Abstract
Introduction: Recent study has shown that renin-angiotensin system plays an important role in the development of acute lung injury (ALI) with high level of angiotensin II (AngII) generated form AngI catalyzed by angiotensin-converting enzyme. AngII plays a major effect mainly through AT1 receptor. Therefore, we speculate inhibition of AT1 receptor may possibly attenuate the lung injury. Losartan, an antagonist of AT1 receptor for angiotensin II, attenuated lung injury by alleviation of the inflammation response in ALI, but the mechanism of losartan in ALI still remains unclear. Methods: Thirty male Sprague-Dawley rats were randomly divided into Control group, ALI group (LPS), and Losartan group (LPS + Losartan). Bronchoalveolar lavage fluid (BALF) and lung tissue were obtained for analysis. The expressions of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), intercellular adhesion molecule-1 (ICAM-1) and caspase-3 were detected by reverse transcriptase polymerase chain reaction (RT-PCR) and western blotting. Results: In ALI group, TNF-α and protein level in BALF, MPO activity in lung tissue, pulmonary edema and lung injury were significantly increased. Losartan significantly reduced LPS-induced increase in TNF-α and protein level in BALF, MPO activity, pulmonary edema and lung injury in LPS-induced lung injury. The mRNA and protein expression levels of LOX-1 were significantly decreased with the administration of losartan in LPS-induced lung injury. Also, losartan blocked the protein levels of caspase-3 and ICAM-1 mediated by LOX-1 in LPS-induced lung injury. Conclusions: Losartan attenuated lung injury by alleviation of the inflammation and cell apoptosis by inhibition of LOX-1 in LPS-induced lung injury.
Keywords: Acute lung injury, caspase-3, lectin-like oxidized low-density lipoprotein receptor-1, losartan, intercellular adhesion molecule-1
Introduction
Acute lung injury (ALI) is a common clinical syndrome characterized by pulmonary inflammation induced by a large variety of inflammatory cells and the damage of pulmonary capillary vessel induced by inflammation infiltration with a high mortality of 40% [1]. Though some research results have implied the potential mechanism in the inflammation during ALI, it still remains unclear that needs further investigation.
It has been recently found that renin-angiotensin system plays an important role in the development of ALI [2]. Angiotensin-converting enzyme (ACE) has key function in the renin-angiotensin system. ACE cleaves angiotensin I to generate angiotensin II (AngII), whereas ACE2 inactivates Ang II as a negative regulator of the system. The study showed that the level of Ang II was increased which contributed to the inflammation during ALI. Losartan, an antagonist of AT1 receptor for angiotensin II, have been reported to attenuate lung injury in ALI [3].
Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), the receptor for oxidized low-density lipoprotein, was expressed in endothelial cells, macrophages or smooth muscle cells and is a Type II membrane protein with a typical C-type lectin structure at the extracellular C-terminus [4,5]. Recent study has shown that blockade of LOX-1 prevented acute lung inflammation and injury, which indicated its valuable therapeutic target in ALI [6]. However, how this LOX-1 has the effect on inflammation in ALI has not yet been elucidated.
In this study, we aimed to investigate the effect of LOX-1 in ALI. We found that losartan, an antagonist of AT1 receptor for angiotensin II, attenuated lung injury by suppression of LOX-1 in LPS-induced ALI.
Methods
Materials
Male Sprague-Dawley rats weighing 210-250 g (Department of Laboratory Animal Center, Chongqing Medical University) were housed under specific pathogen-free conditions in a temperature- and humidity-controlled environment and given free access to food and water with the Guide for the Care and Use of Laboratory Animals. Lipopolysaccharide (LPS, Escherichia coli serotype O111:B4) were purchased from Sigma (St Louis, MO, USA). Antibody against LOX-1 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibody against intercellular adhesion molecule-1 (ICAM-1) was purchased from Abcam (Cambridge, UK). Antibody against caspase-3 was purchased from Cell Signaling Technology (Beverly, MA, USA). Losartan was purchased from Cayman Chemical (Ann Arbor, MI, USA).
The study was approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University.
Animal model and intervention
Rats were randomly divided into Control group, ALI group (LPS), and Losartan group (LPS + Losartan). Rats were anesthetized by intraperitoneal administration of 50 mg/kg sodium pentobarbital. After anesthetization, LPS at a dose of 5.0 mg/kg was administered via intratracheal injection previously described [7]. In Losartan group, losartan at a dose of 8.0 mg/kg was given intraperitoneally after exposure to LPS immediately [3]. Rats in control group were received an equivalent volume of saline. Rats were killed 6 hours after LPS or saline treatment. Blood sample, bronchoalveolar lavage fluid (BALF) and lung tissue were obtained for analysis.
