Abstract
Reactive oxygen species (ROS) generation has been suggested to play a vital role in the initiation and progression of diabetic cardiomyopathy, a major complication of diabetes mellitus. Recent studies reveal that spermine possesses proliferative, antiaging and antioxidative properties. Thus, we hypothesized that spermine could decrease apoptosis via suppressing ROS accumulation induced by high glucose (HG) in cardiomyocytes. Cultured neonatal rat ventricle cardiomyocytes were treated with normal glucose (NG) (5 mM) or HG (25 mM) in the presence or absence of spermine for 48 h. The cell activity, apoptosis, ROS production, T-SOD and GSH activities, MDA content and GSSG level were assessed. The results showed that HG induced lipid peroxidation and the increase of intracellular ROS formation and apoptosis in primary cardiomyocytes. Spermine could obviously improve the above-mentioned changes. Western blot analysis revealed that spermine markedly inhibited HG-induced the phosphorylation of p38/JNK MAPKs and JAK2. Moreover, spermine had better antioxidative and anti-apoptotic effects than N-acetyl-L-cysteine (NAC). Taken together, the present data suggested that spermine could suppress ROS accumulation to decrease cardiomyocytes apoptosis in HG condition, which may be attributed to the inhibition of p38/JNK and JAK2 activation and its natural antioxidative property. Our findings may highlight a new therapeutic intervention for the prevention of diabetic cardiomyopathy.
Keywords: High glucose, spermine, reactive oxygen species, cardiomyocytes, apoptosis
Introduction
Diabetic cardiomyopathy was initially put forward by Rubler et al to illuminate cardiac muscle dysfunction in patients with diabetes who had no other coronary artery diseases [1]. Diabetic cardiomyopathy is one of lethal complications in people with diabetes. Moreover, more than 80% diabetic patients are or will be suffered from diabetic cardiomyopathy. Thus, this situation has been the great concern of the worldwide [2]. In recent years, many data indicate that hyperglycemia is a causal factor of diabetic cardiomyopathy, one of the major cardiac complications in diabetic patients [3,4]. Some hypotheses have been proposed to explain the pathogenesis of diabetic cardiomyopathy: (1) cardiac metabolic disturbances [4]; (2) ROS accumulation [5]; (3) calcium signaling abnormality [6]; (4) mitochondria dysfunction [7]; and (5) chronic low-grade inflammation in the heart [8]. According to these pathogeneses, many therapies were carried out, such as supplying myocardial energy substrate and improving microvascular perfusion [9], inhibiting cardiac inflammation [10], antioxidant treatment [11] or some regents as statins which could not only lower the lipid in cardiovascular diseases [12], but also have antioxidative property [13,14]. However, the treatment effect of these drugs on diabetic cardiomyopathy is always not satisfactory. Hence, exploring new drugs is very essential for scientific workers. Spermine is one kind of polyamines that have a wide distribution, furthermore, possess various functions in mammalian cells, including antioxidative, anticancer, antiaging, regulating cell proliferation, maintaining cell membrane stability, nucleic acid structure and stability and ion channels function, etc. [15,16]. Thus, we assume that spermine may be an antioxidant to treat diabetic cardiomyopathy. But whether spermine has an advantage over other antioxidants needs further investigation.
Materials and methods
Antibodies and reagents
Cell culture medium-Dulbecco modified Eagle medium and fetal bovine serum (FBS) were purchased from Hyclone Laboratories, Inc (HyClone, Logan, UT, USA). Cell-counting kit (CCK-8) was purchased from Dojindo Laboratories (Mashiki-machi, Kumamoto, Japan). 2’7’-dichlorodihydrofluorescin diacetate (DCFH-DA), Hoechst33342, N-acetyl-L-cysteine (NAC), p38 MAPK (mitogen activated protein kinase) inhibitor-SB203580, JNK (c-Jun N-terminal kinase) inhibitor-SP600125, the Janus kinase (JAK2) inhibitor-AG490 were obtained from Beyotime Institute of Biotechnology (Shanghai, China). Malondialdehyde (MDA), total superoxide dismutase (T-SOD), glutathione (GSH) and oxidation glutathione (GSSG) detection kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The Annexin V-FITC Apoptosis Detection Kit was obtained from 4A Biotech, Inc (Beijing, China). Cleaved caspase-3/9, p38, p-p38, JNK and p-JNK antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Bcl-2, cytochrome C, JAK2 antibodies were obtained from Proteintech Group, Inc (Wuhan, China). P-JAK2 antibody was obtained from ABclonal, Inc (Boston, USA). STAT3 (signal transducer and activator of transcription 3), p-STAT3 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All second antibodies were obtained from Zhongshan Golden Bridge Biotechnology (Beijing, China).
Experimental protocols
The following experimental treatments were performed (n=3): (1) normal glucose (NG) (5 mM). (2) NG with spermine (5 μM). (3) HG (25 mM). (4) HG with spermine (5 μM): pretreatment with 5 μM spermine for 1 h before HG condition. Here, the dose of spermine in the study was confirmed through preliminary experiments with CCK-8 assay. In order to investigate signaling pathways of which spermine abated HG-induced ROS production, we devised the following groups: (5) HG and the kinases inhibitors (SB203580, the inhibitor of p38 MAPK, 10 μM; SP600125, the inhibitor of JNK, 10 μM; AG490, the inhibitor of JAK2, 100 μM) or with spermine, preincubation with the inhibitor for 30 min or with spermine for 30 min before HG treatment. These pathways had been reported to be involved in HG-induced ROS generation in the mammal cells [17,18]. To further indentify the antioxidantive activity of spermine, we added another two groups: (6) HG+NAC (a ROS scavenger, a well-known antioxidant, 5 μM) or with spermine, preincubation with NAC or with spermine for 30 min before HG treatment.
Cardiomyocytes culture
The primary cardiomyocytes were separated from one day to three days old Sprague-Dawley rats’ ventricles (The laboratory animal center of Harbin Medical University) by trypsin-EDTA method, briefly [17]. Then, primary ventricular cardiomyocytes were cultured in DMEM containing 10% FBS, 100 g/ml penicillin, 100 g/ml streptomycin, and 2 mM glutamine with low glucose (5 mM). Before the treatment, every other day, the cardiomyocytes’ medium was changed.
