly patients.
Although RCHOP regimen was established as the standard therapy for DLBCL patients, there are still a large number of DLBCL patients who can’t bear the toxicity of doxorubicin, especially in elderly patients. Most common side effects of anthracyclines are nausea, and vomiting, fatigue, hematotoxicity and mucositis. A particular challenge in treatment with anthracyclines is early and late cardiotoxicity [8]. Anthracyclines exhibit cumulative dose related cardiotoxicity. Older patients and those with pre-existing cardiac disease are known to have a greater incidence of both overt heart failure and asymptomatic declines of cardiac parameters [9-11]. A less cardiotoxic approach is critically needed [12].
Modern anticancer regimens have incorporated strategies to reduce the cardiotoxic burden of anthracyclines, such as reducing the cumulative dose of the toxic agents, using the cardioprotective agent dexrazoxane, and incorporating continuous infusion administration schedules.
Previous in-vivo studies have demonstrated the favorable therapeutic activity and safety profile of pegylated liposomal doxorubicin (PLD) [13-24]. It offers an additional strategy for limiting cardiotoxicity that allows localized penetration of the anthracycline molecule selectively through the impaired vasculature, thereby concentrating the delivery of the agent to the tumor. Additionally, the overall peak plasma concentration to which the heart is exposed is reduced with PLD [25].
Thus, we performed a retrospective study to evaluate the efficacy and tolerability of PLD replacing conventional doxorubicin in standard RCHOP regimen (DRCOP) for elderly Diffuse Large B-Cell Lymphoma in China.
Materials and methods
Patients
We reviewed 103 newly diagnosed patients with DLBCL aged between 60 years to 75 years old who were treated with RCHOP or DRCOP regimen in the First Affiliated Hospital of Medical School of Zhejiang University between September 2011 and September 2013. All diagnoses were confirmed by histopathological staining of hematoxylin and eosin (HE) together with the determination of the immunophenotype according to the World Health Organization Classification. Clinical staging and diagnostic methods included a clinical history, physical examination, computed tomography (CT) scans of the chest, abdomen and pelvis, full-digital full-body color Doppler ultrasonic diagnostic analyzer, marrow aspirate and biopsy. Repeat echocardiography imaging was planned before every cycle and after the whole treatment to assess the change in the Left Ventricular Ejection Fractions (LVEF). All patients provided a written consent.
Treatment
Among the 103 patients, 41 cases were treated with DRCOP regimen (median courses was 6; range, 1-8) and 62 cases with RCHOP (median courses was 6; range, 1-8). The median follow-up time was 28 months (range, 2 to 48 months). The regimen of RCHOP or DRCOP consisted of rituximab 375 mg/m2 intravenously (I.V.) on cycle day 1, and/alongside? Cyclophosphamide 750 mg/m2 I.V., PLD 30-40 mg/m2 (maximum, 60 mg) I.V. or doxorubicin 50 mg/m2 I.V. over 1 hour, and vindesine 4.0 mg I.V., on cycle day 2. Additionally patients received prednisone, 100 mg orally, on days 1 to 5 of each cycle. This regimen was implemented every 21 days approximately.
Toxicity and response evaluation
Toxicity was graded using the version 3 of the Common Terminology Criteria for Adverse Events, and assessed from the initiation of the regimen until the end of chemotherapy. Repeat echocardiography imaging was planned before every cycle and after the whole treatment to assess the change in the LVEF. Decreases in the LVEF were graded as follows: grade 1 = LVEF decrease from baseline by 1 to 9 percentage points; grade 2 = LVEF 40% to 49% or LVEF decrease from baseline by 10 to 19 percentage points; grade 3 = LVEF 20% to 39% or LVEF decrease from baseline more than 20 percentage points; and grade 4 = LVEF < 20%. Tumor responses were assessed at the end of treatment and were classified as complete response (CR), unconfirmed complete response (CRU), partial response (PR), stable disease (SD), or progressive disease (PD) according to the International Workshop criteria [26]. CR was defined as the disappearance/eradication of all lesions including radiologic or biologic abnormalities observed at diagnosis and the absence of new ones. CRU was defined as a complete response with the persistence of some radiologic abnormalities, which was regressed in size by at least 75 percent. PR was defined as the regression of all measurable lesions by more than 50 percent, the disappearance of non-measurable lesions, and the absence of new lesions. SD was defined as a regression of any measurable mass by 50 percent or less or no change in the non-measurable lesions, but without further growth of existing lesions or the appearance of new lesions. PD was defined as the appearance of a new lesion, any additional growth of the initial lesion by more than 25 percent, or of any measurable lesion that had previously regressed during treatment by more than 50 percent from its smallest dimensions.