Tumor necrosis factor-α and protein level in BALF
BALF was collected after infusion with 0.9% NaCl for six times in the left lung. After centrifugation at 14,000 rpm for 30 minutes at 4°C, the supernatant was collected for total protein analysis by a protein assay kit (Key-GEN, KeyGEN Bio TECH Co., Nanjing, China). The rest was for tumor necrosis factor-α (TNF-α) analysis according to the manufacturer’s instructions of ELISA kit (Sunbio, Sun Biomedical Technology, Beijing, China).
Myeloperoxidase assay in lung tissue
Inflammatory cell sequestration in lung was analyzed by myeloperoxidase (MPO) activity [8]. MPO activity was determined by a MPO assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
Wet-to-dry ratio analysis
The upper right lungs were weighed to obtain its wet weight, then placed in an oven at 70°C for 72 hours to obtain its dry weight. Later, pulmonary edema was determined by calculating the wet-to-dry ratio.
Lung histology
Lung tissues were harvested and fixed by 10% neutral buffered formalin for 24 hours. Then they were embedded in paraffin and were mounted onto glass slides for hematoxylin and eosin staining after section.
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted by Trizol reagent (Bioss, Beijing, China) according to the manufactures’ instructions. First strand of cDNA was synthesized by reverse transcription. Primer sequences for PCR amplification were: LOX-1 (328 bp), 5’-GACTGGATCTGGCATAAAGA-3’ (forward) and 5’-CCTTCTTCTTCTGACATATGCTG-3’ (reverse); reverse); β-actin (206 bp), 5’-TTCCAGCCTTCCTTCCTGG-3’ (forward) and 5’-TTGCGCTCAGGAGGAGCAAT-3’ (reverse).
Reverse transcription and polymerase chain reaction conditions were 94°C for 60 seconds, 35 cycles of denaturation at 94°C for 30 seconds, annealing at 55°C for 45 seconds and polymerization at 72°C for 60 seconds. Each PCR product was run on a 1.5% agarose gel containing ethidium bromide and was visualized with Gel Imaging System (Bio-Rad, Hercules, CA, USA).
Western blotting
Proteins were obtained with 1 ml of lysis buffer and 1 ml of extraction buffer. 100 μg of each protein sample was separated by 10% SDS-PAGE and transferred to polyvinylidene fluoride membranes. After blocking with 5% nonfat dried milk in Tris-buffered saline and shaking for one hour at room temperature, the membranes were incubated with primary antibodies LOX-1 (1:500), ICAM-1 (1:2000) and caspase-3 (1:1000) overnight, then were incubated with horseradish peroxidase-conjugated secondary antibody (1:2000) for 1 hour. The protein bands were colored by a Western Blot Enhanced Chemiluminescence (ECL) method and were visualized by with Gel Imaging System (Bio-Rad, Hercules, CA, USA).
Statistical analysis
All data were described as mean ± SEM. Statistical analysis was performed by Student’s t-test and one-way analysis of variance (ANOVA) using SPSS 12.0 software. P<0.05 was considered statistically significant.
Results
Effect of losartan on Tumor necrosis factor-α and protein level in BALF
Losartan significantly reduced LPS-induced increase in TNF-α and protein level in BALF, which indicated the effect of attenuation of inflammatory infiltration and edema exudation by losartan in LPS-induced lung injury (Figure 1).
Figure 1.
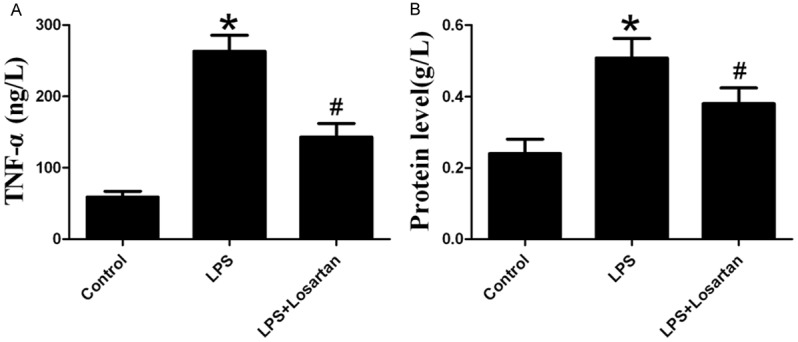
Measurement of TNF-a (A) and protein level (B) in bronchoalveolar lavage fluid 6 hours after LPS or saline treatment (n=10). Data are presented as mean ± S.E.M. *P<0.05 vs LPS; #P<0.05 vs LPS + Losartan.
