Abstract
Focal adhesion kinase (FAK) is known to promote the proliferation, migration and survival of synovial cells and plays an important role in the occurrence, development and pathological process of rheumatoid arthritis (RA). The aim of the present study was to observe FAK changes in synovial cells of rats with collagen-induced arthritis (CIA) and after intervention with disease modifying anti-rheumatic drugs (DMARDs) alone or in combination in a CIA female SD rat model induced by collagen type II. The rats were randomized to 8 groups: normal control group, CIA model control group, methotrexate (MTX, 0.9 mg/kg/w) group, cyclophosphamide (CTX, 24 mg/kg/3 w) group, leflunomide (LEF, 1.2 mg/kg/d) group, MTX + CTX group, LEF + CTX group, and MTX + LEF group. They were intervened with DMARDs alone or in combination for six weeks. The experiment lasted a total of 9 weeks in vivo. Articular inflammation was measured during the process of drug intervention in terms of the degree of swelling degree in the right hind foot using a venire caliper. All animals were sacrificed by breaking the neck after 9 weeks. Then, the ankle was fixed, decalcified, embedded, and HE stained, and prepared into slices to observe pathological changes in the synovial tissue. FAK expression in synovial cells was assayed by immunohistochemistry and the mean optical density (OD) value was measured using the HPIAS-2000 image analysis system. It was found that FAK expression was negative in normal control group, positive in CIA model control group, and decreased in the three DMARD combination treatment groups significantly as compared with that in the three single-drug groups (P < 0.05). FAK expression in LEF + CTX group or MTX + CTX group decreased more significantly than that in MTX + LEF group (P < 0.05), and there was no statistically significant difference between LEF + CTX and MTX + CTX groups. The arthritis index and pathological change in the synovial tissue in LEF + CTX group or MTX + CTX group were improved more significantly than those in MTX + LEF group or single-drug groups. Our results showed that FAK expression was positive in CIA rats, indicating that it played an important role in the pathogenesis of RA, and that intervention with DMARDs could reduce the FAK expression in synovial cells of CIA rats. We hope these findings would contribute to the treatment of RA and other rheumatic diseases by reducing adhesion, proliferation and migration of synovial cells and inhibiting the over-expression of FAK.
Keywords: Focal adhesion kinase, collagen-induced arthritis, rheumatoid arthritis (RA), disease modifying anti-rheumatic drug (DMARD)
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by irreversible joint destruction and disability. The main pathologic features of RA include neovascularization of joint synovial cells and formation of pannus devastating the joints. Fibroblast-like synoviocytes (FLS) are involved in the whole process of inflammation and angiogenesis of RA, resulting in damage to the joints [1,2]. FLS play a key role in the synovial pathological process of RA.
Focal adhesion kinase (FAK) is an intracytoplasmic nonreceptor tyrosine kinase widely expressed in vivo. It is in the intersection of multiple cell signaling pathways and possesses 2 multiple biological activities such as promoting cell proliferation, migration and survival. It also plays an important role in the occurrence, development and progression of RA [3]. The invasive growth of FLS in RA lies in their uncontrolled proliferation. As it is difficult to detect cell apoptosis in the synovial lining layer, FLS are supposed to have the potential of tumor-like proliferation. Based on the above understanding about FLS, we assume that they may prove to be a target for the treatment of RA by reducing the adhesion, proliferation and migration of synovial cells and inhibiting the expression of FAK.
There is a broad consensus about the combination of disease-modifying anti-rheumatic drugs (DMARDs) such as MTX + SSZ, MTX + LEF, MTX + LEF + SSZ for the treatment of RA. Our long-term clinical studies have demonstrated that the combination treatment of MTX or LEF with CTX have a good curative effect and a low rate of adverse reactions, and therefore we supposed that rotary combination of DMARDs would be a new strategy for the treatment of RA [4].
In this study, we observed the expression of FAK in synovial cells in a rat model of collagen-induced arthritis (CIA) after rotary combination of MTX, CTX and LEF, in an attempt to explore the mechanism of FAK in the pathogenesis of RA and the efficacy of rotary combination of DMARDs for the treatment of RA.
