Abstract
Aims: To investigate the roles of CXCL16 and ox-LDL in adriamycin (ADR)-induced nephropathy mice and to explore the mechanism of simvastatin on the renal protective effects of ADR nephropathy. Methods: Fifteen male Balb/c mice were randomly divided into normal control (NC), ADR nephropathy and simvastatin-treated ADR nephropathy (ADR-SIM) groups. ADR nephropathy was induced by a single intravenous injection of ADR into the tail vein. All mice were sacrificed at the end of the 7th week, with the blood, 24-h urine and kidneys collected. The levels of ox-LDL and total cholesterol in the serum, the serum CXCL16, ox-LDL and NF-κB expression were detected. Results: Compared with the NC group, the levels of serum total cholesterol and ox-LDL in the ADR and ADR-SIM groups were significantly higher, the level of serum albumin was significantly lower and the expression of CXCL16, ox-LDL and NF-κB in the renal tissue of ADR and ADR-SIM groups was significantly increased. Compared with the ADR group, the expressions of renal CXCL16, ox-LDL and NF-κB in the ADR-SIM group were significantly decreased. Levels of serum total cholesterol and ox-LDL were not significantly different between the two groups. Conclusions: Simvastatin exerts a protective effect on renal function and structure in mice with ADR nephropathy. The beneficial effects of simvastatin might be related to the decreasing expression of CXCL16 in glomerular podocytes followed by the decreasing endocytosis of ox-LDL in podocytes and inhibition of NF-κB pathway activation.
Keywords: Balb/c mice, adriamycin, nephropathy, CXCL16, ox-LDL, simvastatin
Introduction
Minimal change nephritic syndrome (MCNS) is one of the most common histological subtypes of primary nephritic syndrome (NS) in children. Hyperlipidemia is an important pathophysiological change in children with primary NS and is characterized by high levels of plasma total cholesterol and low density lipoprotein (LDL). In 1982, Moorhead et al. proposed the theory of lipid nephrotoxicity resulting from abnormalities of lipid metabolism in chronic kidney disease [1]. Later, many studies suggested that hyperlipemia and lipid deposition in the kidneys were important risk factors for glomerulosclerosis [2-7]. Adriamycin (ADR)-induced nephropathy, which is very similar to minimal change disease and focal segmental glomerulosclerosis in glomerular disease, in mice is a classic experimental model of kidney disease [8,9].
CXC chemokine ligand 16 (CXCL16), a novel chemokine which was first described in macrophages at sites of atherosclerotic lesions, exists in both transmembrane-bound and soluble forms [10,11]. Several lines of evidence indicate that CXCL16 plays an important role in the pathogenesis of lupus nephritis [12], diabetic nephropathy [5,13] and chronic kidney diseases [14].
Accumulating evidence has suggested that statins confer renal protection in a variety of renal diseases by its pleiotropic effects independent of the cholesterol-lowering effect [15-17]. However, whether statins mediate CXCL16 expression to regulate the accumulation of ox-LDL in the kidneys of mice with ADR nephropathy remains unknown.
In this study, we demonstrated that simvastatin suppressed CXCL16 expression and decreased ox-LDL levels in the kidneys of mice with ADR-induced nephropathy while not significantly influencing serum lipids. We further provided evidence that simvastatin blocked the accumulation of ox-LDL in the kidneys by inhibiting CXCL16 expression, independent of its lipid-lowering effects. These observations suggest that CXCL16 may be a therapeutic target in primary NS.
Materials and methods
Antibodies and reagents
ADR was purchased from Pharmacia Italia S. P.A. (Italy). ox-LDL enzyme-linked immunosorbent assay (ELISA) kit was provided by Shanghai Bluegene Biotech Co., Ltd. (Shanghai, China). The following polyclonal antibodies (pAbs) were used: anti-NF-κB (Boster Biotechnology Company, Wuhan, China), anti-CXCL16 (Abcam, Cambridge, UK), anti-CXCL16 (Biorbyt, Cambridge, UK), anti-Synaptopodin Santa Cruz (Heidelberg, Germany) and anti-ox-LDL (Bioss, Beijing, China).
Experimental animals
Male Balb/c mice (5 weeks old; ADR-susceptible strain) with an average body weight of 18.6 g (Vital River Laboratory Animal Technology Co. Ltd., Beijing, China) were kept under standard conditions (constant temperature 21 ± 2°C, humidity 60 ± 10%, light/dark cycle 12 h) and received water and standard pellet chow. All animal procedures were performed according to the guidelines for the care and use of laboratory animals approved by Shandong University, China.
