Abstract
Recent research demonstrates that the underlying mechanism in immune thrombocytopenia (ITP) is very complex. Lymphocyte function associated antigen-1 (LFA-1) plays important roles in autoimmune diseases. The purpose of this study was to investigate the expression of CD11a on lymphocytes and explore its possible role in ITP. The expression of CD11a on lymphocyte subpopulations (CD3+ T cells, CD3+CD4+ T cells, CD3+CD4- T cells, CD4+Foxp3+ T regulatory cells and CD19+ B cells) were analyzed by flow cytometry. Specific anti-platelet GPIIb/IIIa and/or GPIb/IX autoantibodies were assayed by modified monoclonal antibody specific immobilization of platelet antigens (MAIPA). The mean fluorescence intensity of CD11a on CD3+ T, CD3+CD4- T and CD19+ B lymphocytes were increased in ITP patients compared to healthy controls. No significant difference of CD11a expression on CD3+CD4+ T cells or CD4+Foxp3+ T regulatory cells was found between ITP patients and controls. Our data indicates the possible role of CD11a in the pathogenesis of ITP.
Keywords: Immune thrombocytopenia, LFA-1, CD11a, B cells, T cells
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disease characterized by low platelet counts with or without mucocutaneous bleeding [1]. Like the majority of autoimmune diseases, ITP is an organ-specific disease and abnormalities in the regulation of immune system have been shown to play an important role in the initiation and/or perpetuation of the disease [2]. Autoantibodies reacting against platelet glycoproteins can mediate platelet destruction by the monocyte-macrophage system as well as suppress megakaryocyte proliferation and maturation [3]. Although autoreactive B lymphocytes secreting antiplatelet antibodies are considered as the main defect, substantial evidence suggests that a generalized dysfunction of autoreactive T cells is the critical immunopathological cause of ITP and the antiplatelet autoantibodies are under the control of T cells and the cytokines they produce [4]. In the current management of ITP, corticosteroids and intravenous immunoglobulin are the first-line choice, and many drugs including rituximab have also been tried as second-line treatments. However, the curative effect is unsatisfactory.
Lymphocyte function associated antigen-1 (LFA-1) belonging to the integrin family is composed of the alpha chain CD11a and beta chain CD18 heterologous dimers [5], and expressed on the surface of T lymphocytes, B lymphocytes, monocytes, macrophages and neutrophils. Its major ligand, intercellular adhesion molecule-1 (ICAM-1), belongs to the immunoglobulin superfamily, distributed on the surface of fibroblasts, endothelial cells and activated lymphocytes. The combination of LFA-1 and ICAM-1 can provide coordinated stimulus signal and promote lymphocyte activation, proliferation and differentiation. In the interaction of T cells with antigen-presenting cells (APCs), LFA-1 and its adaptor ICAM-1 directly participate in the formation of immunological synapse that promotes costimulatory function, leading to increased T cell proliferation and cytotoxicity [6]. They also take part in a variety of lymphocytic homing process. In addition, the interaction of LFA-1 with ICAM-1 can also mediate a series of inflammatory responses [7]. CD11a is critical for lymphocyte entry into the lymph nodes [8] and normal development of hematopoietic intermediates [9]. The disruption of LFA-1 activity strongly affects the stability of immune interface [10]. Therefore, LFA-1 plays important roles in inflammatory, immune responses and antigen presentation.
The expression of ICAM-1 and LFA-1 is significantly higher on lymphoid cells and vascular endothelial cells in rheumatoid arthritis (RA), indicating that the combination of LFA-1 and ICAM-1 may play an important role in the progression of RA [11]. The excessive expression of LFA-1 can induce the formation of auto-reactive T cells, resulting in lupus disease in mice. By using LFA-1 monoclonal antibodies in lupus mice the production of autoantibodies could be reduced, the development of autoimmune reaction stopped, and the symptoms of lupus nephritis alleviated [12]. Therefore, LFA-1 may play an important role in the pathogenesis of systemic lupus erythematosus. It has been reported that CD11a was elevated in ITP patients and CD11a could facilitate the survival of CD19+ B cells and promote antibody-mediated platelets destruction [13]. In this study we analyzed the CD11a expression on CD4+ Foxp3+ T cells, CD3+CD4+ T cells, CD3+CD4- T cells and CD19+ B cells, respectively, and compared its expression between ITP patients and healthy controls.
Materials and methods
Patients
Thirty-four patients (eighteen females and sixteen males with a median age of 45 years, Table 1) admitted to our wards were studied. Patients with diabetes, heart disease, pregnancy or any other autoimmune disease were excluded. The diagnosis of ITP was based on the recently reported criteria [14]. Nineteen healthy controls with matched sex and age were also studied. Enrollment took place between February 2013 and January 2014 at the Department of Hematology of our hospital. Informed consent was obtained from each participant. Ethical approval for the study was obtained from the Medical Ethical Committee.
Table 1.