Effect of losartan on neutrophil infiltration in LPS-induced acute lung injury
MPO activity, an indicator of neutrophil activation [9], was significantly increased in LPS-induced lung injury. However, losartan decreased MPO activity in LPS-induced lung injury (Figure 2).
Figure 2.
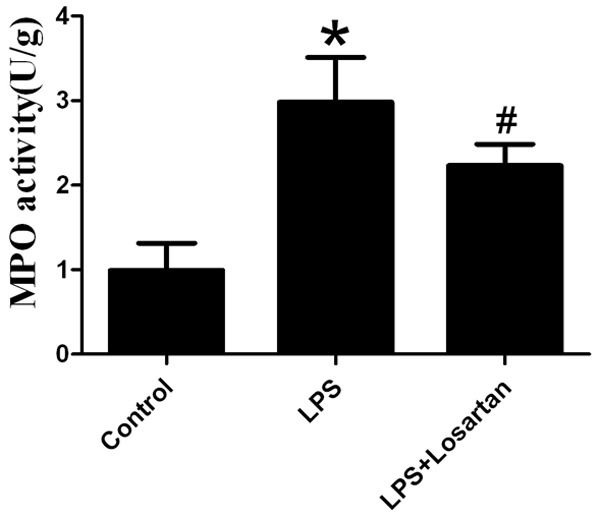
Measurement of myeloperoxidase (MPO) in lung tissue 6 hours after LPS or saline treatment (n=10). Data are presented as mean ± SEM. *P<0.05 vs LPS; #P<0.05 vs LPS + Losartan.
Effect of losartan on pulmonary edema in LPS-induced acute lung injury
Losartan attenuated LPS-induced pulmonary edema in ALI (Figure 3).
Figure 3.
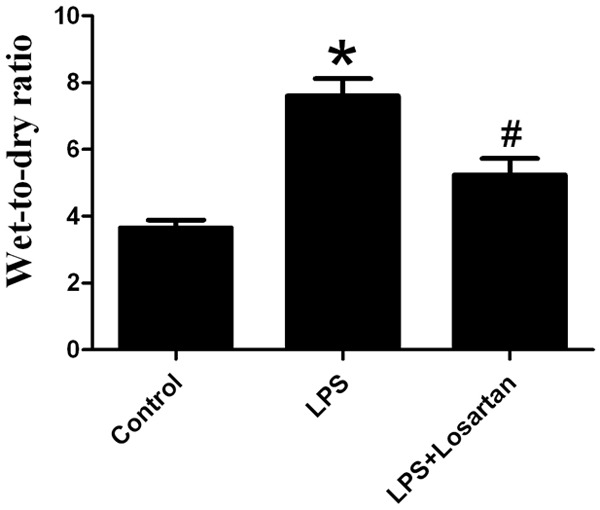
Measurement of pulmonary edema 6 hours after LPS or saline treatment (n=10). Data are presented as mean ± SEM. *P<0.05 vs LPS; #P<0.05 vs LPS + Losartan.
Effect of losartan on lung histology in LPS-induced acute lung injury
The lung tissue was injured with the presence of massive neutrophil infiltration in the interstitial, intra-alveolar edema and hemorrhagic foci in the alveolar spaces, thickening of alveolar septa in LPS group compared with that in control group. Losartan attenuated LPS-induced lung pathologic changes (Figure 4).
Figure 4.
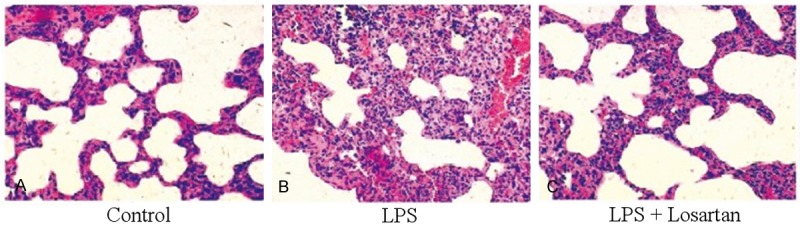
Changes of lung histology 6 hours after LPS or saline treatment. A. Control group: No histologic changes were observed. B. LPS group: massive neutrophil infiltration in the interstitial, intraalveolar edema and hemorrhagic foci in the alveolar spaces, thickening of alveolar septa. C. LPS + Losartan group: Losartan significantly attenuated LPS-induced lung injury. Original magnification: 400×.