Materials and methods
Experimental animals and establishment of the CIA rat model [16-18]
Female SD rats of SPF grade weighing 160-180 g (Experimental Animal Center of Shanxi Medical University, Shanxi, China) were fed normal rat chow and tap water ad libitum with a 12:12 h light-dark cycle (lights on at 19:00 h) at a constant ambient temperature (23±2°C) and humidity (60%±5%). All experimental protocols were approved by the Experimental Animal Care and Use Committee of Shanxi Medical University.
The preparation of the immune mixture
Bovine collagen type II (5 mg) was dissolved in 2.5 ml glacial acetic acid (0.01 mol/L, pH 3.2), kept in the refrigerator at 4°C overnight, emulsionized with 2.5 ml Freund’s complete adjuvant repeatedly, and finally made into an emulsifiable liquid of 1 mg/ml bovine collagen type II.
The primary immunization
The rat was anesthetized with 5 ml/kg 5% chloral hydrate, shaven and sterilized routinely. The animals in the arthritis model group were given 0.5 ml emulsion via intradermal injection, 0.1 ml at one of the five points (three at the back, on at the tail root and one at the vola of any metapodium). Animals in the normal control group were injected with the same amount of saline solution.
Enhanced immunization
The primarily immunized animals were given 0.2 ml emulsion by intraperitoneal injection from the back in two weeks. The steps and methods are the same as previously described. The normal control group was injected with the same amount of saline solution.
Observation indexes including the clinical, X-ray and pathological findings were assessed regularly at the third, fifth and ninth week after immunization.
Rats were randomly divided into 8 groups: ① CIA model control group; ② methotrexate (MTX, 0.9 mg/kg/w) alone group; ③ leflunomide (LEF, 1.2 mg/kg/d) alone group; ④ cyclophosphamide (CTX, 24 mg/kg/3 w) alone group; ⑤ MTX+CTX group; ⑥ LEF+CTX group; ⑦ MTX+LEF group; and ⑧ normal control group without being modeled. Each group contained 10 animals except the CIA model control group (n=9). Medication was started three weeks after the first immunization for six consecutive weeks in all medical intervention groups, and the normal control and CIA model control groups were given the same amount of saline solution via gavage. All animals were sacrificed 9 weeks after immunization.
Measurement observation
Arthritis index
The evaluation of arthritis index (AI) mainly involved the hind ankle joints. The index was observed once before primary immunization, at a 3-day interval in the acute stage for 6 weeks after primary immunization, and then weekly in the chronic stage. Four points were selected for each of the four leg (0 point, no inflammation and joint swelling; 1 point, red spots or mild swelling; 2 points, moderate joint swelling; 3 points, severe joint swelling; 4 points, joint rigidity, deformity or severe dysfunction), totaling 16 points for each animal. Acute arthritis was represented reaching 6-8 points. The degree of joint welling was measured on the basis of the diameter of the right ankle with avernier caliper. The index was observed one time before the primary immunization, and then weekly in the chronic stage.
Pathologic evaluation
All animals were sacrificed 9 weeks after primary immunization. Bilateral ankles were fixed with 10% neutral formalin for 24 h, and decalcified with 10% EDTA for 30 days. After that, ankles were incised longitudinally, paraffin embedded and HE stained for the observation of joint inflammation.
The measurement of FAK expression by immunohistochemistry
The paraffin-embedded sections were dewaxed and dehydrated. Then, the tissue was given effective antigen repair by addition of primary and secondary antibody, incubated for 30 min at room temperature, stained with hematoxylin, DBA developed, and observed for FAK expression under a microscope (×100) by randomly choosing five views. FAK was positive if brownish-yellow granules appeared in the cell membrane and cytoplasm.
Statistical analysis
Enumeration data are expressed with mean ± standard deviation (SD). Comparison between two groups was performed by t test. P < 0.05 was considered statistically significant. Data with normal distribution were analyzed by Kolmogorov-Smirnov test (P > 0.1 conforming to normality). Whether variance was agreed was tested by Levene’s T test (P > 0.1 conforming to constant variances). Multi-group quantitative data were analyzed by single factor analysis of variance. Comparison between two groups was performed by LSD method. Data with non-normal distribution were analyzed by Wilcoxon rank sum test. SPSS 13.0 software was used for statistical analysis, with P < 0.05 indicating a statistically significant difference (double side).