Clinical characteristics of ITP patients
| Patients | Gender | Age (year) | PLT count (× 109) | Anti-GPIIb/IIIa anti-GPIb/IX | Bleeding symptoms |
|---|---|---|---|---|---|
| 1 | Male | 46 | 15 | -/+ | PT, EC |
| 2 | Male | 52 | 25 | -/- | EC, GUH |
| 3 | Male | 25 | 40 | +/- | EC |
| 4 | Female | 57 | 10 | -/- | PT, EC |
| 5 | Female | 24 | 2 | +/- | PT, GH |
| 6 | Male | 39 | 1 | -/- | EC, GU |
| 7 | Male | 46 | 4 | -/+ | EC, GUH |
| 8 | Male | 42 | 16 | +/+ | PT |
| 9 | Male | 19 | 55 | -/- | EC |
| 10 | Female | 66 | 14 | -/- | PT, EC |
| 11 | Female | 49 | 48 | +/- | PT |
| 12 | Male | 41 | 33 | +/+ | EC |
| 13 | Female | 75 | 2 | -/+ | EC, GU |
| 14 | Female | 61 | 5 | -/- | PT, GH |
| 15 | Female | 53 | 6 | +/- | GH |
| 16 | Female | 58 | 10 | -/+ | PT, EC |
| 17 | Fale | 36 | 35 | -/- | EC |
| 18 | Female | 38 | 25 | -/- | GU |
| 19 | Female | 26 | 23 | -/- | PT, EC |
| 20 | Female | 39 | 9 | +/+ | PT, GH |
| 21 | Female | 51 | 14 | -/- | GH |
| 22 | Male | 40 | 22 | -/+ | GUH |
| 23 | Female | 52 | 50 | -/- | EC |
| 24 | Male | 43 | 3 | -/- | EP, GU |
| 25 | Male | 24 | 0 | +/+ | EC, EP, GU |
| 26 | Male | 46 | 7 | -/+ | EC, EP |
| 27 | Female | 39 | 2 | -/- | EP, GU |
| 28 | Male | 42 | 23 | -/- | EC |
| 29 | Female | 78 | 91 | -/+ | PT |
| 30 | Male | 62 | 3 | -/+ | EC, EP, GU |
| 31 | Female | 58 | 13 | -/- | EP, GU |
| 32 | Male | 46 | 63 | -/- | PT |
| 33 | Female | 25 | 61 | +/- | EC |
| 34 | Female | 32 | 51 | -/- | GU |
*PT = petechiae, EC = ecchymoses, EP = epistaxis, GH = gingival hemorrhage, GUH = genitourinary hemorrhage.
Isolation of peripheral blood mononuclear cells (PBMCs)
Eathylene diamine tetraacetic acid (EDTA) anticoagulant venous blood was collected from ITP patients and healthy controls. PBMCs were isolated using Ficoll-Hypaque (Invitrogen, Carlsbad, CA) density gradient Centrifugation at 2,000 rpm for 20 min at 20°C and washed twice with phosphate-buffered saline (PBS).
Flow cytometric analysis
The expression of CD11a on lymphocyte subsets was analyzed by flow cytometry. PBMCs were adjusted to 5 × 106/ml, 100 μl (5 × 105 cells) were incubated at 4°C for 30 min with fluorescence antibodies. The following monoclonal antibodies (eBiosciences, San Diego, CA) were used for surface antigen staining: anti-CD11a monoclonal antibody conjugated to fluorescein isothiocyanate, anti-CD3 conjugated to phycoerythrin (PE), anti-CD4 conjugated to PE-Cy5 and anti-CD19 conjugated to PE-Cy5. Cells were washed with 2 ml PBS and centrifuged at 1500 rpm for 5 min. Cell pellets were then resuspended in 0.3 ml PBS and analyzed by flow cytometer (BD Biosciences, San Jose, CA).
To determine the expression of CD11a on T regulartory cells (Tregs), PE-conjugated Foxp3 antibody reagent kit and a fixation/permeabilization solution and permeabilization buffer (eBiosciences, San Diego, CA) were used for intracellular Foxp3 staining according to the manufacturer’s instructions. After 100 μl PBMCs were incubated at 4°C for 30 min with anti-CD11a monoclonal antibody conjugated to fluorescein isothiocyanate and anti-CD4 conjugated to PE-Cy5, 1 ml of 1 × Foxp3 Fix/Perm buffer solution was added to each tube. The cells were resuspended and incubated in the dark for 20 min, centrifuged and removed of the supernatants and washed twice. Then the cells were resuspended in 1 ml 1 × Foxp3 Perm buffer, incubated in the dark for 15 min. After centrifugation, the supernatant was discarded and the pellet resuspended in 100 μl of 1 × Foxp3 Perm buffer. Appropriate amount of flurochrome conjugated anti-Foxp3 antibody was added and incubated in the dark for 30 min. The cells were washed twice with staining buffer, resuspended in 0.5 ml cell staining buffer and analyzed with flow cytometer. Negative and isotype controls were used for each sample.