CCK-8 assay
CCK-8 assay was performed to analyze the cell viability of primary cardiomyocytes in HG condition. The primary cardiomyocytes were planted into 96 well-plates with a density of 2×104/200 μl and divided into four groups, as above experimental protocols. After 48 h, the cell medium with FBS was removed. Then the cells were washed with phosphate buffer solution (PBS) twice, and added the CCK-8 agent and DMEM free FBS cocktail (1:10) 100 μl every well for 1 h in the dark at 37°C before measuring OD450 value.
Hoechst33342 and Annexin V/PI staining
The primary cardiomyocytes were planted into 35 mm culture dishes with 5×104/ml. After treated as previous protocols, the cells were washed with PBS for three times. Then the cells were stained with 5 mg/L Hoechst33342 for 20 min at 37°C in the dark. Next, the cells were washed with PBS for three times to remove Hoechst33342 stain. The cell nuclei were observed and visualized by an inverted fluorescence microscope. The number of apoptotic nuclei was calculated in 10 randomly selected areas for each experimental group. The data were expressed as the percentage of apoptotic cells relative to the total number of cells (apoptotic cells/total cells ×100%). Images (×200 magnification) were captured.
Meanwhile, cell apoptosis was also assessed by the Annexin V/PI staining apoptosis kit according to the manufacturer’s instructions. Following indicated treatments as mentioned above, the cells were digested with trypsin free of EDTA and centrifuged at 1000 rpm for 5 min. Then the cells were washed twice with cold PBS and suspended in 100 μl 1× binding buffer at a density of 1×106 cells/ml. Subsequently, 5 μl Annexin V for 15 min and 10 μl propidium iodide (PI) for 5 min were added to incubate the cells in dark at room temperature (RT). Cellular fluorescence was measured by flow cytometry analysis. Annexin V/FITC and propidium iodide double stain were used to evaluate the percentages of apoptosis. Annexin V- and PI- cells were used as controls. Annexin V+ and PI- cells were designated as early apoptotic and Annexin V+ and PI+ cells displayed as late apoptotic. Annexin V- and PI+ cells represented necrotic.
MDA and total SOD, total GSH and GSSG assay
The samples were obtained from the medium supernatant of primary cardiomyocytes (200 μl every sample) treated as above designed. The SOD, GSH activity and MDA and GSSG levels were measured by colorietric analysis using a spectrophotometer with the associated detection kits.
Measurement of intracellular ROS
The peroxide-sensitive fluorescent probe-DCFH-DA was used to measure intracellular ROS production and accumulation. After treated as above mentioned, primary cardiomyocytes were washed with DMEM for three times, incubated for 20 min at 37°C in DMEM with free FBS containing 10 μM DCFH-DA to label intracellular ROS, and then washed again. DCFH-DA was detected by fluorescence microscope (excitation wavelength: 488 nm, emission wavelength: 510 nm). Images (×200 magnification) were captured randomly.
Western blot assay
After designated treatments, cell extracts were prepared with RIPA lysis buffer with PMSF (99:1) for 30 min and centrifuged at 14,000× g for 15 min at 4°C. Total protein in the supernatant was quantified using a BCA protein assay kit according to the manufacturer’s instructions. Protein samples (50-100 μg per lane) were subjected to 8-15% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose filter membrane. The membranes were blocked with 5% skim milk in TBST (TBS containing 0.05% Tween-20) for 1 h at RT, incubated with primary antibody overnight at 4°C, washed with TBST three times for every 10 min, and then incubated with the secondary alkaline phosphatase (AP)-labeled antibodies for 1 h at RT. The bands were visualized by enhanced chemiluminescence reagents. The results were quantified by Quantity One Software. The antibody sources and dilutions used were as follows: antibodies against p38 (1:1000), p-p38 (1:1000), JNK (1:1000), p-JNK (1:1000), cleaved-caspase3 (1:1000), cleaved-caspase9 (1:1000), bcl-2 (1:1000), cytochrome c (1:1000), JAK2 (1:1000), p-JAK2-Y1007 (1:500), STAT3 (1:1000), GAPDH (1:1000) and AP-conjugated secondary mouse, rabbit ant goat antibodies (1:1000).
Statistical analysis
Quantitative data were reported as the mean ± standard error of mean (SEM) from at least three independent experiments. Comparisons were performed by Student’s t test or oneway analysis of variance (ANOVA) followed by Tukey’s multiple comparison test with Prism 5.00 software. Values that reached a P<0.05 level of significance were considered statistically significant.
Results
Identification of primary cardiomyocytes
To guarantee the accuracy of the study, primary cardiomyocytes were identified. First, after primary cardiomyocytes separated successfully, under an inverted microscope, cells appeared many shapes-spindle-shaped, triangular, irregular star-shaped and rhythmic beat after 2 days. Second, when the cells were cultured for 4 days, more than 90% cells were stained positive for α-actin (green) with immunofluorescence assay (DAPI stained for nuclei (blue)), which could further confirm that the cells were cardiomyocytes (Figure 1A) [19]. Image (×400) was obtained randomly. Finally, α-actin expression measured by western blot assay was visible in primary cardiomyocytes (Figure 1B).
Figure 1.
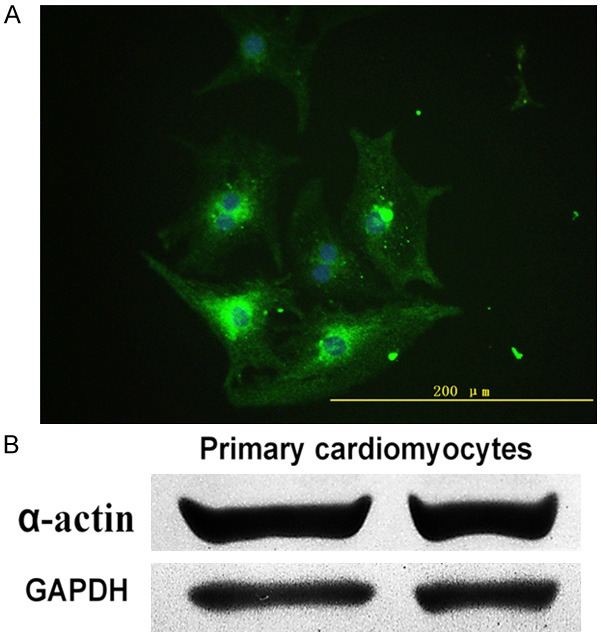
Primary cardiomyocytes were identified. A. Cultured primary cardiomyocytes presented positive α-actin stain (green) and DAPI stain for nuclei (blue) with fluorescence microscopy (×400). B. α-actin expression in primary cardiomyocytes was determined.