Experimental protocol and sample collection
The mice were randomly divided into the following groups (n=5 for each group): normal control group (NC group), ADR nephropathy group (ADR group) and simvastatin-treated ADR nephropathy group (ADR-SIM group). The ADR and ADR-SIM groups were induced by a single, slow intravenous injection of ADR (10.5 mg/kg body weight, diluted in 0.9% saline) into the tail vein. The NC group was injected with the same volume of 0.9% saline.
All mice were individually placed in metabolic cages for 24-h urine collection after 1 week of ADR injection. The mice with 24-h proteinuria levels of ≥ 50 mg/kg were included in this study. Mice in the ADR-SIM group were administered an oral gavage of 4 mg/kg simvastatin dissolved in 0.9% saline once per day beginning 1 week after ADR treatment. This dosage was selected because it did not affect serum lipid levels in a preliminary experiment. Both NC and ADR groups were treated with a comparable volume of 0.9% saline once per day by oral gavage. One week after ADR treatment and again at the end of the experiment (7 weeks), each of the mice was individually placed in metabolic cages, and the 24-h urine was collected. Following the last urine collection at the end of the experiment, all of the mice were anesthetized via intraperitoneal injection of 100 mg/kg sodium pentobarbital. Subsequently, blood was collected from the heart, and the kidneys were harvested. All experiments were performed with the approval of the Animal Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong University, China.
Urinary protein and blood sample analysis
The 24-h urinary protein excretion was measured using the Coomassie brilliant blue method [18]. Serum albumin and total cholesterol were measured using an automatic biochemical analyzer (Olympus AU5400; Tokyo, Japan). Serum level of ox-LDL was detected by ELISA at the end of the 7th week of the experiment. All specimens were processed and analyzed according to the instructions supplied by the manufacturer.
Histopathological examination
For histological examination, kidney tissues were fixed in 4% buffered paraformaldehyde immediately after the mice were sacrificed, embedded in paraffin, and then cut into 5-μm thick sections and stained with periodic acid-Schiff (PAS). Renal morphologic lesions were identified on ten randomly selected, non-overlapping specimens from each mouse at 400× magnification. All specimens were analyzed blindly by the same investigator. Glomerular and tubulointerstitial morphologic lesions were graded on a scale of 0 to 3+: 0, absence of lesions; 1+, lesions involving up to 25% of the component considered; 2+, lesions involving 25 to 50% of the component; 3+, lesions involving 50% or more of the component. The Chronicity Index (CI) was evaluated according to the sum of the following four components: (1) glomerulosclerosis, (2) fibrous crescents, (3) tubular atrophy and (4) interstitial fibrosis [19].
For ultrastructural examination, the renal cortex was fixed in 3% glutaraldehyde for 2 h immediately after the mice were sacrificed, post-fixed in 1% osmium tetroxide for 1 h, washed in acetone, and then embedded in Epon 812. The ultra-thin sections (60-70 nm) were stained with uranyl acetate and lead citrate, and then examined under a JEM-2000EX transmission electron microscope (JEOL Ltd., Tokyo, Japan).
Immunohistochemical assay
The upper pole of the right kidney was fixed in 10% formaldehyde immediately after the mice were sacrificed, dehydrated in graded alcohol, and then embedded in paraffin. Paraffin sections (5 μm) were deparaffinized in an environmentally-safe cleaning agent, rehydrated in graded alcohol, and then washed in phosphate-buffered saline (pH 7.4). Antigen retrieval was performed by boiling the tissue sections in water for 15 min in 0.01 mol/L sodium citrate buffer (pH 6.0) at 100°C and then cooling at 25°C for 2 h. Tissue sections were treated with 3% H2O2 for 30 min at 37°C to quench endogenous peroxide activity. After blocking the sections with 10% goat serum for 1 h at 37°C, the sections were incubated with rabbit anti-mouse NF-κB pAb at 4°C overnight, followed by incubation with horseradish peroxidase-labeled polymer conjugated to secondary goat anti-rabbit antibody at 37°C for 30 min. Sections were developed with diaminobenzidine (DAB) reagent, and then the nuclei were counterstained with hematoxylin. The primary antibody was substituted for PBS as a negative control, while the positive control was verified by confirming positively-stained tissue specimens. Images were collected using an ECLIPSE Ti scanning microscope (Nikon, Japan) and analyzed according to immunohistochemical score (IHS), as described previously by Soslow RA [20]. The slices were independently evaluated by two experienced pathologists.
Immunofluorescence analysis
For immunofluorescence analysis, the lower pole of the left kidney was cut into 5-μm sections and then fixed in acetone for 5-10 min. After incubation with 10% donkey serum for 1 h, tissue sections were incubated with primary antibodies overnight at 4°C, followed by incubation with secondary antibody at 37°C for 30 min. Nuclei were stained with 4’-6-diamidino-2-phenylindole, and slides were mounted in Fluoromount G (Southern Biotech, Birmingham, USA). Evaluation was performed using an ECLIPSE Ti inverted fluorescence microscope (Nikon, Japan) and analyzed using Image-Pro Plus 6.0 software (Media Cybernetic, Silver Spring, USA), as described previously by Zhang [18].