Modified monoclonal antibody specific immobilization of platelet antigens (MAIPA) assay
All plasma samples were stored at -20°C prior to use. The MAIPA assay of ITP patients with specific anti-platelet GPIIb/IIIa and/or GPIb autoantibodies was carried out as previously described in detail by Hou et al [15].
Statistical analysis
Data were expressed as mean ± standard deviation (SD). The difference between means was tested by independent-student t tests. The Pearson correlation test was conducted to identify univariate associations. All tests were performed by SPSS 16.0 system. Differences with a P<0.05 were determined as statistically significant.
Results
The expression of CD11a was analyzed by flow cytometry. Figure 1 showed the expression of CD11a on lymphocyte subsets. CD3+CD4+ T cells (R2), CD3+CD4- T cells (R3), CD4+foxp3+ Treg cells (R4) and CD19+ B cells (R5) were gated by flow cytometry. Figure 1E-H showed histogram of the expression of CD11a gated on CD3+CD4+ T cells, CD3+CD4- T cells, CD4+foxp3+ Treg cells and CD19+ B cells.
Figure 1.
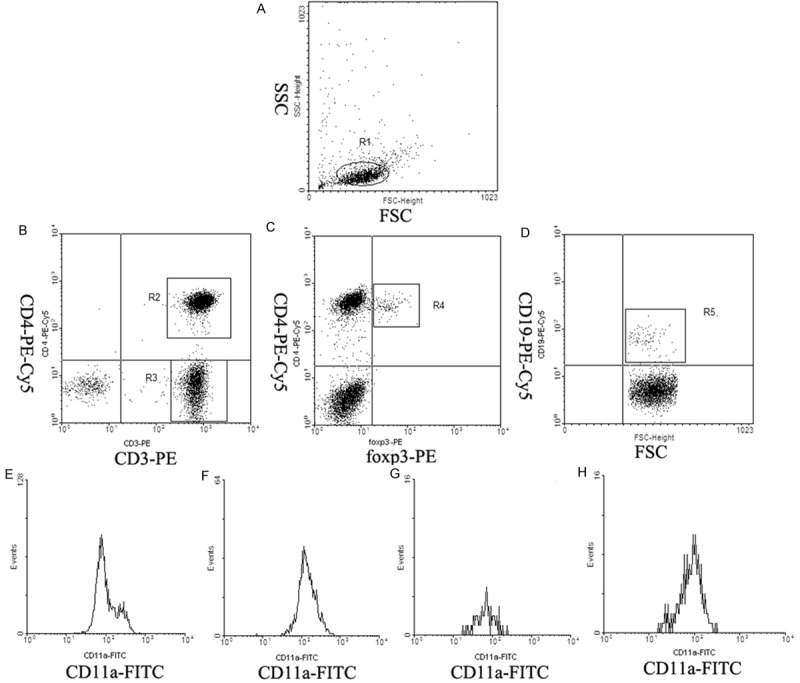
The expression of CD11a on lymphocyte subsets. (A) Lymphocytes were gated by flow cytometry (R1). (B)CD3+CD4+ T cells (R2) and CD3+CD4- T cells (R3) were gated by flow cytometry. (C) CD4+foxp3+ Treg cells (R4) were gated by flow cytometry. (D) CD19+ B cells (R5) were gated by flow cytometry. (E-H) showed histogram of the expression of CD11a gated on R2 (E), R3 (F), R4 (G) and R5 (H).
Expression of CD11a on T cells
CD11a was expressed on all lymphocyte subsets in patients and healthy controls. The expression of CD11a mean fluorescence intensity(MFI) on total T cells (CD3+) were increased in ITP patients compared to healthy controls (mean ± SD, 221.47 ± 68.33 & 165.57 ± 43.93, respectively, P<0.01) (Figure 2).
Figure 2.
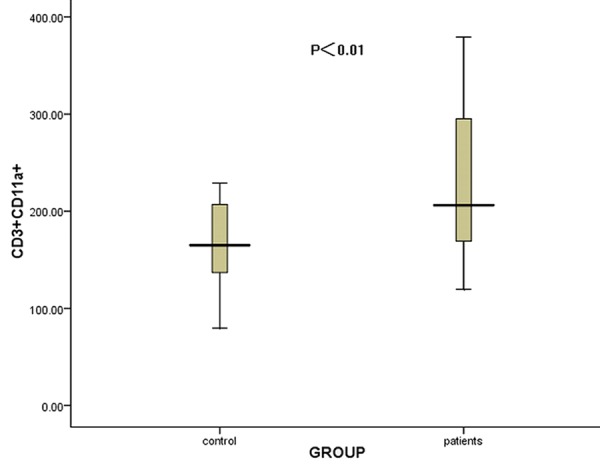
The comparison of the CD11a MFI on CD3+ T cells between ITP patients and controls. The expression of CD11a on CD3+ T cells was significantly increased in ITP patients compared to controls (P<0.01).