Spermine ameliorated cell injury in HG-treated primary cardiomyocytes
To explore the effect of spermine on HG-induced cytotoxicity in primary cardiomyocytes, the CCK-8 assay was performed. As presented in Figure 2A, the cells 25 mM glucose (HG) for 48 h obviously caused a decrease of cell viability, compared with NG (5 mM) group (P<0.01). However, pretreatment of 5 μM spermine for 1 h dramatically reverted the decrease of cell viability induced by HG in primary cardiomyocytes (P<0.01).
Figure 2.
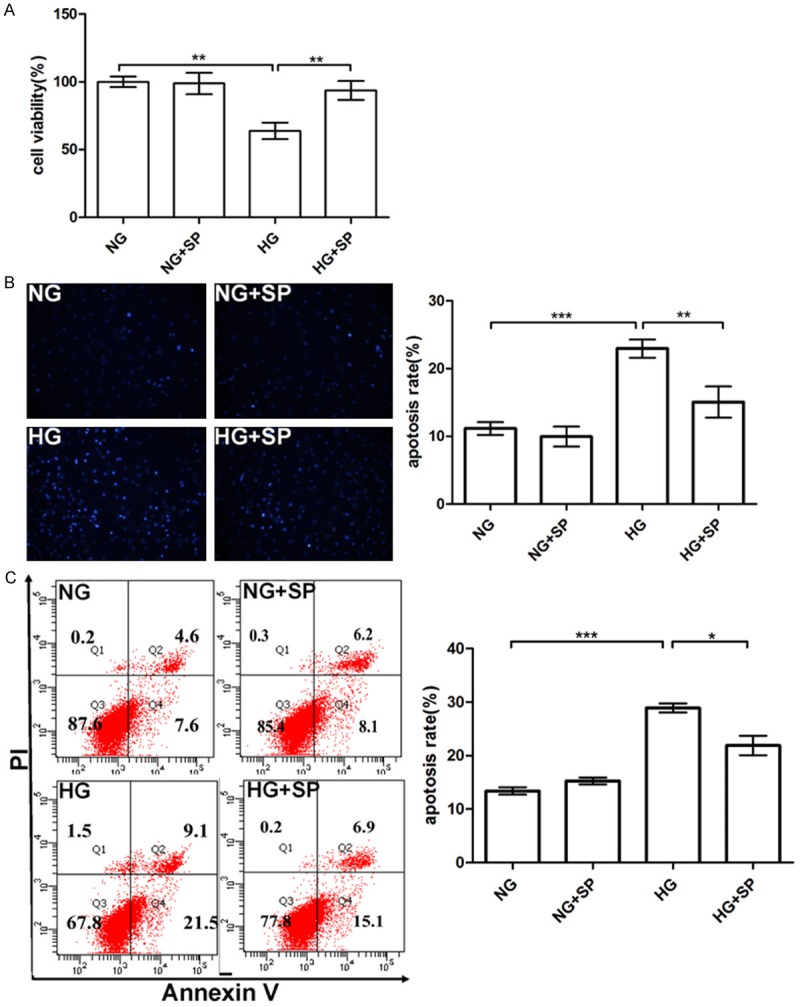
Effects of spermine on HG-induced primary cardiomyocytes injury. A. The cell viability of primary cardiomyocytes. B. Primary cardiomyocytes apoptosis induced by HG detected by Hoechst33342 staining with fluorescence microscopy, quantitative analysis the apoptosis rate of four groups by nuclei counting. C. The apoptosis of primary cardiomyocytes in HG condition was determined by flow cytometry. Data are presented as means ± SEM; *P<0.05 vs. control group (NG or HG group ); **P<0.01 vs. control group (NG or HG group); ***P<0.001 vs. control group (NG or HG group ).
To further confirm the influence of spermine on HG-treated primary cardiomyocytes, Hoechst33342 staining and flow cytometry analysis were used. As evidenced in Figure 2B, primary cardiomyocytes’ nuclei stained by Hoechst33342 in HG condition showed apoptotic phenotypes compared with NG group [20]. Moreover significant differences were observed between the cells subjected to HG and NG. However, the cell apoptosis rate was considerably decreased by pretreatment with spermine (P<0.05).
Flow cytometry analysis is used to detect phosphatidylserine (by Annexin V staining) exposed on the outer cell membrane in combination with nuclear PI staining, which is externalized in cells undergoing apoptosis [21]. As observed in Figure 2C, the Annexin V-positive events (the early and late apoptosis) in HG group (28.9±1.47%) were increased, compared with NG group (13.4±1.2%) (P<0.001). However, pretreatment with spermine in primary cardiomyocytes (21.9±3.15%), the apoptosis rate reached a comparable attenuation with HG group (P<0.05).
In the study, according to the results of Hoest3342 staining and Annexin V staining, the apoptosis rate was a little difference in NG group. We speculated that the analysis of Hoest33342 staining was more subjective.
Spermine affected HG-induced alterations of cytochrome c release, bcl-2 and cleaved caspase3/9 protein expressions in primary cardiomyocytes
As shown in Figure 2, spermine declined HG-induced apoptosis in primary cardiomyocytes. HG group displayed an obvious increase of cytochrome c in the cytosolic (cyto-Cyt C) fraction compared with the NG group. In parallel with this, mitochondrial cytochrome c (mt-Cyt C) showed a slight decreasing level (Figure 3A). However, the anti-apoptotic protein bcl-2 was significantly decreased (P<0.05) (Figure 3B). Meanwhile, HG notably promoted the activation of both caspase3 and caspase9 in primary cardiomyocytes. Interestingly, preincubation with spermine, as expected, inhibited the HG-induced cytochrome c release into the cytosol, increased bcl-2 expression, and abolished the activation of caspase3/9 (P<0.05) (Figure 3B).
