Abstract
Objective: To evaluate the relationship between BRAF mutation and factors influencing the prognosis of papillary thyroid microcarcinoma (PTMC). Methods: Clinical data from patients with PTMC were subjected to retrospective analysis. A total of 86 patients were included, and the BRAFV600E mutation was identified in surgically dissected tissues. Results: The incidence of BRAF mutation in patients with PTMC was 65.1% (56/86). Both univariate and multivariate analyses indicated a correlation between BRAF mutation and lymph node metastasis (P = 0.057). For patients with tumors ≤ 10 mm in diameter, BRAF mutation had no effect on lymph node metastasis (P > 0.05). No lymph node metastasis was found in patients with tumors ≤ 5 mm in diameter. Conclusion: BRAF gene mutation is an independent predictive risk factor for central lymph node metastasis in patients with PTMC. For patients with preoperative BRAF mutation positivity, it is important to perform central lymph node dissection (CLND) and lymphatic and adipose tissues should be routinely removed. However, in patients without BRAF mutation and tumors ≤ 5 mm in diameter, the necessity of prophylactic CLND should be reevaluated.
Keywords: Papillary thyroid microcarcinoma, BRAF gene, gene mutation, lymph node metastasis
Introduction
Papillary thyroid microcarcinoma (PTMC) refers to papillary thyroid carcinoma (PTC) with a diameter ≤ 10 mm and accounts for more than 50% of all thyroid cancers [1]. The v-raf murine sarcoma viral oncogene homolog B1 (BRAF) gene mutation is the most common genetic event in thyroid carcinoma, with the highest prevalence in PTC (29-88%) [2]. PTMC exhibits relatively indolent behavior. Studies have found that BRAF mutation is strongly associated with PTMC invasiveness. However, the relationships between the BRAFV600E mutation and some clinicopathological features of PTMC remain controversial. This study examined the BRAFV600E mutation status in samples from a total of 86 PTMC patients and also retrospectively analyzed the clinical data from these patients.
Materials and methods
Patients and samples
Paraffin-embedded specimens from a total of 86 PTMC patients at our hospital collected between October 2011 and October 2013 were used in this study. All patients included in this study met the following requirements: (1) Unilateral tumor confirmed by ultrasound before surgery; (2) PTC diagnosis by both intraoperative rapid pathology and postoperative pathology; and (3) treatment via ipsilateral lobectomy, isthmectomy, and prophylactic ipsilateral central lymph node dissection (CLND).
Methods
Eluent containing DNA was obtained from 1-2 paraffin-embedded tissue sections (8 µm in thickness) per sample using a DNA kit (Aide Biotechnology Inc., Xiamen, China) according to the manufacturer’s instructions. The BRAFV600E mutation was detected by analyzing real time polymerase chain reaction signals using a human BRAFV600E mutation detection kit (Fluorescence PCR’ Aide Biotechnology Inc., Xiamen, China), to determine whether the sample carried a mutation-positive gene.
Statistical analysis
The SPSS 13.0 statistical software package (SPSS, Inc., Chicago, IL, USA) was used for all analyses. Normally distributed measurement data were expressed as means ± standard deviations (SD), and non-normally distributed data were expressed as medians (quartiles). Enumeration data were expressed as frequencies and rates. The independent samples t-test (normally distributed data), rank-sum test (non-normally distributed data), or χ2 test (enumeration data) was used to compare 2 variables. Univariate and multivariate logistic regression analyses were used to evaluate the influences of clinicopathological factors on BRAFV600E mutation and lymph node metastasis (lymph node metastasis as the dependent variable). The level of significance was defined as P < 0.05.
Results
Clinicopathological data from patients with thyroid cancer
The clinical and pathological features of patients are shown in Table 1. A total of 86 cases of unilateral PTMC in 8 male and 78 female patients (male: female patient ratio = 1:9.75; age range = 25-73 years, average age = 43.7 ± 10.3 years) were included in this study.
Table 1.
Clinicopathological characteristics of 86 patients with PTMC
| Clinicopathologic characteristics | Patients (n) | Percentage (%) |
|---|---|---|
| Gender | ||
| Male | 8 | 9.3 |
| Female | 78 | 90.7 |
| Age, yr | 43.7 ± 10.3 | |
| ≥ 45 | 41 | 47.7 |
| < 45 | 45 | 52.3 |
| Tumor size, mm | 6.8 ± 2.2 | |
| 5 < and ≤ 10 | 58 | 67.4 |
| ≤ 5 | 28 | 32.6 |
| Multifocality | ||
| Multiple (≥ 2) | 5 | 5.8 |
| Single | 81 | 94.2 |
| Extrathyroid invasion | ||
| Yes | 3 | 3.5 |
| No | 83 | 96.5 |
| Hashimoto’s disease | ||
| Yes | 17 | 19.8 |
| No | 69 | 80.2 |
| Lymph node metastases | ||
| Yes | 22 | 25.6 |
| No | 64 | 74.4 |
| T stage* | ||
| T3 | 2 | 2.3 |
| T1 | 84 | 97.7 |
| BRAFV600E mutation | ||
| Yes | 56 | 65.1 |
| No | 30 | 34.9 |
TNM stage is based on the AJCC Cancer Staging Manual, 7th edition (2010).