The CD11a MFI on CD3+CD4- T lymphocytes were increased in ITP patients compared to healthy controls (259.97 ± 88.79 & 193.58 ± 46.30, respectively, P<0.05) (Figure 3A). While no significant difference of CD11a MFI on CD3+CD4+ T lymphocytes was found between patients and controls (160.69 ± 50.84 & 143.40 ± 21.69, respectively, P>0.05) (Figure 3B).
Figure 3.
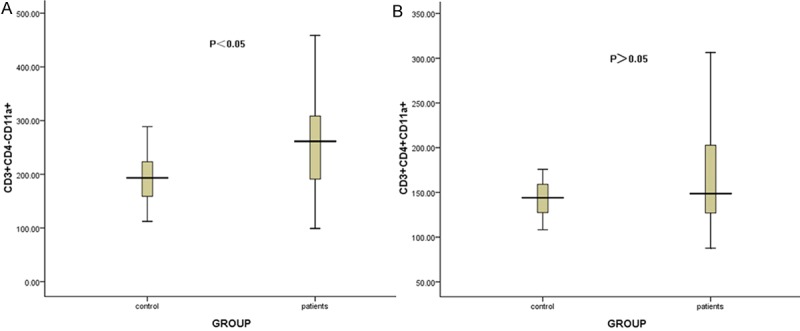
The comparison of the CD11a MFI on CD3+CD4- and CD3+CD4+ T lymphocytes between ITP patients and healthy controls. A showed the comparison of the CD11a MFI on CD3+CD4- T lymphocytes between ITP patients and healthy controls (P<0.05). B showed that CD11a expression on CD3+CD4+ T lymphocytes was not significantly different between patients and controls (P>0.05).
Expression of CD11a on Treg cells
The percentage of CD4+foxp3+ T cells in ITP patients was obviously lower than that in normal controls (P<0.05, data not shown). No significant difference of CD11a MFI on CD4+foxp3+ Treg cells was found between patients and controls (160.69 ± 50.84 & 143.40 ± 21.69, respectively, P>0.05) (Figure 4).
Figure 4.
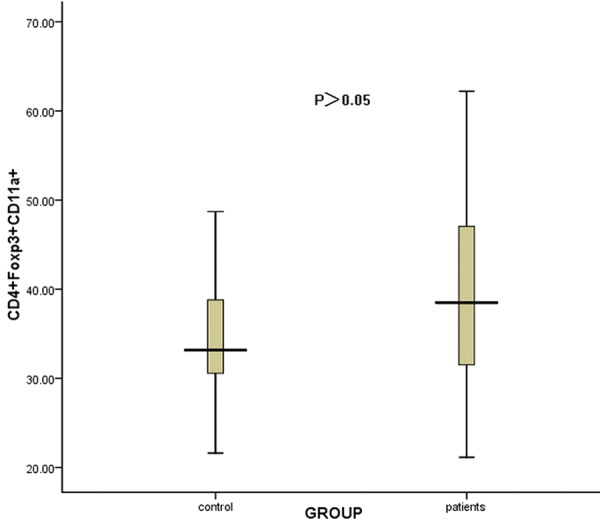
The comparison of the CD11a MFI on CD4+foxp3+ Treg cells between ITP patients and controls. No significant difference of the CD11a MFI on CD4+foxp3+ Treg cells was found between patients and controls (P>0.05).
Expression of CD11a on B cells
The percentage of CD19+ B lymphocytes in peripheral blood of ITP patients was significantly increased compared with that in healthy controls (P<0.05, data not shown). The CD11a MFI on CD19+ B lymphocytes was increased in ITP patients compared to controls (75.84 ± 27.61 & 56.26 ± 21.05, respectively, P<0.05) (Figure 5).
Figure 5.
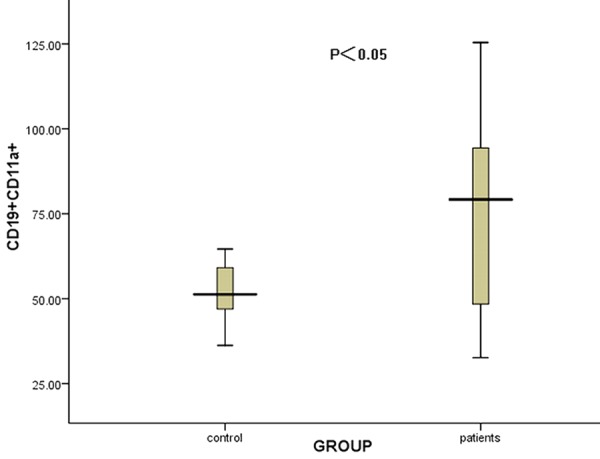
The comparison of the CD11a expression on CD19+ B lymphocytes between ITP patients and controls. The CD11a expression on CD19+ B lymphocytes was significantly increased in ITP patients compared to controls (P<0.05).