Statistics methods
This analysis is based on the data obtained during the follow-up from January 2011 to December 2014. Overall survival (OS) was defined as the time from diagnosis to death or the last follow-up. OS was assessed using the Kaplan-Meier method and compared between risk groups using the chi-square test. P values less than 0.05 were considered to be statistically significant. The statistical software SPSS19.0 was used for all the statistical analysis.
Results
Patient characteristics
The clinical characteristics are summarized in Table 1. The patients’ average age was 67.0 years in the DRCOP group, as compared to 67.5 years in the RCHOP group. The numbers of male patients were 20 (48.8%) and 34 (54.8%) in the DRCOP and RCHOP groups respectively. There were 10 (24.3%) diagnosed as GCB-like (germinal center B-cell-like) DLBCL in the DRCOP group, and there were 19 (30.6%) diagnosed as GCB-like DLBCL in RCHOP group.
Table 1.
Baseline characteristics between DRCOP and RCHOP groups
| Factors | Subgroups | DRCOP group | RCHOP group | P value |
|---|---|---|---|---|
| Average age | 67.0 y | 67.5 y | 0.577 | |
| Gender | Male | 20 (48.8%) | 34 (54.8%) | 0.547 |
| Female | 21 (51.2%) | 28 (45.2%) | ||
| IPI | 0-1 | 4 (9.8%) | 12 (19.3%) | 0.268 |
| 2 | 13 (31.7%) | 16 (25.8%) | 0.515 | |
| 3 | 14 (34.1%) | 20 (32.3%) | 0.842 | |
| 4-5 | 10 (24.4%) | 14 (22.6%) | 0.832 | |
| Genetic subtype | GCB | 10 (24.3%) | 19 (30.6%) | 0.490 |
| NON-GCB | 31 (75.7%) | 43 (69.4%) | ||
IPI international prognostic index; GCB germinal center B-cell-like.
Treatment delivery and toxicity
All the 103 patients were evaluated for hematotoxicity and cardiotoxicity. The grade 3 or greater hematotoxicity and cardiotoxicity observed during treatment are summarized in Tables 2, 3 and 4. All the patients haven’t been managed with pegfilgrastim in our study.
Table 2.
Hematotoxicity and cardiotoxicity observed in DRCOP and RCHOP groups
| Factors | Subgroups | DRCOP group | RCHOP group | P value |
|---|---|---|---|---|
| Hematotoxicity | Grade 3 | 5 (12.2%) | 9 (14.5%) | 0.737 |
| Grade 4 | 30 (73.2%) | 43 (69.4%) | 0.677 | |
| Grade 3-4 | 35 (85.4%) | 52 (83.9%) | 0.838 | |
| Cardiotoxicity | Grade 3 | 3 (7.3%) | 13 (21.0%) | 0.061 |
| Grade 4 | 1 (2.4%) | 4 (6.5%) | 0.354 | |
| Grade 3-4 | 4 (9.8%) | 17 (27.4) | 0.029 |
Table 3.