Figure 3.
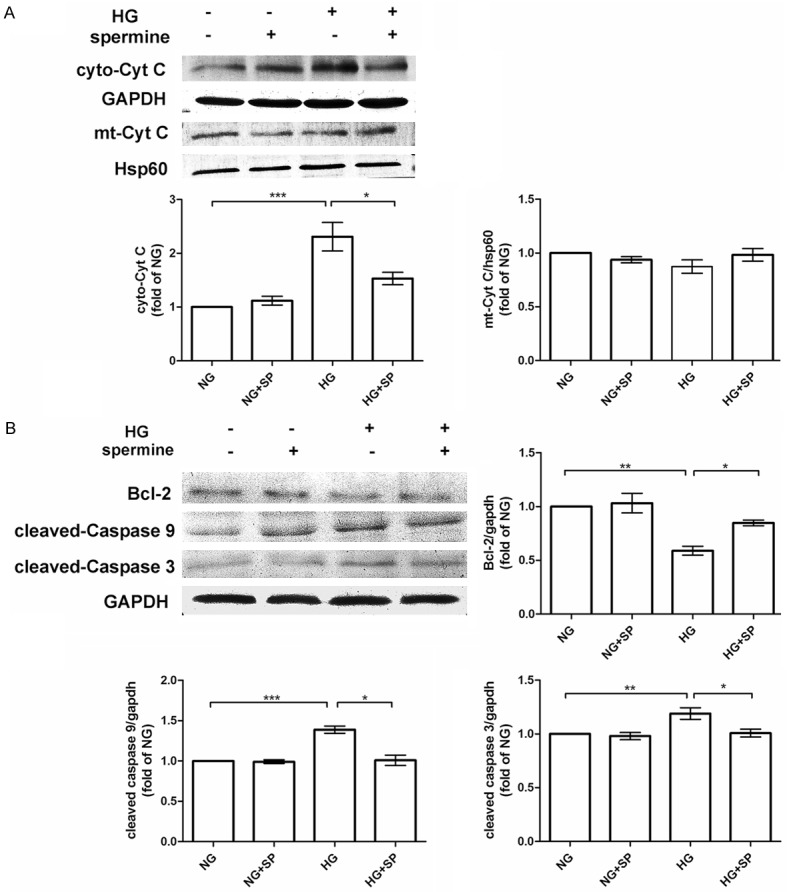
Effects of spermine on HG-induced cytochrome C release, bcl-2 decrease, and caspases activation in primary cardiomyocytes. A. Protein levels of cyto-Cyt C, mt-Cyt C in the NG, NG+SP, HG, HG+SP groups, as evaluated by western blot assay. GAPDH was used as a loading control for total cell lysate. Hsp60 was used as a loading for mitochondria (mt) protein. B. Protein levels of bcl-2 and cleaved caspase-3/9 in the four groups assessed by western blot assay. Quantification of cyto-Cyt C, mt-Cyt C, bcl-2, and cleaved caspase-3/9 expression compared with control protein. Data are presented as means ± SEM; n = 3 per group; *P<0.05 vs. control group (NG or HG group); **P<0.01 vs. control group; ***P<0.001 vs. control group.
Spermine reduced ROS generation in HG treated primary cardiomyocytes
It is well-known that ROS overproduction could impair cell [22]. The increase of ROS was due to both decreased or inadequate antioxidant and increased production in diabetic kidney disease [23]. So, first, whether spermine could increase antioxidative activity, we examined T-SOD, MDA, GSH and GSSG in HG-treated primary cardiomyocytes. After 48 h treatment, the total superoxide dismutase (T-SOD) activity in medium supernatant was markedly decreased in HG group compared to NG group (P<0.05) (Figure 4A), as well as the ratio of GSH/T-GSH (Figure 4C). Conversely, the increases of MDA contents, the index of lipid peroxidation, was also confirmed (P<0.05) (Figure 4B), consistent with GSSG/T-GSH ratio (P<0.05) (Figure 4D). Of interest, those changes were significantly ameliorated by spermine supplementation (P<0.05). Second, as shown in Figure 4E, treatment with HG for 48 h contributed to a marked elevation in accumulation of intracellular ROS in primary cardiomyocytes (P<0.001), compared with NG group. Nevertheless, pretreatment with spermine prior to HG exposure significantly attenuated intracellular ROS levels (P<0.01). Images (×200) were obtained, 10 random pictures for each group.
Figure 4.
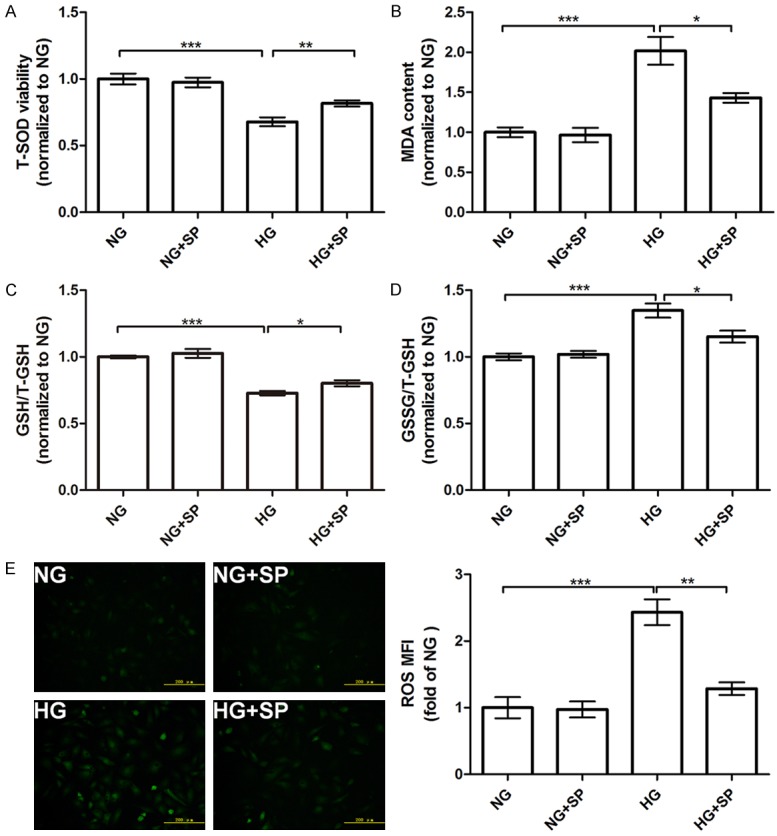
Effects of spermine on T-SOD, MDA, GSH, GSSG changes and ROS generation in HG-treated primary cardiomyocytes. A. T-SOD activity; B. MDA content; C. GSH/T-GSH ratio; D. GSSG/T-GSH ratio. All parameters were detected by the corresponding kits. E. DCFH-DA staining followed by photofluorography was carried out to observe intracellular ROS levels. The mean fluorescence intensity (MFI) of DCFH-DA staining of four groups in right panel was quantitative analysis with Image-Pro Plus software. Data are presented as mean ± SEM. *P<0.05, **P<0.01, ***P<0.001 vs. control group (NG or HG group).