Correlation of CD11a expression between different lymphocyte subsets
The CD11a expression on CD3+CD4- T cells was positively correlated with that on CD19+ B cells (P<0.05, r = 0.365) (Figure 6), while no significant correlation of CD11a between other lymphocyte subsets was found (P>0.05).
Figure 6.
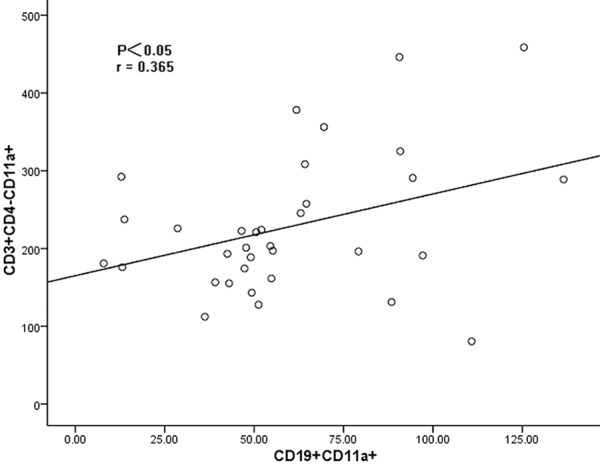
The correlation between the CD11a expression on CD3+CD4- cells and that on CD19+ cells. The CD11a expression on CD3+CD4- cells was positively correlated with that on CD19+ cells (r = 0.365, P<0.05).
Correlation between CD11a expression with platelet counts or anti-platelet autoantibodies
No significant correlation was found between CD11a expression on lymphocyte subsets with platelet counts or anti-platelet autoantibodies in ITP patients (P>0.05).
Discussion
ITP is an autoimmune disease characterized by low platelet count, increased bleeding tendency, blood smear showing a decreased number of platelets, bone marrow test showing an increased number and immature of megakaryocytes. Autoantibodies against platelet surface glycoproteins such as GPIIb/IIIa and GPIb/IX complexes, and cytotoxic T cell mediated platelet destruction play important roles in its pathophysiology. Recent research demonstrates that the underlying mechanism in ITP is very complex and includes: decreased number and defective suppressive function of Tregs, Th1/Th2 imbalance, increase of Th17 cells and IL-17 levels, elevated Th22 cells, decreased B regulatory cells, and so on [16-21].
In the current study the expression of CD11a on all lymphocyte subsets were analyzed in ITP. We found the expression of CD11a on CD3+ T cells, CD3+CD4- T lymphocytes and CD19+ B lymphocytes were increased in ITP patients compared to healthy controls. CD11a on CD3+CD4+ T lymphocytes and CD4+Foxp3+ Tregs were not significantly different between patients and controls.
During adaptive immunity, T cells become activated and proliferated in lymphoid organs when encountering antigens presented by APCs. After activation, T cells arrive at the site of inflammation and exert active roles in peripheral tissues [22]. T-cell recruitment, which is controlled by the combination of adhesion molecules and their receptors, is crucial for T-cell circulation among lymphoid organs and peripheral tissues [23]. Dysregulation of T cell activity and cytokine abnormalities have been found to be a contributing factor to many autoimmune diseases, including ITP [24]. LFA-1 is the predominant cell adhesion molecule present on T cells [25] and plays crucial roles in lymphocyte trans-endothelial migration, immunologic synapse and T-cell activation and proliferation through interaction with ICAM-1 on APCs [26]. LFA-1 can decrease the threshold of TCR signals and antigen doses required for T cell activation and proliferation in vitro [25,27]. Increasing CD11a expression in T cells by transfection causes autoreactivity in vitro and an autoimmune disease in mice [28]. In our study CD11a was expressed on all T cells and the expression of CD11a was increased on CD3+ T cells in ITP patients compared with healthy controls, confirming LFA-1 plays a critical role on T cell activation and increased CD11a expression on T cells may induce autoreactivity in ITP.
T cells are broadly classified as either helper T cells (Th cells, CD4+) or cytotoxic T cells (Tc cells, CD8+). Th cells play a key role in determining whether autoantibodies are produced. They are separated into Th1 cells and Th2 cells, depending upon the specific cytokines which the cells secrete in response to antigenic stimulation [29]. There were some studies showing an increased Th1/Th2 ratio in ITP patients compared with controls [18]. In addition to their roles in adaptive immunity, memory T cells, CD8+ T cells in particular, are also involved in innate immunity, becoming activated and proliferated under cytokine stimulation in the absence of antigens. In some research, it may be speculated that Tc cells play a part in at least some patients with ITP, Tc cells may directly lyse platelets and possibly suppress megakaryopoiesis. CD11a appears to play a direct role on effector CD8 T cell subset development, presumably through augmentation of T cell-APC interactions [30]. In the current study, the expression of CD11a on CD3+CD4- T lymphocytes was increased in ITP patients compared to healthy controls and that on CD3+CD4+ T lymphocytes was not significantly different between patients and controls. We speculated that Th1 cells bias and Tc cells could both participate in the occurrence of ITP and Tc cells may lyse platelets partly through LFA-1-dependent pathway. However, CD4+ T cells may play a part in LFA-1-independent manner.