Hematotoxicity grade
| Grade | |||||
|---|---|---|---|---|---|
|
| |||||
| 1 | 2 | 3 | 4 | 5 | |
| Hematotoxicity | |||||
| WBC | 3.0-4.0*10E9/L | 2.0-3.0*10E9/L | 1.0-2.0*10E9/L | < 1.0*10E9/L | Death |
| Neutrophil | 1.5-2.0*10E9/L | 1.0-1.5*10E9/L | 0.5-1.0*10E9/L | < 0.5*10E9/L | Death |
| Hemoglobin | 100-120 g/L | 80-100 g/L | 65-80 g/L | < 65 g/L | Death |
| Platelet | 75-99*10E9/L | 50-75*10E9/L | 10-50*10E9/L | < 10*10E9/L | Death |
Table 4.
Cardiotoxicity grade
| Grade | Cardiotoxicity |
|---|---|
| 1 | LVEF decrease from baseline by 1-9% |
| 2 | LVEF 40-49% or LVEF decrease from baseline by 10-19% |
| 3 | LVEF 20-39% or LVEF decrease from baseline ≥ 20% |
| 4 | LVEF < 20% |
| 5 | Death |
In this retrospective study, 35 (85.4%) and 52 (83.9%) of patients experienced grade 3 or 4 hematotoxicity in the DRCOP and the RCHOP group respectively. There was no statistical difference in hematotoxicity between the RCHOP and DRCOP groups (P = 0.534).
The LVEF was evaluated before therapy and at therapy completion. A total of 4 patients (9.8%) had a documented decrease in LVEF (grade 3, n = 3 [7.3%]; grade 4, n = 1 [2.4%]) at the end of the treatment evaluations in the DRCOP group; while 17 patients (27.4%) were accredited to have a decrease in LVEF (grade 3, n = 12 [19.4%]; grade 4, n = 4 [6.5%]) in the RCHOP group. Moreover, there were less grade 3-4 cardiotoxicity in patient treated with DRCOP (9.8%) than RCHOP (27.4%, P = 0.029).
Response and survival
In these 103 patients, 41 cases were treated with RDCOP regimen and 62 cases with RCHOP regimen. All the patients completed our follow up, and the median follow-up time was 28 months (range, 2 to 48 months). Remission was evaluated using the IWG response criteria for lymphoma at the end of chemotherapy. CR, CRU and PR was present in 26 (63.4%), 3 (7.3%), 5 (12.2%) patients respectively in the DRCOP group. In the RCHOP group, we observed a CR in 36 patients (58.1%), a CRU in 5 patients (8.1%), and a PR in 7 patients (11.3%). There was no statistical difference in the CR/CRU or overall response rate between the DRCOP and the RCHOP group (P = 0.624, 0.497, Table 5). Kaplan-Meier curve showed there was no statistical difference of OS between the DRCOP and group) The OS in the DRCOP group (78.0%) and the RCHOP (72.6%) also showed no difference statistically relevant (P = 0.787, Figure 1).
Table 5.
Response between DRCOP and RCHOP groups
| Response | DRCOP group | RCHOP group | P value |
|---|---|---|---|
| CR | 26 (63.4%) | 36 (58.1%) | 0.587 |
| CRU | 3 (7.3%) | 5 (8.1%) | 0.890 |
| PR | 5 (12.2%) | 7 (11.3%) | 0.889 |
| SD | 5 (12.2%) | 12 (19.4%) | 0.338 |
| PD | 2 (4.9%) | 2 (3.2%) | 0.671 |
| CR/CRU | 29 (70.7%) | 41 (66.1%) | 0.624 |
| Overall response | 34 (82.9%) | 48 (77.4%) | 0.497 |
Figure 1.