Effect of losartan on the expression of LOX-1 in LPS-induced acute lung injury
The mRNA and protein expression levels of LOX-1 in rat lung showed significant increases by LPS treatment, but the mRNA and protein expression levels of LOX-1 were significantly decreased with the administration of losartan in LPS-induced lung injury (Figure 5).
Figure 5.
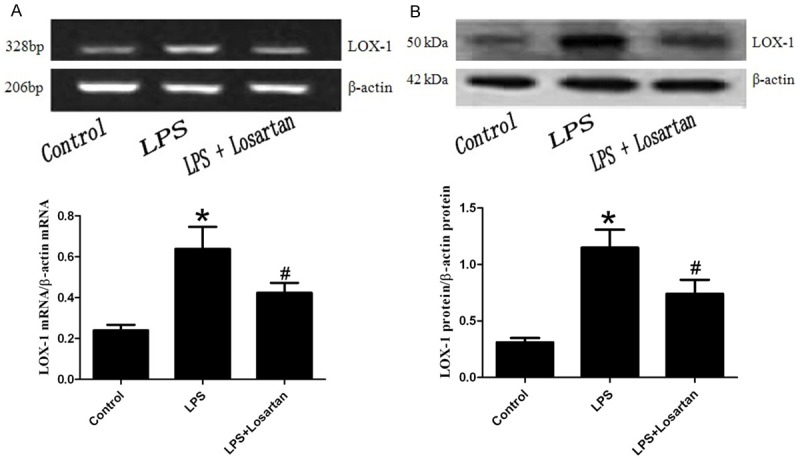
Expressions of LOX-1 in rat lung was measured by RT-PCR (A) and western blotting (B) 6 hours after LPS or saline treatment (n=5). Data are presented as mean ± S.E.M. *P<0.05 vs LPS; #P<0.05 vs LPS + Losartan.
Effect of losartan on the expression of caspase-3 and ICAM-1 in LPS-induced acute lung injury
To further investigate the effect of LOX-1 in LPS-induced lung injury by losartan treatment, the protein levels of caspase-3 and ICAM-1 were performed by western blotting. The result showed that losartan blocked the protein levels of caspase-3 and ICAM-1 mediated by LOX-1, which implied the apoptotic and inflammatory effect of LOX-1 in LPS-induced lung injury (Figure 6).
Figure 6.
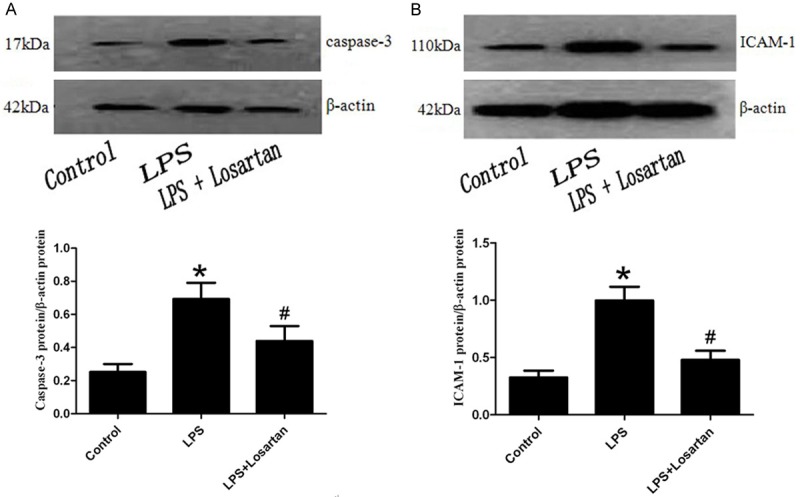
Expressions of caspase-3 and intercellular adhesion molecule-1 (ICAM-1) in rat lung was measured by western blotting 6 hours after LPS or saline treatment (n=5). Data are presented as mean ± SEM. *P<0.05 vs LPS; #P<0.05 vs LPS + Losartan.
Discussion
Mass aggregation of neutrophils in the lung leads to the release of oxygen free radicals, protease and other inflammatory mediators, which led to the inflammatory injury of alveolar epithelial cells and pulmonary capillary endothelial cells [10]. Uncontrolled systemic inflammatory response is the root cause of ALI.