Tregs are a subset of T cells, representing 5-10% of peripheral CD4+ T cells. Marked by their expression of CD4, CD25, CD127 and Foxp3, they appear to play a critical role in maintaining peripheral tolerance by suppressing both cell-mediated and antibody-mediated responses [31]. Evidence indicated that levels of circulating Tregs in patients with acute and chronic ITP have been significantly reduced compared to healthy controls [32,33]. In ITP, platelet autoreactive T cells have decreased apoptosis, underwent clonal expansion and created a cytokine imbalance that can lead to lower levels and abnormal function of Tregs [34]. Defective Tregs appear to be critical to the pathogenesis of ITP by breaking self-tolerance, allowing the autoimmune process to progress [17]. This shift in self-tolerance was believed to contribute to autoimmune-mediated platelet destruction. LFA-1 on the human Tregs is critical for their suppressor-function. Tran DQ found that Tregs could not exert its inhibition with blocking anti-human CD11a or anti-human CD18a monoclonal antibody, and Tregs in patients lack of LFA-1 could not restrain T cell activation [35,36]. As the researcher points out, antigen-activated Tregs exert suppression by two distinct steps: initial LFA-1-dependent formation of Treg aggregates on immature dendritic cells (DCs) and subsequent LFA-1- and CTLA-4-dependent active down-modulation of CD80/86 expression on DCs. Both steps prevent antigen-reactive naive T cells from being activated by antigen-presenting DCs, resulting in specific immune suppression and tolerance [37]. In our study we detected the number of CD4+Foxp3+ Tregs and the expression of CD11a on Tregs, the results verified the decreased number of Tregs in ITP. However, the expression of CD11a in ITP was not significantly different compared with healthy controls. Reasons for this finding may include too little sample number, insensitive methods and so on. In addition, we speculated that Tregs may also exert its inhibition through other pathways in ITP.
The interaction of LFA-1 and its ligands provides essential adhesive and co-stimulatory signals required for the initiation of immune responses. B cells are generally considered to be positive regulators of the immune response because of their capability to produce antibodies, including autoantibodies. Autoantibodies against platelets really play a major role in ITP. Increased levels of circulating B cells excreting anti-GPIIbIIIa antibodies in ITP patients have been reported [38]. The dysregulation of B cell development is also associated with ITP. LFA-1/ICAMs interaction transduces biochemical signals for T-cell-dependent B cell activation and immunoglobulin production and lowers the threshold of B cell activation by facilitating B cell adhesion and synapse formation [39]. Anti-LFA-1 added to culture strongly inhibits antibody responses although B-cell proliferative responses are only partially mitigated [40]. We speculated that CD11a over-expression on CD19+ cells in ITP could provide sufficient B cell stimulatory signals to activate B cells and produce autoantibodies.
The positive correlation of CD11a expressions between CD3+CD4- cells and CD19+ cells indicated that Tc cells and B cells could play their roles through LFA-1 participated common pathway in ITP. In our study the CD11a expressions on all lymphocyte cells were not relevant with the platelet counts or anti-platelet autoantibodies in ITP, indicating that the expression levels of CD11a on lymphocyte cells can not reflect the severity of the disease.
Taken together, elevated expression of CD11a on CD3+CD4- cells and CD19+ cells indicate its possible role in the pathogenesis of ITP, even though further experimentation is needed to elucidate the basic mechanism by which LFA-1 exert its function.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (81100335, 81202307 and 81100417).
Disclosure of conflict of interest
None.