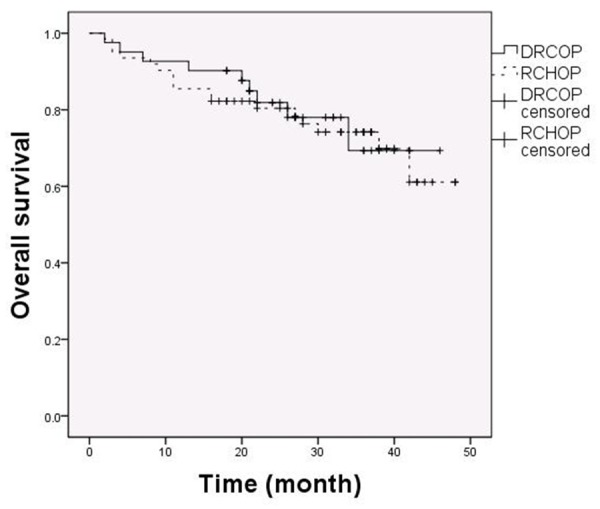
OS between DRCOP and RCHOP group. From 103 patients, 41 were treated with DRCOP and 62 with RCHOP. There was no statistical difference of OS between the DRCOP (78.0%) and RCHOP (72.6%) groups (P = 0.787).
Discussion
In this study, we addressed the question whether the substitution of conventional doxorubicin by PLD is a feasible and effective therapeutic option in elderly patients with DLBCL. We retrospectively analyzed 103 elderly patients with DLBCL who were treated with either RCHOP or DRCOP regimen in our hospital between January 2011 and June 2013. In these 103 patients, 41 cases were treated with RDCOP regimen and 62 cases with RCHOP regimen. All the patients completed a mean follow-up period of 28 months (range, 2 to 48 months). There was no statistical difference in the CR/CRU, OR or OS between the DRCOP and RCHOP group. Therefore our finding shows that DRCOP offers similar oncologic efficacy in the treatment of DLBCL as that of RCHOP. The CR/CRU and OS of RCHOP group in our study were similar as GELA LNH-98.5 trial [5]. Data about clinical trials of DLBCL patients treated with liposomal doxorubicin are scarce. Yasuhiro Oki and colleagues published an open label, single arm, phase II trial revealing that the CR/CRU rate in elderly patient with DLBCL on DRCOP regimen was 78%, and the overall response rate was 86%. The estimated 5-year EFS rate was 53% and the estimated 5-year OS rate was 70% [27]. In our study the DRCOP group showed a similar response rate and survival time.
We also evaluated the hematotoxicity and cardiotoxicity between the DRCOP and RCHOP regimens. In our study, we didn’t manage patients with pedfilgrstim, so we can see more grade 3-4 hematotoxicity occurred in our study than other studies such as GELA LNH-98.5, RICOVER-60 [5,6] and so on. But our study suggested that there was no statistical difference in hematotoxicity between the two groups. However, there were less grade 3-4 cardiotoxicity in patients treated with DRCOP (9.8%) than RCHOP regimen (27.4%, P = 0.029). DRCOP may be a more secure treatment for elderly DLBCL patients.
Conclusion
Our findings in this study indicate that the DRCOP regimen offers similar oncologic efficacy when weighed against the standard RCHOP regimen in elderly DLBCL patients. This study also insinuates that DRCOP regimen might be a more secure treatment for elderly DLBCL patients who have additional risk factors for cardiac diseases.
Acknowledgements
This work was supported in part by the Research Plan of Education Administration of Zhejiang Province, China (No. Y201120523).
Disclosure of conflict of interest
None.