BRAFV600E mutation detection results
The results of gene mutation are shown in Figure 1. Of the 86 evaluated cases of PTMC, 56 were positive for BRAF mutation and the mutation prevalence was 65.1% (56/86), as shown in Table 1.
Figure 1.
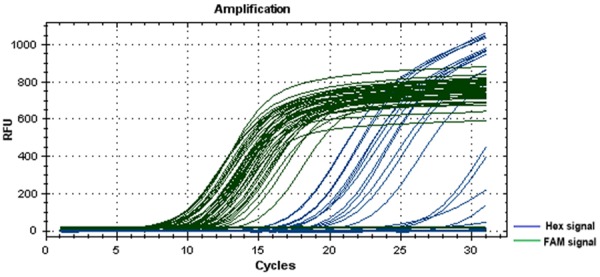
Gene mutation test results (real time-polymerase chain reaction signal curves). Note: The taq enzyme corresponding to the Hex signal is DNA specific, whereas the taq enzyme corresponding to the FAM signal is BRAF gene specific. The elevated Hex signal curves indicate successful DNA extraction. Some elevated FAM signal curves indicate BRAF mutation positivity.
Association of BRAF mutation with clinicopathological features of PTMC
The relationships between BRAF mutation and clinicopathological features of PTMC are shown in Table 2. The univariate analysis indicated a trend toward a correlation between BRAF mutation and lymph node metastasis (P = 0.057). The multivariate analysis indicated a significant correlation between BRAF mutation and lymph node metastasis (P < 0.05).
Table 2.
Univariate and multivariate analyses of the BRAFV600E mutation and clinicopathological features in PTMC
| Variable (n = 126) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
| ||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Gender (male/female) | 1.7 (0.3-8.9) | 0.542 | 2.2 (0.3-13.9) | 0.416 |
| Age, yr (≥ 45 vs. < 45) | 1.1 (0.4-2.6) | 0.891 | 1.7 (0.6-4.6) | 0.304 |
| Tumar size, mm (5 < and ≤ 10 vs. ≤ 5) | 1.7 (0.7-4.2) | 0.283 | 1.4 (0.5-3.4) | 0.494 |
| Multifocality (multiple/single) | 2.2 (0.2-20.9) | 0.482 | 3.0 (0.3-33.2) | 0.365 |
| Extrathyroidal invasion (yes/no) | 1.1 (0.1-12.4) | 0.954 | 0.7 (0.1-9.7) | 0.793 |
| Hashimoto’s disease (yes/no) | 0.5 (0.2-1.5) | 0.244 | 0.6 (0.2-1.9) | 0.396 |
| LNM (yes/no) | 3.1 (0.9-10.1) | 0.057 | 3.7 (1.0-13.5) | 0.045 |
| T stage (T3/T1) | 0.5 (0.1-8.7) | 0.655 | 0.2 (0.01-5.1) | 0.339 |
Note: Age and tumor size were assessed as continuous variables in both the univariate and multivariate analyses.
The relationship between BRAF mutation and central lymph node metastasis
The correlation between BRAF mutation and features of central lymph node metastasis is shown in Table 3. Compared with BRAF negative patients, a particular trend toward correlation between BRAF mutation and the number of central lymph node metastases was observed in BRAF mutation positive patients, as well as the ratio of the number of metastatic lymph nodes to the number of total dissected lymph nodes (P = 0.057). However, the mutation status was not significantly associated with the number of lymph nodes dissected from the patients (P > 0.05).
Table 3.
Correlation of central lymph node metastasis with BRAFV600E mutation
| BRAFV600E mutation | P-value | |||
|---|---|---|---|---|
|
| ||||
| Positive | Negative | |||
| LNM (n/%) | Yes | 18 (81.8) | 4 (18.2) | 0.057 |
| No | 38 (59.4) | 26 (40.6) | ||
| Lymph node dissection (n, mean ± s.d.) | 4.5 ± 3.5 | 4.2 ± 2.8 | 0.634 | |
| LNM (n) | 0 (0, 1) | 0 (0, 0) | 0.057 | |
| LNM/lymph node dissection (%) | 0 (0, 29.9) | 0 (0, 0) | 0.057 | |
Note: Data were abnormally distributed and are expressed as medians and inter-quartile ranges (P25-P75); these were analyzed using the Wilcoxon Rank Sum W Test.
Correlations between tumor size, BRAF mutation, and central lymph node metastasis are shown in Table 4. In patients with tumors ≤ 10 mm in diameter, the BRAF mutation status was not associated with lymph node metastasis (P > 0.05). Furthermore, no metastases were found in patients negative for the BRAF mutation or those with tumors ≤ 5 mm in diameter.