References
- 1.McMillan R. Immune-mediated thrombocytopenias: focus on chronic immune thrombocytopenic purpura. Semin Hematol. 2007;44:S1–2. doi: 10.1053/j.seminhematol.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 2.McKenzie CG, Guo L, Freedman J, Semple JW. Cellular immune dysfunction in immune thrombocytopenia (ITP) Br J Haematol. 2013;163:10–23. doi: 10.1111/bjh.12480. [DOI] [PubMed] [Google Scholar]
- 3.Stasi R. Immune Thrombocytopenia: Pathophysiologic and Clinical Update. Semin Thromb Hemost. 2012;38:454–62. doi: 10.1055/s-0032-1305780. [DOI] [PubMed] [Google Scholar]
- 4.Kawana M, Ikeda Y. The role of autoreactive TCells in the pathogenesis of idiopathic thrombocytopenic purpura. Int J Hematol. 2005;81:106–12. doi: 10.1532/ijh97.04176. [DOI] [PubMed] [Google Scholar]
- 5.Ghislin S, Obino D, Middendorp S, Boggetto N, Alcaide-Loridan C, Deshayes F. LFA-1 and ICAM-1 expression induced during melanomaendothelial cell co-culture favors the transendothelial migration of melanoma cell lines in vitro . BMC Cancer. 2012;12:455. doi: 10.1186/1471-2407-12-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parameswaran N, Suresh R, Bal V, Rath S, George A. Lack of ICAM-1 on APCs during T cell priming leads to poor generation of central memory cells. J Immunol. 2005;175:2201–11. doi: 10.4049/jimmunol.175.4.2201. [DOI] [PubMed] [Google Scholar]
- 7.Lisby S, Ralfkiaer E, Rothlein R, Vejlsgaard G. Intercellular adhesion molecule-1 (ICAM-1) expression correlated to inflammation. Br J Dermatol. 1989;120:479–84. doi: 10.1111/j.1365-2133.1989.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 8.Park EJ, Peixoto A, Imai Y, Goodarzi A, Cheng G, Carman CV, von Andrian UH, Shimaoka M. Distinct roles for LFA-1 affinity regulation during T-cell adhesion, diapedesis, and interstitial migration in lymph nodes. Blood. 2010;115:1572–81. doi: 10.1182/blood-2009-08-237917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bose TO, Colpitts SL, Pham QM, Puddington L, Lefrançois L. CD11a Is Essential for Normal Development of Hematopoietic Intermediates. J Immunol. 2014;193:2863–72. doi: 10.4049/jimmunol.1301820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pribila JT, Quale AC, Mueller KL, Shimizu Y. Integrins and T cell mediated immunity. Annu Rev Immunol. 2004;22:157–80. doi: 10.1146/annurev.immunol.22.012703.104649. [DOI] [PubMed] [Google Scholar]
- 11.Kakimoto K, Nakamura T, Ishii K, Takashi T, Iigou H, Yagita H, Okumura K, Onoue K. The effect of anti-adhesion molecule antibody on the development of collagen-induced arthritis. Cell Immunol. 1992;142:326–37. doi: 10.1016/0008-8749(92)90294-y. [DOI] [PubMed] [Google Scholar]
- 12.Sela U, Mauermann N, Hershkoviz R, Zinger H, Dayan M, Cahalon L, Liu JP, Mozes E, Lider O. The inhibition of autoreactive T cell functions by a peptide based on the CDR1 of an anti-DNA autoantibody is via TGF-beta mediated suppression of LFA-1 and CD44 expression and function. J Immunol. 2005;175:7255–63. doi: 10.4049/jimmunol.175.11.7255. [DOI] [PubMed] [Google Scholar]
- 13.Ma L, Zhou Z, Jia H, Zhou H, Qi A, Li H, Wang H, Zhang L, Yang R. Effects of CD70 and CD11a in Immune Thrombocytopenia Patients. J Clin Immunol. 2011;31:632–42. doi: 10.1007/s10875-011-9539-1. [DOI] [PubMed] [Google Scholar]
- 14.Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190–207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 15.Hou M, Peng J, Shi Y, Zhang C, Qin P, Zhao C, Ji X, Wang X, Zhang M. Mycophenolate mofetil (MMF) for the treatment of steroid-resistant idiopathic thrombocytopenic purpura. Eur J Haematol. 2003;70:353–7. doi: 10.1034/j.1600-0609.2003.00076.x. [DOI] [PubMed] [Google Scholar]
- 16.Provan D. Immune thrombocytopenia. Hematology. 2013;18:242–243. doi: 10.1179/1024533213Z.000000000181. [DOI] [PubMed] [Google Scholar]
- 17.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–58. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Stasi R, Del Poeta G, Stipa E, Evangelista ML, Trawinska MM, Cooper N, Amadori S. Response to B-cell depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood. 2007;110:2924–30. doi: 10.1182/blood-2007-02-068999. [DOI] [PubMed] [Google Scholar]