References
- 1.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J Jr, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson W, Grever MR, Byrd JC, Botstein D, Brown PO, Staudt LM. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 2.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thieblemont C, Grossoeuvre A, Houot R, Broussais-Guillaumont F, Salles G, Traullé C, Espinouse D, Coiffier B. Non-Hodgkin’s lymphoma in very elderly patients over 80 years. A descriptive analysis of clinical presentation and outcome. Ann Oncol. 2008;19:774–779. doi: 10.1093/annonc/mdm563. [DOI] [PubMed] [Google Scholar]
- 4.Maartense E, Kluin-Nelemans HC, Noordijk EM. Non-Hodgkin’s lymphoma in the elderly. A review with emphasis on elderly patients, geriatric assessment, and future perspectives. Ann Hematol. 2003;82:661–670. doi: 10.1007/s00277-003-0722-1. [DOI] [PubMed] [Google Scholar]
- 5.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, ederlin P, Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 6.Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, Reiser M, Nickenig C, Clemens M, Peter N, Bokemeyer C, Eimermacher H, Ho A, Hoffmann M, Mertelsmann R, Trümper L, Balleisen L, Liersch R, Metzner B, Hartmann F, Glass B, Poeschel V, Schmitz N, Ruebe C, Feller AC, Loeffler M German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60) Lancet Oncol. 2008;9:105–116. doi: 10.1016/S1470-2045(08)70002-0. [DOI] [PubMed] [Google Scholar]
- 7.Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, Dakhil SR, Woda B, Fisher RI, Peterson BA, Horning SJ. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J. Clin. Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 8.Simnek T, Strba M, Popelov O, Adamcov M, Hrdina R, Gersl V. Anthracycline-induced cardiotoxicity: overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Rep. 2009;61:154–171. doi: 10.1016/s1734-1140(09)70018-0. [DOI] [PubMed] [Google Scholar]
- 9.Ewer MS, Ewer SM. Troponin I provides insight into cardiotoxicity and the anthracycline-trastuzumab interaction. J. Clin. Oncol. 2010;28:3901–4. doi: 10.1200/JCO.2010.30.6274. [DOI] [PubMed] [Google Scholar]
- 10.Lotrionte M, Biondi-Zoccai G, Abbate A, Lanzetta G, D’Ascenzo F, Malavasi V, Peruzzi M, Frati G, Palazzoni G. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am J Cardiol. 2013;112:1980–4. doi: 10.1016/j.amjcard.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 11.Sarkozy C, Coifer B. Diffuse large B-cell lymphoma in the elderly: a review of potential difficulties. Clin Cancer Res. 2013;19:1660–9. doi: 10.1158/1078-0432.CCR-12-2837. [DOI] [PubMed] [Google Scholar]
- 12.Suter TM, Ewer MS. Cancer drugs and the heart: importance and management. Eur Heart J. 2013;34:1102–11. doi: 10.1093/eurheartj/ehs181. [DOI] [PubMed] [Google Scholar]
- 13.Northfelt DW, Martin FJ, Working P, Volberding PA, Russel J, Newman M, Amantea MA, Kaplan LD. Doxorubicin encapsulated in liposomes containing surface-bound polyetylene glycol: pharmacokinetics, tumor localization, and safety in patients with AIDS-related Kaposi’s sarcoma. J Clin Pharmacol. 1996;36:55–63. doi: 10.1002/j.1552-4604.1996.tb04152.x. [DOI] [PubMed] [Google Scholar]
- 14.Gabizon A, Catane R, Uziely B, Kaufman B, Safra T, Cohen R, Martin F, Huang A, Barenholz Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulalated in polyetylene-glycol coated liposomes. Cancer Res. 1994;54:987–992. [PubMed] [Google Scholar]
- 15.Bogner JR, Kronawitter U, Rolinski B, Truebembach K, Goebel FD. Liposomial doxorubicin in the treatment of advanced AIDS related Kaposi sarcoma. J Acquir Immune Defic Syndr. 1994;7:463–C468. [PubMed] [Google Scholar]