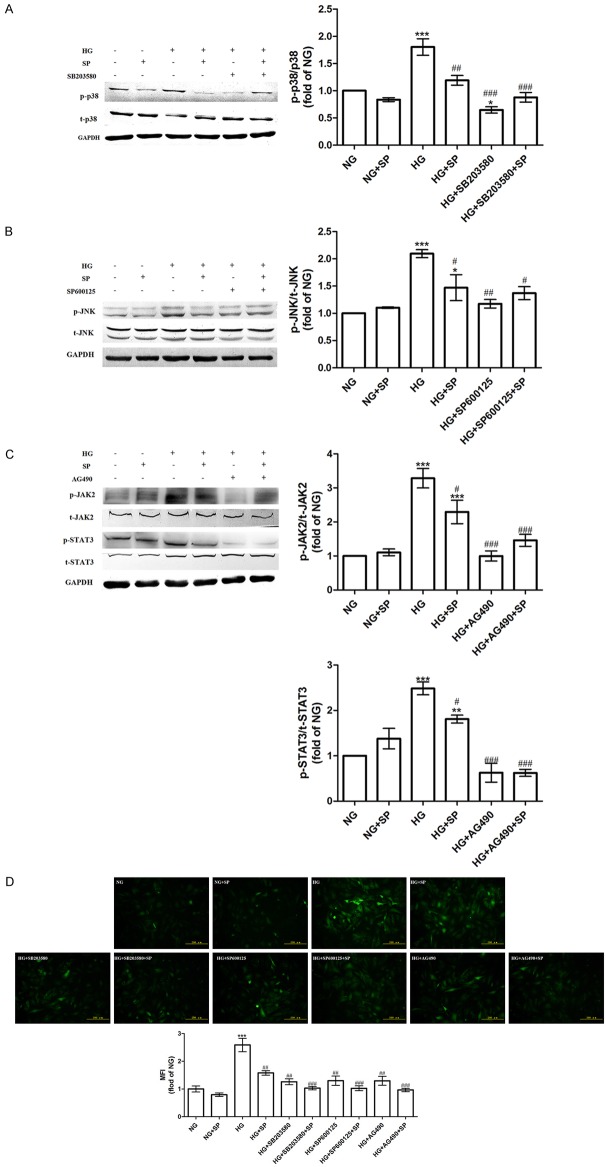
Spermine suppressed activation of p38/JNK and JAK2 to decrease ROS generation in primary cardiomyocytes in HG condition
It was reported that p38/JNK and JAK2/STAT3 pathways could be activated by ROS in HG condition [24-27]. However, p38/JNK and JAK2/STAT3 pathways were also relative to ROS generation induced by HG [17,18]. Here, to detect the corresponding mechanism of spermine on inhibiting excessive oxidative stress induced by HG, some upstream pathways of inducing ROS generation-p38/JNK and JAK2/STAT3 pathways were investigated. According to treatments for investigating relative signaling pathways mentioned above, as shown in Figure 5A-C, the protein expressions of phosphorylation p38 (p-p38), p-JNK, p-JAK2, p-STAT3 were significantly increased in HG group, while spermine-pretreatment apparently decreased the expressions of p-p38, p-JNK, p-JAK2, p-STAT3 (P<0.05), as well as pathway inhibitors (P<0.05). To explore the relationship between elevated ROS generation and p38/JNK and JAk2/STAT3 activation, the ROS production was assessed when the special pharmacological inhibitors (SB203580, SP600125 and AG490) or with spermine were given to the cells in HG condition. It was found that pretreatment of the cells with pharmacological inhibitors of p38, JNK and JAK2 pathways, respectively, blocked intracellular ROS accumulation induced by HG (P<0.05), as well as spermine (Figure 5D). However, compared pharmacological inhibitors and spermine group to pharmacological inhibitors group, there was a little decrease on ROS accumulation.
Figure 5.
Effects of spermine on activation of p38, JNK, JAK2 and STAT3 in HG-treated primary cardiomyocytes. A. Western blot analysis of p-p38 and p38. B. Western blot analysis of p-JNK and JNK. C. Western blot analysis of p-JAK2, JAK2 and p-STAT3 and STAT3. The statistical bar graph (right panel) showed the comparison of phospho-kinase levels that had been normalized to the total kinases levels. D. Primary cardiomyocytes were pretreated with p38 inhibitor SB203580, JNK inhibitor SP600125, and JAK2 pathway inhibitor AG490 or with spermine in HG condition and intracellular ROS level was measured. Data are presented as the means ± SEM; n = 3 per group; *P<0.05, **P<0.01, ***P<0.001 vs. NG group; #P<0.05, ##P<0.01, ###P<0.001 vs. HG group.