- 19.Hu Y, Ma DX, Shan NN, Zhu YY, Liu XG, Zhang L, Yu S, Ji CY, Hou M. Increased number of Tc17 and correlation with Th17 cells in patients with immune thrombocytopenia. PLoS One. 2011;6:e26522. doi: 10.1371/journal.pone.0026522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao J, Chen C, Zeng L, Li L, Li X, Li Z, Xu K. Elevated plasma IL-22 levels correlated with Th1 and Th22 cells in patients with immune thrombocytopenia. Clin Immunol. 2011;141:121–3. doi: 10.1016/j.clim.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Zhong H, Bao W, Boulad N, Evangelista J, Haider MA, Bussel J, Yazdanbakhsh K. Defective regulatory B cell compartment in patients with immune thrombocytopenia. Blood. 2012;16:3318–25. doi: 10.1182/blood-2012-05-432575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 23.Lu H, Smith CW, Perrard J, Bullard D, Tang L, Shappell SB, Entman ML, Beaudet AL, Ballantyne CM. LFA-1 is sufficient in mediating neutrophil emigration in Mac-1-deficient mice. J Clin Invest. 1997;99:1340–50. doi: 10.1172/JCI119293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao J, Chen C, Li L, Ling-yu Z, Zhen-yu L, Zhiling Y, Wei C, Hai C, Sang W, Kai-lin X. Effects of high-dose dexamethasone on regulating interleukin-22 production and correcting Th1 and Th22 polarization in immune thrombocytopenia. J Clin Immunol. 2012;3:523–9. doi: 10.1007/s10875-012-9649-4. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Shibuya K, Yamashita Y, Shirakawa J, Shibata K, Kai H, Yokosuka T, Saito T, Honda S, Tahara-Hanaoka S, Shibuya A. LFA-1 decreases the antigen dose for T cell activation in vivo. Int Immunol. 2008;20:1119–27. doi: 10.1093/intimm/dxn070. [DOI] [PubMed] [Google Scholar]
- 26.Wu H, Rodgers JR, Perrard XY, Perrard JL, Prince JE, Abe Y, Davis BK, Dietsch G, Smith CW, Ballantyne CM. Deficiency of CD11b or CD11d results in reduced staphylococcal enterotoxin-induced T cell response and T cell phenotypic changes. J Immunol. 2004;173:297–306. doi: 10.4049/jimmunol.173.1.297. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki J, Yamasaki S, Wu J, Koretzky GA, Saito T. The actin cloud induced by LFA-1-mediated outside-in signals lowers the threshold for Tcell activation. Blood. 2007;109:168–75. doi: 10.1182/blood-2005-12-020164. [DOI] [PubMed] [Google Scholar]
- 28.Yung R, Powers D, Johnson K, Amento E, Carr D, Laing T, Yang J, Chang S, Hemati N, Richardson B. Mechanisms of drug-induced lupus. II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J Clin Invest. 1996;97:2866–71. doi: 10.1172/JCI118743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone: I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 30.Bose TO, Pham QM, Jellison ER, Mouries J, Ballantyne CM, Lefrançois L. CD11a Regulates Effector CD8 T Cell Differentiation and Central Memory Development in Response to Infection with Listeria monocytogenes. Infect Immun. 2013;81:1140–51. doi: 10.1128/IAI.00749-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3 + regulatory T cells in the human immune system. Nat Rev Immunol. 2010;7:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 32.Fahim NM, Monir E. Functional role of CD4+ CD25+ regulatory T cells and transforming growth factor-beta1 in childhood immune thrombocytopenic purpura. Egypt J Immunol. 2006;1:173–87. [PubMed] [Google Scholar]
- 33.Liu B, Zhao H, Poon MC, Han Z, Gu D, Xu M, Jia H, Yang R, Han ZC. Abnormality of CD4(+) CD25(+) regulatory T cells in idiopathic thrombocytopenic purpura. Eur J Haematol. 2007;2:139–43. doi: 10.1111/j.1600-0609.2006.00780.x. [DOI] [PubMed] [Google Scholar]
- 34.Yu J, Heck S, Patel V, Levan J, Yu Y, Bussel JB, Yazdanbakhsh K. Defective circulating CD25 regulatory T cells in patients with chronic immune thrombocytopenic purpura. Blood. 2008;4:1325–8. doi: 10.1182/blood-2008-01-135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tran DQ, Shevach EM. Therapeutic potential of FOXP3(+) regulatory T cells and their interactions with dendritic cells. Hum Immunol. 2009;70:294–9. doi: 10.1016/j.humimm.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tran DQ, Glass DD, Uzel G, Darnell DA, Spalding C, Holland SM, Shevach EM. Analysis of adhesion molecules, target cells, and role of IL-2 in human FOXP3+ regulatory T cell suppressor function. J Immunol. 2009;182:2929–38. doi: 10.4049/jimmunol.0803827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A. 2008;105:10113–8. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen JF, Yang LH, Chang LX, Feng JJ, Liu JQ. The clinical significance of circulating B cells secreting anti-glycoprotein IIb/IIIa antibody and platelet glycoprotein IIb/IIIa in patients with primary immune thrombocytopenia. Hematology. 2012;5:283–90. doi: 10.1179/1607845412Y.0000000014. [DOI] [PubMed] [Google Scholar]
- 39.Carrasco YR, Fleire SJ, Cameron T, Dustin ML, Batista FD. LFA-1/ICAM-1 interaction lowers the threshold of B cell activation by facilitating B cell adhesion and synapse formation. Immunity. 2004;20:589–99. doi: 10.1016/s1074-7613(04)00105-0. [DOI] [PubMed] [Google Scholar]
- 40.Owens T. A role for adhesion molecules in contact-dependent T help for B cells. Eur J Immunol. 1991;21:979–83. doi: 10.1002/eji.1830210418. [DOI] [PubMed] [Google Scholar]