Results
Successful establishment of the CIA rat model
Chronic progressive symmetric peripheral arthritis was always associated with systemic symptoms (Figure 1), pathologically characterized by hyperplastic synovitis with destruction of the cartilage and bone (Figure 2). Osteoporosis, destruction of the cartilage and bone, narrowing of the joint space and destruction and fusion of the joints were observed on radiographs (Figure 3).
Figure 1.
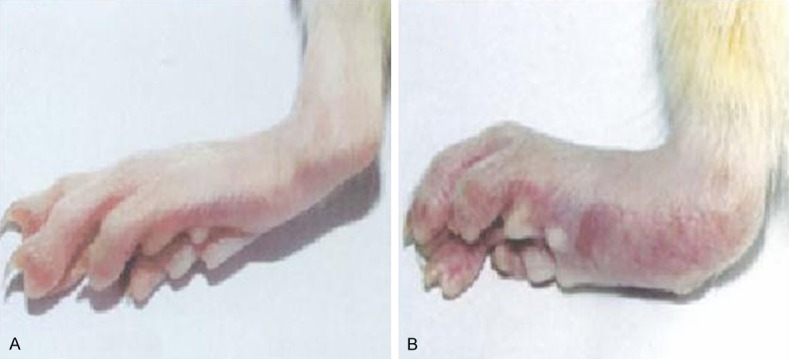
Morphological comparison of the joints between the CIA model group and the normal control group. Articular swelling in CIA model group (B) is more serious than that in normal control group (A).
Figure 2.
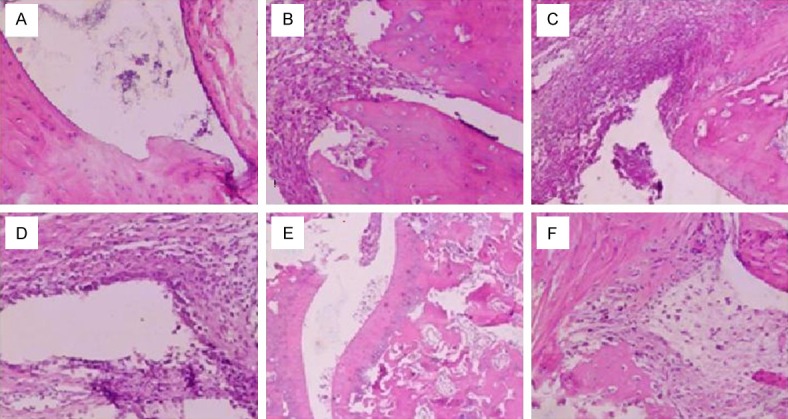
Histopathology in CIA model group is different from that in normal control group. A. The synovial membrane structure is clear in normal control group, without hyperplasia and infiltration of inflammatory cells. B. The synovial membrane structure is deranged in CIA model group. C. Synovial cells proliferate in CIA model group. D. There is obvious infiltration of inflammatory cells in CIA model group. E, F. There is formation of the pannus and destruction of the cartilage and bone in the synovial membrane.
Figure 3.
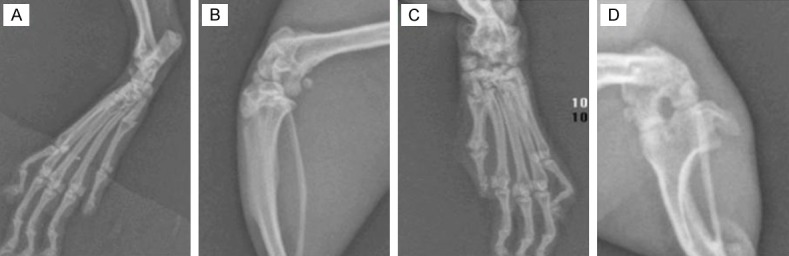
X-ray expression of joints: the ankle (A) and the knee (B) are normal in normal control group. Destruction of the cartilage and bone, and narrowing of the joint space or even fusion are observed in the ankle (C) and knee (D) of CIA model rats after 9 weeks.