It has been recently found that renin-angiotensin system plays an important role in the development of ALI. The level of Ang II was increased in the lung during ALI, which further promoted the inflammatory response by binding to the AT1 receptor [3]. Recent study has shown that ACE had a protective effect in oleic-induced lung injury [11]. Losartan is a blocker of AT1 receptor, which can block the bacterial-mediated neutrophil activation and aggregation in rat lung so as to attenuate lung injury in ALI [3,12,13]. In our study, administration of losartan significantly alleviated TNF-α and protein level in BALF, MPO activity in lung tissue in the rat model of ALI, which indicated its anti-inflammatory response by blocking of inflammatory factor in LPS-induced lung injury. Also, losartan attenuated LPS-induced lung pathologic changes by HE staining.
LOX-1 is a C-type plant lectin family with type 2 membrane protein structure, which has a high degree of homology with the natural killer cell receptors, of which the main function is the identification of target cells and the activation of natural killer cells [14]. LOX-1 is not only an oxidized low density the plasma lipoprotein receptor, but also a cell adhesion molecule involving the inflammatory response. In addition, LOX-1 is able to be combined with the neutrophils, which contributes to the neutrophil-mediated acute inflammatory response [15,16]. LOX-1 neutralizing antibodies can reduce the exudation of the white blood cells and protein and decrease the mortality of endotoxin-shock animals [17]. Recent study has shown that LOX-1 participated the LPS-mediated ALI, which suggested an important factor in ALI; also a variety of inflammatory mediators can promote LOX-1 protein expression [18,19]. In the study, the mRNA and protein expressions of LOX-1 were increased in LPS-induced lung injury, but the expression level of LOX-1 were decreased by losartan intervention, which suggested that LOX-1 could be induced by LPS and protective effect of losartan in ALI was possibly achieved by inhibiting LOX-1 expression via AT1 receptor pathway. In addition, ICAM-1 is an important factor involved neutrophil aggregation in inflammation [20]. Caspase-3 reflects the apoptosis [21]. In the study, losartan was able to decrease the LOX-1-mediated expressions of ICAM-1 and caspase-3 in LPS-induced lung injury, which was consistent with the previous results [19].
Conclusions
In this study, the results suggested that losartan attenuated lung injury by alleviation of the inflammation and cell apoptosis by inhibition of LOX-1 in LPS-induced lung injury.
Acknowledgements
This study was supported by Chongqing Health Bureau of scientific research project (No. 2008-2-224).
Disclosure of conflict of interest
None.
References
- 1.MacCallum NS, Evans TW. Epidemiology of acute lung injury. Curr Opin Crit Care. 2005;11:43–49. doi: 10.1097/00075198-200502000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, Qiu HB, Yang Y, Wang L, Ding HM, Li HP. Losartan, an antagonist of AT1 receptor for angiotensin II, attenuates lipopolysaccharideinduced acute lung injury in rat. Arch Biochem Biophys. 2009;481:131–136. doi: 10.1016/j.abb.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 5.Chen M, Masaki T, Sawamura T. LOX-1, the receptor for oxidized low-density lipoprotein identified from endothelial cells: implications in endothelial dysfunction and atherosclerosis. Pharmacol Ther. 2002;95:89–100. doi: 10.1016/s0163-7258(02)00236-x. [DOI] [PubMed] [Google Scholar]
- 6.Zhang P, Liu MC, Cheng L, Liang M, Ji HL, Fu J. Blockade of LOX-1 prevents endotoxin-induced acute lung inflammation and injury in mice. J Innate Immun. 2009;1:358–365. doi: 10.1159/000161070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang JL, Lee HW, Lee HS, Pack IS, Chong Y, Castranova V, Koh Y. Genistein prevents nuclear factor-kappa B activation and acute lung injury induced by lipopolysaccharide. Am J Respir Crit Care Med. 2001;164:2206–2212. doi: 10.1164/ajrccm.164.12.2104017. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia M, Wong FL, Fu D, Lau HY, Moochhala SM, Moore PK. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 2005;19:623–625. doi: 10.1096/fj.04-3023fje. [DOI] [PubMed] [Google Scholar]