- 16.Harrison M, Tomlinson D, Stewart S. Liposomalentrapped doxorubicin: an active agent in AIDS-related Kaposi’s sarcoma. J. Clin. Oncol. 1995;13:914–920. doi: 10.1200/JCO.1995.13.4.914. [DOI] [PubMed] [Google Scholar]
- 17.James ND, Coker RJ, Tomlinson D, Harris JR, Gompels M, Pinching AJ, Stewart JS. Liposomal doxorubicin: an effective new treatment for Kaposi’s sarcoma in AIDS. Clin Oncol (R Coll Radiol) 1994;6:294–296. doi: 10.1016/s0936-6555(05)80269-9. [DOI] [PubMed] [Google Scholar]
- 18.Berry G, Billingham M, Alderman E, Richardson P, Torti F, Lum B, Patek A, Martin FJ. The use of cardiac biopsy to demonstrate reduced cardiotoxicity in AIDS Kaposi’s Sarcoma patients treated with pegylated liposomal doxorubicin. Ann Oncol. 1998;9:711–716. doi: 10.1023/a:1008216430806. [DOI] [PubMed] [Google Scholar]
- 19.Wollina U, Graefe T, Kaatz M. Pegylated doxorubicin for primary cutaneous T-cell lymphoma: a report on ten patients with follow-up. J Cancer Res Clin Oncol. 2001;127:128–134. doi: 10.1007/s004320000178. [DOI] [PubMed] [Google Scholar]
- 20.Aviles A, Neri N, Castaneda C, Talavera A, Huerta-Guzman J, Gonzalez M. Pegylated liposomal doxorubicin in combination chemotherapy in the treatment of previously untreated aggressive diffuse large-B-cell lymphoma. Med Oncol. 2002;19:55–58. doi: 10.1385/MO:19:1:55. [DOI] [PubMed] [Google Scholar]
- 21.Chanan-Khan A, Islam T, Bernstein SH. CDOP (cyclophosphamide, doxil, vincristine, prednisone) as a front-line regimen for elderly patients with intermediate grade non-Hodgkin’s lymphoma (IG-NHL) Blood. 2001;98:4681a. [Google Scholar]
- 22.Martino R, Perea G, Caballero MD, Mateos MV, Ribera JM, de Oteyza JP, Arranz R, Terol MJ, Sierra J, San Miguel JF. Cyclophosphamide, pegylated liposomal doxorubicin (Caelyx), vincristine and prednisone (CCOP) in elderly patients with diffuse large B-cell lymphoma: results from a prospective phase II study. Haematologica. 2002;87:822–827. [PubMed] [Google Scholar]
- 23.Tsavaris N, Kosmas C, Vadiaka M, Giannouli S, Siakantaris MP, Vassilakopoulos T, Pangalis GA. Pegylated liposomal doxorubicin in the CHOP regimen for older patients with aggressive (stagesIII/IV) non-Hodgkin’s lymphoma. Anticancer Res. 2002;22:1845–1848. [PubMed] [Google Scholar]
- 24.Tulpule A, Khan AU, Mohrbacher AF. A phase II trial of pegylated liposomal doxorobucin ,rituximab, cyclophosphamide, vincristine ,and prednisone (DR-COP) in aggressive B-cell nonHodgkin’s lymphoma. ASCO. 2004;23:6688a. [Google Scholar]
- 25.Yildirim Y, Gultekin E, Avci ME, Inal MM, Yunus S, Tinar S. Cardiac safety profile of pegylated liposomal doxorubicin reaching or exceeding lifetime cumulative doses of 550 mg/m2 in patients with recurrent ovarian and peritoneal cancer. Int J Gynecol Cancer. 2008;18:223–7. doi: 10.1111/j.1525-1438.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- 26.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas: NCI Sponsored International Working Group. J. Clin. Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 27.Oki Y, Ewer MS, Lenihan DJ, Fisch MJ, Hagemeister FB, Fanale M, Romaguera J, Pro B, Fowler N, Younes A, Astrow AB, Huang X, Kwak LW, Samaniego F, McLaughlin P, Neelapu SS, Wang M, Fayad LE, Durand JB, Rodriguez MA. Pegylated Liposomal Doxorubicin Replacing Conventional Doxorubicin in Standard R-CHOP Chemotherapy for Elderly Patients With Diffuse Large B-Cell Lymphoma: An Open Label, Single Arm, Phase II Trial. Clin Lymphoma Myeloma Leuk. 2015;15:152–8. doi: 10.1016/j.clml.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