Swelling and arthritis index (AI) observation of rats in CIA model and medical intervention groups
AI in all medical intervention groups was statistically different from that in the CIA model control group (P < 0.01). AI in MTX + CTX group and LEF + CTX group was minimal in all experimental groups, and was statistically different from that in MTX, LEF and CTX alone groups and MTX + LEF group (P < 0.05). The degree of metapodial swelling in all intervention groups was statistically different from that in CIA model control group (P < 0.05). Metapodial swelling in all three combination treatment groups was improved the most markedly as compared with the other groups (P < 0.05) (Table 1). AI in MTX + CTX group and LEF + CTX group was minimal, followed by MTX + LEF group after 9 weeks (Figures 4, 5).
Table 1.
AI comparison in the extremities of CIA rats
| Group | N | AI (Mean ± SD) | |||
|---|---|---|---|---|---|
|
|
|||||
| 0 W | 3 W | 5 W | 9 W | ||
| A | 8 | 0 | 7.06±0.21 | 7.79±1.15 | 7.63±0.89 |
| B | 10 | 0 | 6.85±0.16 | 7.33±1.12 | 5.80±0.62a |
| C | 9 | 0 | 6.95±0.23 | 7.29±0.78 | 5.77±0.87a |
| D | 9 | 0 | 6.78±0.32 | 6.91±0.81 | 5.98±0.79a |
| E | 10 | 0 | 6.90±0.25 | 6.21±0.64 | 3.71±0.93a,b,c |
| F | 10 | 0 | 6.83±0.29 | 6.34±0.61 | 3.87±0.66a,b,c |
| G | 10 | 0 | 6.95±0.27 | 6.67±0.68 | 4.73±0.82a,b |
A: CIA model control group; B: MTX group; C: LEF group; D: CTX group; E: MTX + CTX group; F: LEF + CTX group; G: MTX + LEF group; H: normal control group.
P < 0.05;
P < 0.01 vs. 3 W;
P < 0.05 vs. 5 W.
Figure 4.
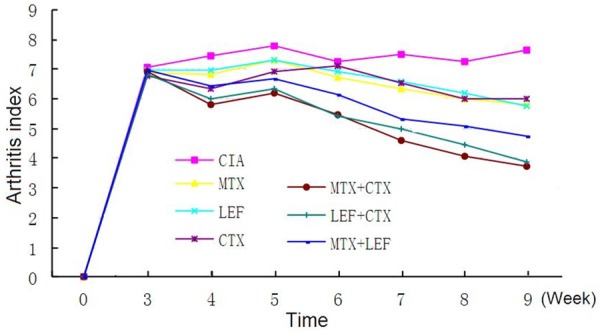
Dynamic observation of the arthritis index in CIA rats for 9 weeks.
Figure 5.
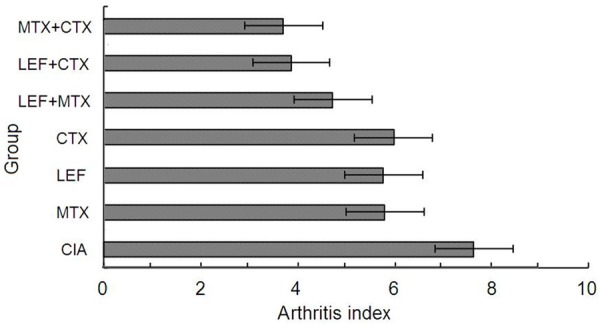
Comparison of the arthritis index in CIA rats at the 9th week. AI in MTX + CTX group and LEF + CTX group is the lowest of the three combination treatment groups after 9 weeks.
Morphological observationsin CIA and medical intervention groups after HE staining
The synovial membrane structure was clear without hyperplasia in the normal control group. There was no obvious infiltration of lymphocytic cells and plasma cells inthe synovial membrane. The level of synovial cells in CIA model group was increased to 6-10 layers significantly, where cells were deranged with obvious infiltration of inflammatory cells, formation of pannus and destruction of the cartilage and bone in the synovial membrane. The synovial membrane structure disorder in single drug groups was less than that in CIA model groups. The synovial cell of level in single drug groups reaches 3-4 layers with cell derangement and severe infiltration of inflammatory cells, and destruction of the cartilage and bone. The condition in the three combination treatment groups was improved significantly as compared with CIA model group. There was no obvious hyperplasia of synovial cells (1-2 layers), or cell derangement. In addition, the degree of inflammatory cell infiltration and destruction of the cartilage and bone were also improved markedly (Figure 6).