Spermine possessed better protective properties compared with NAC
To verify the antioxidative activity of spermine, we contrasted it to a well-known antioxidant-NAC [28]. First, we confirmed that ROS overproduction could induce cell apoptosis, as reported [24]. Here, we examined cleaved caspase3 expression. As shown in Figure 6A, NAC obviously decreased cardiomyocytes apoptosis induced by HG through scavenging ROS, compared with HG group (P<0.05). It revealed that ROS could lead to cell apoptosis with the reductio ad absurdum. Moreover, we found that spermine possessed better anti-apoptotic effect than NAC in HG-treated cardiomyocytes (P<0.05). Second, through examining ROS production, as shown in Figure 6B, spermine had better anti-oxidantive effect than NAC. When NAC and spermine were coexisting, the protective effects were more changeable than NAC group in HG condition, slightly ameliorative compared with spermine group in HG situation. This suggested that spermine and NAC might be synergistic action. Otherwise they may decrease ROS accumulation through different patterns or scavenging different types of ROS. Third, as shown in Figure 6C, when NAC was added, we observed that the phosphorylation of kinases was decreased in HG situation. However, this change in spermine group was more mutative than NAC group in HG condition. Moreover, the changes of phosphorylation protein expressions in HG with spermine group, HG with NAC group, and HG with NAC and spermine group were consistent with ROS examination.
Figure 6.
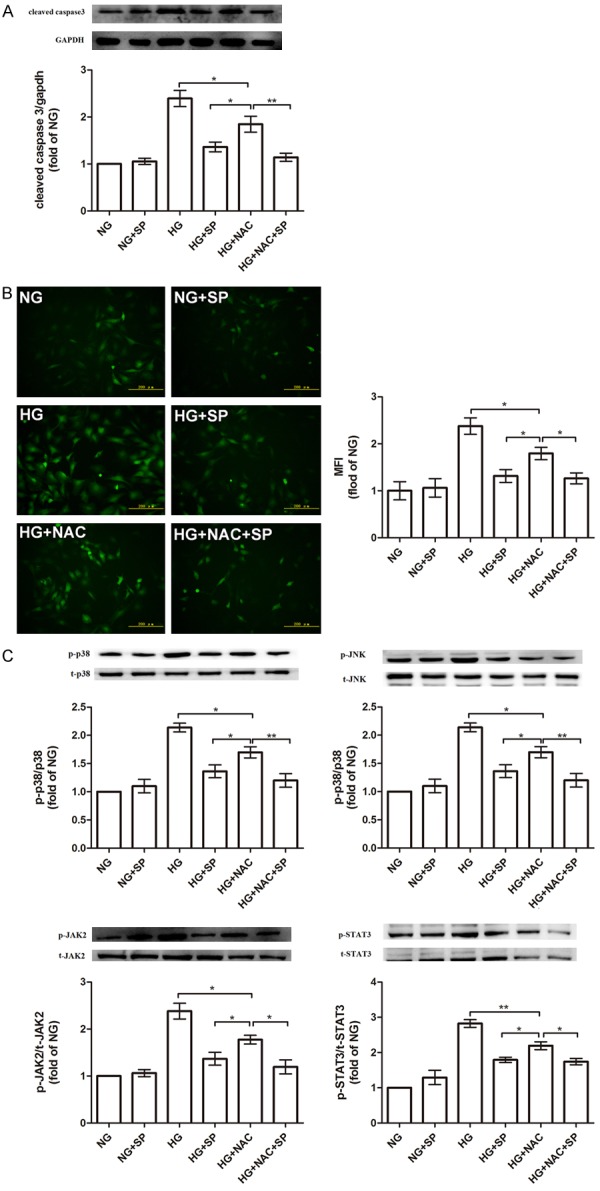
Examine the protective effect of spermine through comparing with NAC (a well-known antioxidant). A. the anti-apoptotic effect comparison with spermine and NAC. Cells were pretreated as described, and then the protein expression of cleaved caspase3 was examined. B. the antioxidantive effect comparison with spermine and NAC. Cells were pretreated as described, and then intracellular ROS level were analyzed. C. spermine inhibited the activation of p38/JNK and JAK2 pathways to decrease ROS generation, not only scavenged ROS to suppress p38/JNK and JAK2 pathways as NAC. Data are presented as the means ± SEM; n = 3 per group; *P<0.05, **P<0.01. control group (HG or HG+NAC group).
Discussion
In this study, spermine could decrease cardiomyocytes apoptosis induced by HG. It is widely-known that HG could lead to ROS overproduction, resulting in excessive oxidative stress. We found that spermine could significantly attenuate the expressions of phosphorylation form of these kinases (p38/JNK, and JAK2) to decrease ROS accumulation, consistent with their special inhibitors. And compared with NAC, a ROS scavenging agent, spermine exerted better antioxidative effect and anti-apoptotic effect. It has been reported that spermine is nature antiglycation agents, inhibits advanced glycation end products (AGEs) formation in diabetes mellitus [29]. Thus, spermine may decrease detrimental products-AGEs formation, and then alleviate cardiomyocytes injury in HG condition.
Some scientific workers reported that spermine could promote apoptosis or delay/resist apoptosis, depending on cell types [30]. In some physiologic apoptosis progress, spermine exerts protective effects on promoting apoptosis [31]. In contrast, in pathological condition, spermine could delay or prevent cell apoptosis [32]. Here, it was probable that spermine could decrease cardiomyocytes apoptosis induced by HG due to its special protective effects. However, further studies are needed to clarify this point. In the other hand, spermine could act as antioxidant due to its high content of positive charges amine [33,34]. Spermine is not only an antioxidant, but also essential for organization the structure and function of chromatin [35]. In the present work, when spermine was added, ROS generation was significantly decreased. It is possible that spermine neutralizes negative charges due to its positive charges amine. It was reported that spermine had a profound effect on lipoperoxidation, did not affect enzymatic activity [32]. In contrast, in the present study, when HG-treated cardiomyocytes were in presence of spermine, the lipoperoxidation level was decreased and the antioxidantive enzymatic activity (T-SOD and GSH) was increased. Here, spermine could increases enzyme scavenger to scavenge ROS.
In addition, NAC, a thiol-containing radical scavenger and glutathione precursor, is a broad-spectrum and short-term ROS scavenger [36]. Moreover, NAC could not affect pathways directly, as all known. In present work, we found that spermine could significantly inhibited the phosphorylation of kinases expressions, compared with NAC. We suspected that spermine could inhibit the activation of p38/JNK MAPK and JAK2 to decrease ROS formation, and the decrease of ROS could suppress the phosphorylation of p38/JNK MAPK and JAK2 conversely, as shown in Figure 7. Thereby, there may be a positive feedback to reduce ROS accumulation. Moreover, if spermine is metabolized in the cells, this feedback function maybe still work. But this viewpoint needs further investigated. Thus, the antioxidative property of spermine may be more effective and more targeting. The present study has several limitations. Firstly, it should be emphasized that these experiments were performed with primary cardiomyocytes in vitro. Thus, our observations cannot be fully speculated to the in vivo environment. Secondly, we focused on only three pathways of inducing ROS accumulation, but other pathways may also have a role. Further studies are required in the future.