Table 4.
Correlations of tumor size and BRAFV600E mutation with lymph node metastasis
| Tumor size, mm | Mutation | Lymph node metastases | P-value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Yes (n) | Percentage, % | No (n) | Percentage, % | |||
| ≤ 5 | 0.238 | |||||
| Positive | 3 | 18.7 | 13 | 81.3 | ||
| Negative | 0 | 0 | 12 | 100 | ||
| 5 < and ≤ 10 | 0.251 | |||||
| Positive | 15 | 37.5 | 25 | 62.5 | ||
| Negative | 4 | 22.2 | 14 | 77.8 | ||
Discussion
The mechanism by which BRAF mutation affects tumor onset and progression may be related to abnormal activation of the MAP kinase pathway [3]. Zheng et al. [4] found that BRAF mutation was closely related to a poor clinicopathological outcome and could lead to an increase in PTMC recurrence. Through the multivariate analysis conducted in this study, BRAF mutation was found only to be associated with lymph node metastasis (P < 0.05). It is safe to believe that BRAF mutation is an independent predictor of central lymph node metastasis. This result is consistent with the study results published by Lin et al [5]. Also, the results of this study suggest the importance of CLND in preoperatively detected BRAF mutation-positive PTMC patients and that lymphatic and adipose tissues should be routinely removed. However, a study by Cheng [6] showed that BRAF mutation could not be used as an independent predictor of lymph node metastasis in PTMC, and this represents a current area of contention.
The present study further analyzed the relationships of lymph node metastasis with BRAF mutation and tumor size. Again, a certain correlative trend was found between lymph node metastasis and BRAF mutation (P = 0.057). Although the presence of mutation was not associated with an increased number of surgically dissected central lymph nodes, it was related to an increased number and frequency of metastatic lymph nodes, indicating the effect and predictive value of BRAF mutation with respect to central lymph node metastasis. Moreover, the study also found that for patients with tumors ≤ 10 mm in diameter, BRAF mutation did not correlate with lymph node metastasis (P > 0.05); furthermore, no metastases were found in patients negative for BRAF mutation and those with tumors ≤ 5 mm in diameter. Currently, very few studies have discussed how BRAF mutation influences surgical strategies for PTC patients. Although prophylactic CLND is well accepted in the field as a routine procedure for PTC patients [7], CLND can increase the incidence of complications during surgery, and therefore, the patient indications for CLND remains under study. This study speculated that the necessity of CLND should be reconsidered in BRAF mutation-negative patients with tumors ≤ 5 mm in diameter.
In summary, this study found a BRAFV600E mutation prevalence of 65.1% in PTMC patients and determined that BRAFV600E mutation is an independent predictor of central lymph node metastasis in PTMC. For patients determined to be BRAF mutation-positive during preoperative examinations, the importance of CLND should be seriously considered and lymphatic adipose tissues should be routinely removed. However, the necessity of CLND should be reevaluated for mutation-negative patients with tumors ≤ 5 mm in diameter.
Disclosure of conflict of interest
None.
References
- 1.Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, Zhang Y, Bai Y, Zhu C, Guo GL, Rothman N. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control. 2009;20:525–531. doi: 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim KH, Kang DW, Kim SH, Seong IO, Kang DY. Mutations of the BRAF gene inpapillary thyroid carcinoma in a Korean population. Yonsei Med J. 2004;45:818–821. doi: 10.3349/ymj.2004.45.5.818. [DOI] [PubMed] [Google Scholar]
- 3.Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006;6:292–306. doi: 10.1038/nrc1836. [DOI] [PubMed] [Google Scholar]
- 4.Zheng X, Wei S, Han Y, Li Y, Yu Y, Yun X, Ren X, Gao M. Papillary microcarcinoma of the thyroid: clinical characteristics and BRAF(V600E) mutational status of 977 cases. Ann Surg Oncol. 2013;20:2266–2273. doi: 10.1245/s10434-012-2851-z. [DOI] [PubMed] [Google Scholar]
- 5.Lin KL, Wang OC, Zhang XH, Dai XX, Hu XQ, Qu JM. The BRAF mutation is predictive of aggressive clinicopathological characteristics in papillary thyroid microcarcinoma. Ann Surg Oncol. 2010;17:3294–3300. doi: 10.1245/s10434-010-1129-6. [DOI] [PubMed] [Google Scholar]
- 6.Cheng S, Serra S, Mercado M, Ezzat S, Asa SL. A high-throughput proteomic approach provides distinct signatures for thyroid cancer behavior. Clin Cancer Res. 2011;17:2385–2394. doi: 10.1158/1078-0432.CCR-10-2837. [DOI] [PubMed] [Google Scholar]
- 7.Shi CL, Shi TF, Qin HD. Pattern and Predictive Factors of Neck Lymph Node Metastasis in Papillary Thyroid Carcinoma. Chin J Bases Clin General Surg. 2014;21:29–34. [Google Scholar]