Figure 6.
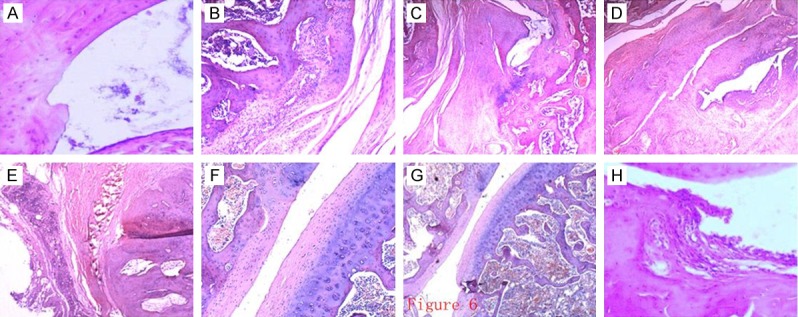
Morphological observation of each group after HE staining. (A) The synovial membrane structure is clear in normal control group, without hyperplasia and evidence of inflammatory cell infiltration. (B) The level of synovial cells is increased, with deranged cells and obvious infiltration of inflammatory cells in CIA model group. The synovial membrane structure disorder of single drug group using MTX (C), LEF (D) or CTX (E) relieved compared with CIA model group. The condition of the cycle combination group using LEF+CTX (F), MTX+CTX (G) and MTX+LEF (H) relieved compared with the single-drug groups.
Comparison of FAK expression by immunohistochemistry in CIA and medical intervention groups
The expression of FAK was negative in normal control group; strongly positive in CIA model control group; decreased in single medication treatment groups as compared with CIA model group; and further decreased in the three combination treatment groups as compared with the three single treatment groups (Figure 7).
Figure 7.
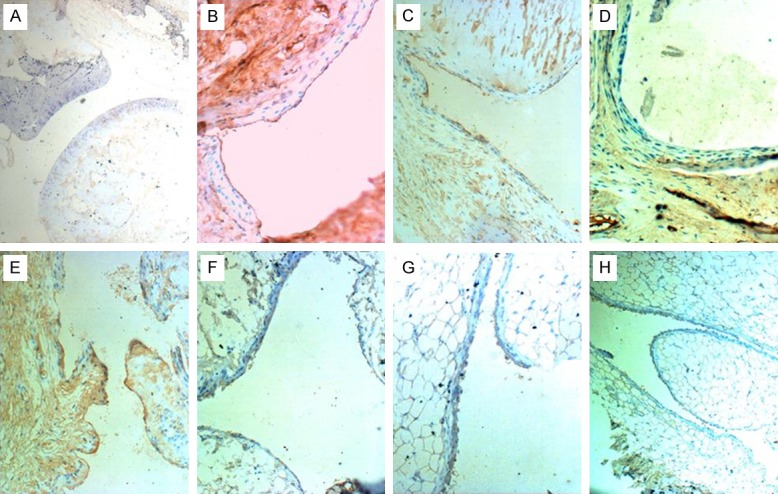
Comparison of FAK expression in all groups by immunohistochemistry. (A) The expression of FAK is negative in normal control group. (B) FAK expression is excessive and strongly positive in CIA model control group. FAK in the single-drug groups using MTX (C), LEF (D) or CTX (E) alone is positive and decreased as compared with CIA model group. FAK is weakly positive in DMARD combination treatment groups using LEF+CTX (F), MTX+CTX (G) and MTX+LEF (H) and decreased significantly as compared with the single-drug groups.
Comparison of FAK expression between the normal and CIA model control groups in terms of the mean optical density
The mean OD in CIA model control group was significantly higher than that in normal control group (P < 0.05) (Table 2).
Table 2.