Figure 7.
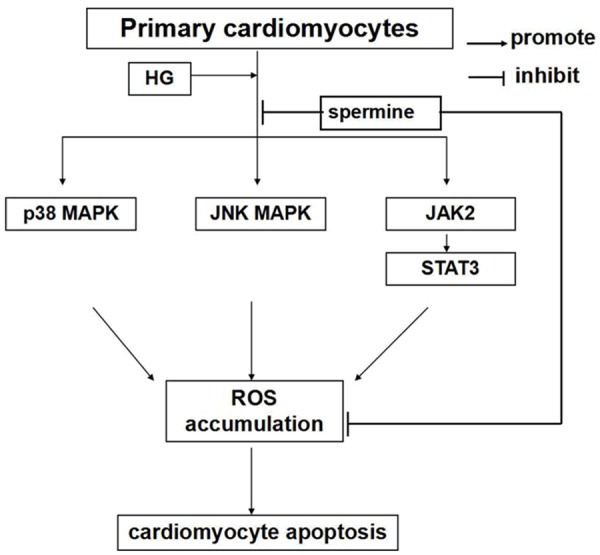
Diagram representing the proposed mechanism of antioxidative activity of spermine on HG induced ROS generation in cardiomyocytes. HG induced cardiomyocytes apoptosis by p38 mitogen-activated protein kinase (MAPK), JNK MAPK, and JAK2 pathways to increase ROS generation. Spermine could inhibit ROS production through reducing the activity of three pathways and scavenge ROS directly to decrease cardiomyocytes apoptosis.
Conclusion
The most important findings of this study demonstrate the antioxidative activity of spermine was more advantage over NAC. Spermine could not only scavenge ROS to decrease HG-induced cardiomyocytes injury via its natural property, but also inhibit p38/JNK MAPKs and JAK2 pathways to decline ROS formation. Therefore, spermine may be a new and effective antioxidant to treat diabetic cardiomyopathy.
Acknowledgements
This research is supported by the National Natural Science Foundation of China (No. 81270311).
Disclosure of conflict of interest
None.
References
- 1.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 2.Selvaraju V, Joshi M, Suresh S, Sanchez JA, Maulik N, Maulik G. Diabetes, oxidative stress, molecular mechanism, and cardiovascular disease-an overview. Toxicol Mech Methods. 2012;22:330–5. doi: 10.3109/15376516.2012.666648. [DOI] [PubMed] [Google Scholar]
- 3.Joshi M, Kotha SR, Malireddy S, Selvaraju V, Satoskar AR, Palesty A, McFadden DW, Parinandi NL, Maulik N. Conundrum of pathogenesis of diabetic cardiomyopathy: role of vascular endothelial dysfunction, reactive oxygen species, and mitochondria. Mol Cell Biochem. 2014;386:233–49. doi: 10.1007/s11010-013-1861-x. [DOI] [PubMed] [Google Scholar]
- 4.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–23. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 5.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee TI, Chen YC, Kao YH, Hsiao FC, Lin YK, Chen YJ. Rosiglitazone induces arrhythmogenesis in diabetic hypertensive rats with calcium handling alteration. Int J Cardiol. 2013;165:299–307. doi: 10.1016/j.ijcard.2011.08.072. [DOI] [PubMed] [Google Scholar]
- 7.Fillmore N, Mori J, Lopaschuk GD. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br J Pharmacol. 2014;171:2080–90. doi: 10.1111/bph.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palomer X, Salvado L, Barroso E, Vazquez-Carrera M. An overview of the crosstalk between inflammatory processes and metabolic dysregulation during diabetic cardiomyopathy. Int J Cardiol. 2013;168:3160–72. doi: 10.1016/j.ijcard.2013.07.150. [DOI] [PubMed] [Google Scholar]
- 9.von Bibra H, Hansen A, Dounis V, Bystedt T, Malmberg K, Ryden L. Augmented metabolic control improves myocardial diastolic function and perfusion in patients with non-insulin dependent diabetes. Heart. 2004;90:1483–4. doi: 10.1136/hrt.2003.020842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuentes-Antras J, Ioan AM, Tunon J, Egido J, Lorenzo O. Activation of toll-like receptors and inflammasome complexes in the diabetic cardiomyopathy-associated inflammation. Int J Endocrinol. 2014;2014:847827. doi: 10.1155/2014/847827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipinski B. Hydroxyl radical and its scavengers in health and disease. Oxid Med Cell Longev. 2011;2011:809696. doi: 10.1155/2011/809696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmood D, Jahan K, Habibullah K. Primary prevention with statins in cardiovascular diseases: A Saudi Arabian perspective. J Saudi Heart Assoc. 2015;27:179–91. doi: 10.1016/j.jsha.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luther JM, Brown NJ. The renin-angiotensin-aldosterone system and glucose homeostasis. Trends Pharmacol Sci. 2011;32:734–9. doi: 10.1016/j.tips.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miwa S, Watada H, Omura C, Takayanagi N, Nishiyama K, Tanaka Y, Onuma T, Kawamori R. Anti-oxidative effect of fluvastatin in hyperlipidemic type 2 diabetic patients. Endocr J. 2005;52:259–64. doi: 10.1507/endocrj.52.259. [DOI] [PubMed] [Google Scholar]
- 15.Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61:880–94. doi: 10.1002/iub.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nowotarski SL, Woster PM, Casero RA Jr. Polyamines and cancer: implications for chemotherapy and chemoprevention. Expert Rev Mol Med. 2013;15:e3. doi: 10.1017/erm.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai KH, Wang WJ, Lin CW, Pai P, Lai TY, Tsai CY, Kuo WW. NADPH oxidase-derived superoxide anion-induced apoptosis is mediated via the JNK-dependent activation of NF-kappaB in cardiomyocytes exposed to high glucose. J Cell Physiol. 2012;227:1347–57. doi: 10.1002/jcp.22847. [DOI] [PubMed] [Google Scholar]
- 18.Zheng Z, Chen H, Zhao H, Liu K, Luo D, Chen Y, Yang X, Gu Q, Xu X. Inhibition of JAK2/STAT3-mediated VEGF upregulation under high glucose conditions by PEDF through a mitochondrial ROS pathway in vitro. Invest Ophthalmol Vis Sci. 2010;51:64–71. doi: 10.1167/iovs.09-3511. [DOI] [PubMed] [Google Scholar]