- 9.Jin SW, Zhang L, Lian QQ, Liu D, Wu P, Yao SL, Ye DY. Posttreatment withaspirin-triggered lipoxin A4 analog attenuates lipopolysaccharideinducedacute lung injury in rats: the role of heme oxygenase-1. Anesth Analg. 2007;104:369–377. doi: 10.1213/01.ane.0000252414.00363.c4. [DOI] [PubMed] [Google Scholar]
- 10.Ayyagari VN, Januszkiewicz A, Nath J. Effects of nitrogen dioxide on the expression of intercellular adhesion molecule-1, neutrophil adhesion, and cytotoxicity: studies in human bronchial epithelial cells. Inhal Toxicol. 2007;19:181–194. doi: 10.1080/08958370601052121. [DOI] [PubMed] [Google Scholar]
- 11.He X, Han B, Mura M, Xia S, Wang S, Ma T, Liu M, Liu Z. Angiotensin-converting enzyme inhibitor captopril prevents oleic acid-induced severe acute lung injury in rats. Shock. 2007;28:106–111. doi: 10.1097/SHK.0b013e3180310f3a. [DOI] [PubMed] [Google Scholar]
- 12.Shen L, Mo H, Cai L, Kong T, Zheng W, Ye J, Qi J, Xiao Z. Losartan prevents sepsis-induced acute lung injury and decreases activation of nuclear factor kappaB and mitogen-activated protein kinases. Shock. 2009;31:500–506. doi: 10.1097/SHK.0b013e318189017a. [DOI] [PubMed] [Google Scholar]
- 13.Yao S, Feng D, Wu Q, Li K, Wang L. Losartan attenuates ventilator-induced lung injury. J Surg Res. 2008;145:25–32. doi: 10.1016/j.jss.2007.03.075. [DOI] [PubMed] [Google Scholar]
- 14.Tanigawa H, Miura S, Matsuo Y, Fujino M, Sawamura T, Saku K. Dominant-negative lox-1 blocks homodimerization of wild-type lox-1-induced cell proliferation through extracellular signal regulated kinase 1/2 activation. Hypertension. 2006;48:294–300. doi: 10.1161/01.HYP.0000229825.98545.5e. [DOI] [PubMed] [Google Scholar]
- 15.Lee WJ, Ou HC, Hsu WC, Chou MM, Tseng JJ, Hsu SL, Tsai KL, Sheu WH. Ellagic acid inhibits oxidized LDL-mediated LOX-1 expression, ROS generation, and inflammation in human endothelial cells. J Vasc Surg. 2010;52:1290–1300. doi: 10.1016/j.jvs.2010.04.085. [DOI] [PubMed] [Google Scholar]
- 16.Lee HS, Lee MJ, Kim H, Choi SK, Kim JE, Moon HI, Park WH. Curcumin inhibits TNFalphainduced lectin-like oxidised LDL receptor-1 (LOX-1) expression and suppresses the inflammatory response in human umbilical vein endothelial cells (HUVECs) by an antioxidant mechanism. J Enzyme Inhib Med Chem. 2010;25:720–729. doi: 10.3109/14756360903555274. [DOI] [PubMed] [Google Scholar]
- 17.Honjo M, Nakamura K, Yamashiro K, Kiryu J, Tanihara H, McEvoy LM, Honda Y, Butcher EC, Masaki T, Sawamura T. Lectin-like oxidized LDL receptor-1 is a cell-adhesion molecule involved in endotoxin-induced inflammation. Proc Natl Acad Sci U S A. 2003;100:1274–1279. doi: 10.1073/pnas.0337528100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang P, Liu MC, Cheng L, Liang M, Ji HL, Fu J. Blockade of LOX-1 prevents endotoxin-induced acute lung inflammation and injury in mice. J Innate Immun. 2009;1:358–365. doi: 10.1159/000161070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibata Y, Kume N, Arai H, Hayashida K, Inui-Hayashida A, Minami M, Mukai E, Toyohara M, Harauma A, Murayama T, Kita T, Hara S, Kamei K, Yokode M. Mulberry leaf aqueous fractions inhibit TNF-alpha-induced nuclear factor kappaB (NF-kappaB) activation and lectin-like oxidized LDL receptor-1 (LOX-1) expression in vascular endothelial cells. Atherosclerosis. 2007;193:20–27. doi: 10.1016/j.atherosclerosis.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Vainer B, Berliner S, Nielsen OH. Spontaneous aggregation of leukocytes in active ulcerative colitis might be ICAM-1 dependent. Inflamm Res. 2004;53:458–461. doi: 10.1007/s00011-004-1280-2. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Chai L, Cai Z, Jin LJ, Chen Y, Wu H, Sun Z. Expression of survivin and caspase 3 in oral squamous cell carcinoma and peritumoraltissue. Asian Pac J Cancer Prev. 2012;13:5027–5031. doi: 10.7314/apjcp.2012.13.10.5027. [DOI] [PubMed] [Google Scholar]