Comparison of FAK expression between normal and CIA model control groups in terms of mean OD
| Group | N | Mean ± SD | F | P |
|---|---|---|---|---|
| G | 8 | 2.645±0.425 | 70.319 | 0.000 |
| H | 10 | 1.234±0.322 |
Comparison of FAK expression between CIA model control group and the three combination medical treatment groups in terms of the mean optical density
The mean OD in the three combination medical treatment groups was lower than that in CIA model control group (P < 0.05) (Table 2). In addition, LEF + CTX and MTX + CTX groups were statistically different from MTX + LEF group (P < 0.05), and there was no significant different between LEF + CTX and MTX + CTX groups (Table 3).
Table 3.
Comparison of FAK expression between CIA model control group and the three combination treatment groups
| Group | N | Mean ± SD | F | P |
|---|---|---|---|---|
| A | 8 | 2.645±0.425 | 15.236 | 0.000 |
| E | 10 | 1.954±0.233 | ||
| F | 10 | 2.003±0.091 | ||
| G | 10 | 2.360±0.169 |
Comparison of FAK expression between the three single-drug groups and the three combination medical treatment groups in terms of the mean optical density
There was no statistically significant difference in FAK expression between the three single-drug (MTX, LEF or CTX) groups and the three combination medical treatment (MTX + LEF, LEF + CTX or MTX + CTX) groups (P < 0.05) (Table 4).
Table 4.
The average optical density Comparison of FAK expression between the three single-drug groups and the three combination treatment groups in terms of RFI
| Group | N | Mean ± SD | F | P |
|---|---|---|---|---|
| B | 10 | 2.240±0.207 | 4.481 | 0.002 |
| C | 9 | 2.265±0.409 | ||
| D | 9 | 2.236±0.225 | ||
| E | 10 | 1.954±0.233 | ||
| F | 10 | 2.003±0.091 | ||
| G | 10 | 2.360±0.169 |
Discussion
Focal adhesion kinase was first discovered in chicken embryo fibroblast cells transfected with V-Src by Schaller et al. [5], and later it was found widely existing in vivo. It is a highly conserved protein in evolution, with tyrosine kinase activity. It is activated by quickly assembled and tyrosine phosphorylation when stimulated by the cancer gene or integrin. Activated FAK can phosphorylate a variety of downstream molecules by binding with them and participate in many signaling pathways by activating the downstream pathways [6]. FLS plays a key role in the synovial pathological process of RA [1,2] and is involved in the whole process of inflammation and angiogenesis. FLS also has the characteristics of tumor-like proliferation. FLS are a major constituent of the hyperplastic synovial pannus and the extensive expression of the variant surviving protein in the pannus would induce the pannus to undergo abnormal apoptosis, invading and damaging the joint. Thus, unbalance of proliferation and apoptosis is the basic reason of synovial hyperplasia in RA. FAK inhibits cell apoptosis [7], causing hyperplasia of FLS by preventing apoptosis, leading to destruction of the cartilage and bone. Therefore, FAK may play an important role in the development of RA.
Immunohistochemistry of the present study showed that expression of FAK was strongly positive in the CIA model control group, and negative in the normal control group, and the difference in the mean OD of FAK expression between the two groups is statistically significant. Lambetal [16] used immunoblot analysis and Western blot to study the expression of pFAK, pPyk2, pSrc, pPaxillin and pPLC phosphor-marked in RA and normal synovial cells and found that the expressions of these proteins were increased in RA synovial cells as compared with those in RA synovial tissues, especially in RA synovial cells treated by TNF2α. These proteins are important to cell adhesion, migration and differentiation of macrophages into osteoclasts. Matsumoto et al. [9] demonstrated that intra-articular infection of FAK-negative adenovirus could reduce monocyte recruitment in rat adjuvant-induced arthritis (AIA). Studies [10] have shown that FAK can promote inflammatory angiogenesis and is involved in the formation of RA synovial pannus, which is consistent with the results of the present study, suggesting that FAK plays an important role in synovial hyperplasia and development of RA.