- 19.Ji Q, Liu H, Mei Y, Wang X, Feng J, Ding W. Expression changes of ionic channels in early phase of cultured rat atrial myocytes induced by rapid pacing. J Cardiothorac Surg. 2013;8:194. doi: 10.1186/1749-8090-8-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong Y, Wu J, Huang Y, Shen S, Han X. Nonylphenol induces apoptosis in rat testicular Sertoli cells via endoplasmic reticulum stress. Toxicol Lett. 2009;186:84–95. doi: 10.1016/j.toxlet.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Jaruga E, Salvioli S, Dobrucki J, Chrul S, Bandorowicz-Pikula J, Sikora E, Franceschi C, Cossarizza A, Bartosz G. Apoptosis-like, reversible changes in plasma membrane asymmetry and permeability, and transient modifications in mitochondrial membrane potential induced by curcumin in rat thymocytes. FEBS Lett. 1998;433:287–93. doi: 10.1016/s0014-5793(98)00919-3. [DOI] [PubMed] [Google Scholar]
- 22.Styskal J, Van Remmen H, Richardson A, Salmon AB. Oxidative stress and diabetes: what can we learn about insulin resistance from antioxidant mutant mouse models? Free Radic Biol Med. 2012;52:46–58. doi: 10.1016/j.freeradbiomed.2011.10.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanton RC. Oxidative stress and diabetic kidney disease. Curr Diab Rep. 2011;11:330–6. doi: 10.1007/s11892-011-0196-9. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Mo H, Guo R, You Q, Huang R, Wu K. Inhibition of the leptin-induced activation of the p38 MAPK pathway contributes to the protective effects of naringin against high glucoseinduced injury in H9c2 cardiac cells. Int J Mol Med. 2014;33:605–12. doi: 10.3892/ijmm.2014.1614. [DOI] [PubMed] [Google Scholar]
- 25.Ong JY, Yong PV, Lim YM, Ho AS. 2-Methoxy-1,4-naphthoquinone (MNQ) induces apoptosis of A549 lung adenocarcinoma cells via oxidation-triggered JNK and p38 MAPK signaling pathways. Life Sci. 2015;135:158–64. doi: 10.1016/j.lfs.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Schieffer B, Luchtefeld M, Braun S, Hilfiker A, Hilfiker-Kleiner D, Drexler H. Role of NAD(P)H oxidase in angiotensin II-induced JAK/STAT signaling and cytokine induction. Circ Res. 2000;87:1195–201. doi: 10.1161/01.res.87.12.1195. [DOI] [PubMed] [Google Scholar]
- 27.Modesti A, Bertolozzi I, Gamberi T, Marchetta M, Lumachi C, Coppo M, Moroni F, Toscano T, Lucchese G, Gensini GF, Modesti PA. Hyperglycemia activates JAK2 signaling pathway in human failing myocytes via angiotensin IImediated oxidative stress. Diabetes. 2005;54:394–401. doi: 10.2337/diabetes.54.2.394. [DOI] [PubMed] [Google Scholar]
- 28.Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6:593–7. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 29.Gugliucci A, Menini T. The polyamines spermine and spermidine protect proteins from structural and functional damage by AGE precursors: a new role for old molecules? Life Sci. 2003;72:2603–16. doi: 10.1016/s0024-3205(03)00166-8. [DOI] [PubMed] [Google Scholar]
- 30.Seiler N, Raul F. Polyamines and apoptosis. J Cell Mol Med. 2005;9:623–42. doi: 10.1111/j.1582-4934.2005.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nitta T, Igarashi K, Yamashita A, Yamamoto M, Yamamoto N. Involvement of polyamines in B cell receptor-mediated apoptosis: spermine functions as a negative modulator. Exp Cell Res. 2001;265:174–83. doi: 10.1006/excr.2001.5177. [DOI] [PubMed] [Google Scholar]
- 32.Chirino-Galindo G, Mejia-Zepeda R, Palomar-Morales M. Change in lipoperoxidation but not in scavenging enzymes activity during polyamine embryoprotection in rat embryo cultured in hyperglycemic media. In Vitro Cell Dev Biol Anim. 2012;48:570–6. doi: 10.1007/s11626-012-9548-2. [DOI] [PubMed] [Google Scholar]
- 33.Belle NA, Dalmolin GD, Fonini G, Rubin MA, Rocha JB. Polyamines reduces lipid peroxidation induced by different pro-oxidant agents. Brain Res. 2004;1008:245–51. doi: 10.1016/j.brainres.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 34.Das KC, Misra HP. Hydroxyl radical scavenging and singlet oxygen quenching properties of polyamines. Mol Cell Biochem. 2004;262:127–33. doi: 10.1023/b:mcbi.0000038227.91813.79. [DOI] [PubMed] [Google Scholar]
- 35.Bjelakovic G, Stojanovic I, Jevtovic Stoimenov T, Pavlovic D, Kocic G, Rossi S, Tabolacci C, Nikolić J, Sokolović D, Bjelakovic LJ. Metabolic correlations of glucocorticoids and polyamines in inflammation and apoptosis. Amino Acids. 2010;39:29–43. doi: 10.1007/s00726-010-0489-3. [DOI] [PubMed] [Google Scholar]
- 36.Hung KY, Liu SY, Kao SH, Huang JW, Chiang CK, Tsai TJ. N-acetylcysteine-mediated antioxidation prevents hyperglycemia-induced apoptosis and collagen synthesis in rat mesangial cells. Am J Nephrol. 2009;29:192–202. doi: 10.1159/000155657. [DOI] [PubMed] [Google Scholar]