RA is a systemic autoimmune disease characterized by irreversible joint destruction and disability. There is a broad consensus about the combination use of DMARDs (such as MTX + SSZ, MTX + LEF and MTX + LEF + SSZ) in the treatment of RA, especially in the initial stage of the disease. Since the early 1990s, our department had conducted studies on a variety of DMARDs combination schemes and developed a new therapeutic protocol of DMARDs combination based on the dynamics of cell proliferation by referring to the successful experience of combination chemotherapy in leukemia [4]. The new therapeutic protocol mainly included the use of the cycle specificity drug MTX or LEF in combination with the cycle non-specific drug CTX. The drugs are given intermittently in small doses. The new therapeutic protocol shows a better curative effect and a lower adverse reaction rate in the treatment of RA as compared with the other therapeutic protocols. As MTX/LEF and CTX act in different cell cycles without overlapping side effects and all drugs are given intermittently in small doses, there are fewer adverse reactions and it also gives normal tissues and organs the time to recover. For example, the incidence of liver damage in MTX + CTX group is only 8.5%, which is much lower than 35% in MTX + LEF group, indicating that the cycle combination protocol has good security.
Our previous study [11-14] found that the apoptosis of synovial cells and the expression of mutant p53 and cyclin D1 mRNA were reduced more effectively in MTX + CTX group as compared with those in the single-drug group. Variance analysis with factorial design confirmed the synergistic effect between MTX and CTX. This study showed that FAK expression of synovial cells was enhanced in collagen-induced arthritis rats, and reduced by DMARD intervention in the combination treatment groups, especially in LEF + CTX and MTX + CTX groups, as compared with that in single-drug and MTX + LEF groups. AI and synovial pathological changes were also improved significantly in LEF + CTX and MTX + CTX groups. These findings are consistent with the results of previous studies [11-14]. FAK can degrade p53 via the ubiquitin pathway. P53 protein as a transcription factor plays an important role in regulating cell cycle, inducing apoptosis and preventing proliferation of abnormal cells through regulation on the transcription and expression of a series of downstream target genes. Studies [15] have shown that p53, especially mutant p53, plays a key role in the pathogenesis of RA, suggesting that the MTX + CTX group may work on RA through inhibiting the expression of mutant p53 and proliferation of synovial cells, and inducing apoptosis. Presumably, FAK degrades p53 by preventing apoptosis through the FAK-p53 pathway. The synergistic effect of CTX and LEF/MTX combination in controlling the occurrence and development of RA may be through blocking the FAK-p53 pathway, inducing apoptosis and inhibiting the proliferation of synovial cells via inhibition of FAK expression.
FAK plays an important role in the hyperplasia of synovial cells and the occurrence and development of RA. Regulation of FAK activity directly affects cell apoptosis. The therapeutic effect of DMARDs is associated with the inhibition of FAK expression in synovial cells. The therapeutic protocol using MTX or LEF in combination with CTX has a better curative effect and a lower adverse reaction rate in RA treatment as compared with the other therapeutic protocols. We hope that the findings of the present study would evoke a new breakthrough in the treatment of RA and other rheumatic diseases by reducing adhesion, proliferation and migration of synovial cells and inhibiting the over-expression of FAK.
Acknowledgements
We’d like to thank the laboratory of the rheumatism department of the Second Hospital of Shanxi Medical University.
Disclosure of conflict of interest
None.
Authors’ contribution
QL, QZS, XCZ, WPZ and TTZ carried out the immunoassays. HYG, HYW and JL participated in the design of the study and performed the statistical analysis. JMZ and XFL conceived of the study, and participated in its design and coordination and helped draft the manuscript. All authorshave read and approved the final manuscript.
Abbreviations
- FAK
Focal adhesion kinase
- CIA
Collagen induced arthritis
- DMARDs
Disease modifying anti-rheumatic drugs
- RA
Rheumatoid arthritis
- SD
Sprague-Dawley
- MTX
Methotrexate
- CTX
Cyclophosphamide
- LEF
Leflunomide
- SSZ
Sulphasalazine
- FLS
Fibeblast like synovioeytes
- AIA
Adjuvant induced arthritis
- OA
Osteoarthritis
- SLE
Systemic lupus erythematosus
References
- 1.Szekanecz Z, Koch AE. Angiogenesis and its targeting in rheumatoid arthritis. Vascul Pharmacol. 2009;51:1–7. doi: 10.1016/j.vph.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ospelt C, Gay S. The role of resident synovial cells in destructive arthritis. Best Pract Res Clin Rheumatol. 2008;22:239–252. doi: 10.1016/j.berh.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Lv Q, Song QZ, Gao HY, Li XF. The research of focal adhesion kinase in rheumatoid arthritis. Chin J Rheumatol. 2013;17:88–90. [Google Scholar]
- 4.Li XF. The new idea of cycle combination therapy for rheumatoid arthritis. Chin J Rheumatol. 2008;12:491–492. [Google Scholar]
- 5.Meng XN, Wang BC, Jin Y. Focal adhesion kinase. Int J Genetics. 2007:394–397. [Google Scholar]
- 6.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 7.van Nimwegen MJ, Huigsloot M, Camier A, Burg D, van de Water B. Focal adhesion kinase and protein kinase B cooperate to suppress doxorubicin-induced apoptosis of breast tumor cells. Mol Pharmacol. 2006;70:1330–1339. doi: 10.1124/mol.106.026195. [DOI] [PubMed] [Google Scholar]
- 8.Lamb J, Ramaswamy S, Ford HL, Contreras B, Martinez RV, Kittrell FS, Zahnow CA, Patterson N, Golub TR, Ewen ME. A mechanism of cyclinD1 action encoded in the patterns of gene expression in human cancer. Cell. 2003;114:323–334. doi: 10.1016/s0092-8674(03)00570-1. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto Y, Tanaka K, Hirata G, Hanada M, Matsuda S, Shuto T, Iwamono Y. Possible involvement of the vascular endothelial growth factor-Flt-1-focal adhesion kinase pathway in chemotaxis and the cell proliferation of osteoclast precursor cells in arthritic joints. J Immunol. 2002;168:5824–5831. doi: 10.4049/jimmunol.168.11.5824. [DOI] [PubMed] [Google Scholar]
- 10.Shahrara S, Castro-Rueda HP, Haines GK, Koch AE. Differential expression of the FAK family kinases in rheumatoid arthritis and osteoarthritis synovial tissues. Arthritis Res Ther. 2007;9:R112. doi: 10.1186/ar2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li XF, Zhang LY, Niu HQ, Ru JL, Wang CH, Gao JF. Evaluating the short-term efficacy and safety of methotrexate and cyclophosphamide in patients with rheumatoid arthritis: a multi-center, randomized, single-blinded and controlled study. Abstract from the 13th Congress of the Asia Pacific League of Associations. The 13th Congress, A133. [Google Scholar]
- 12.Wang CH, Li XF, Zhang FC, Liang ME, Qu SJ. Effect of combination MTX with CTX on rats of collagen-induced arthritis. Chin J Rheumatol. 2009;13:581–582. [Google Scholar]
- 13.Qu SJ, Li XF, Wang CH, Liang ME. Effect of combination MTX with CTX on p53 expression in FLS of CIA rats. Chin J Rheumatol. 2009;13:42–44. [Google Scholar]
- 14.Li XF, Zhang LY, Niu HQ, Ru JL, Wang CH, Gao JF. Simpleblind random controlled clinical trial of combination MTX with CTX on rheumatoid arthritis. Chin J Rheumatol. 2010;14:110–114. [Google Scholar]
- 15.Ru JL, Li XF, Wang X, Niu HQ, Zhang LY. Effect of combination MTX with CTX on cellcycle and cyclinD1 in marrow and peripheral blood lymphocyte of rats. Chin J Rheumatol. 2009;13:541–544. [Google Scholar]
- 16.Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146:857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brand DD, Kang AH, Rosloniec EF. The mouse model of collagen-induced arthritis. Methods Mol Med. 2004;102:295–312. doi: 10.1385/1-59259-805-6:295. [DOI] [PubMed] [Google Scholar]
- 18.Schett G, Middleton S, Bolon B, Stolina M, Brown H, Zhu L, Pretorius J, Zack DJ, Kostenuik P, Feige U. Additive bone-protective effects of anabolic treatment when used in conjunction with RANKL and tumor necrosis factor inhibition in two rat arthritis models. Arthritis Rheum. 2005;52:1604–1611. doi: 10.1002/art.21021. [DOI] [PubMed] [Google Scholar]