Western blot analysis
Renal cortex samples (100 mg) were lysed in protein lysis buffer for protein. Equal amounts of protein (100 μg/lane) were fractionated by SDS-PAGE (10%) and transferred to PVDF membranes. The membranes were then incubated with rabbit anti-mouse CXCL16 pAb or with rabbit anti-NF-kB pAb followed by species-specific horseradish peroxidase-coupled secondary antibodies. Antibody to β-actin was used to confirm the equal loading of samples.
The immune complexes were visualized under the Image Quant LAS 4000 Mini (GE Healthcare Life Sciences, Pittsburgh, USA). Multi Gauge 3.2 software was used for quantification.
Statistical analysis
The software used for statistical analysis was SPSS for Windows 19.0. The data are expressed as mean ± S.D. The two-tailed t-test was used to compare means among two groups. A value of P < 0.05 was considered a statistically significant difference between two groups.
Results
Effects of simvastatin on urinary protein, serum albumin, total cholesterol and ox-LDL of ADR mice
As shown in Figure 1, the ADR and ADR-SIM mice developed marked proteinuria, hypoalbuminemia and hyperlipidemia. The levels of 24-h urinary protein in the ADR and ADR-SIM groups were significantly higher than that in the NC group (P < 0.05), and the level of 24-h urinary protein in the ADR-SIM group was significantly lower than that in the ADR group (P < 0.05) (Figure 1A). The levels of serum albumin in the ADR and ADR-SIM groups were lower than that in the NC group (P < 0.05), and the level of serum albumin in the ADR-SIM group was significantly higher than that in the ADR group (P < 0.05) (Figure 1B). The levels of serum total cholesterol in the ADR and ADR-SIM groups were higher than that in the NC group (P < 0.05), though the level of serum total cholesterol in the ADR-SIM group was not significantly lower than that in the ADR group (P > 0.05) (Figure 1C). To our surprise, differences in serum ox-LDL levels among groups were consistent with that of serum total cholesterol (Figure 1D). The mean body weight of mice in the ADR and ADR-SIM groups was significantly lower than that of the NC group (P < 0.05), and the body weight of mice in the ADR-SIM group was significantly higher than that of the ADR group (P < 0.05) (Figure 1E).
Figure 1.
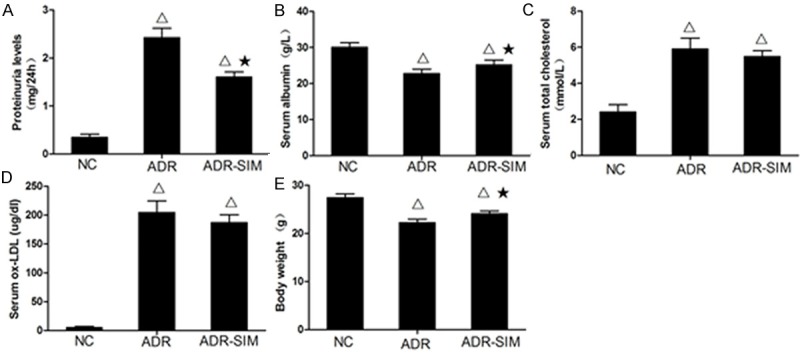
Differences in 24-h urine protein, blood protein and mean weight between groups. Levels of 24-hour urine protein (A), serum albumin (B), total cholesterol (C), serum ox-LDL (D) and body weight (E) in different groups. Data represent mean ± S.D. P < 0.05 vs. NC group (Δ), P < 0.05 vs. ADR group (★).
Effects of simvastatin on histopathological findings of ADR mice
The histology of NC group kidneys was normal under light microscopy (Figure 2A). The ADR group developed severe proliferation of mesangial cells, marked expansion of mesangial matrix, glomerular tufts adhering to Bowman’s space and focal segmental sclerosis (Figure 2B). The focal expansion of renal tubules, increase of local micro droplets in the tubular cells and thickening of the vascular wall were also noted in the kidneys of the ADR group. Compared with the ADR group, lesions were markedly reduced in the ADR-SIM group yet did not completely restore to a normal level (Figure 2C). Compared with the NC group, the CI of the ADR and ADR-SIM groups was significantly increased (P < 0.05), whereas the CI of the ADR-SIM group was significantly decreased compared to the ADR group (P < 0.05) (Figure 2D) yet did not completely restore to a normal level.
Figure 2.
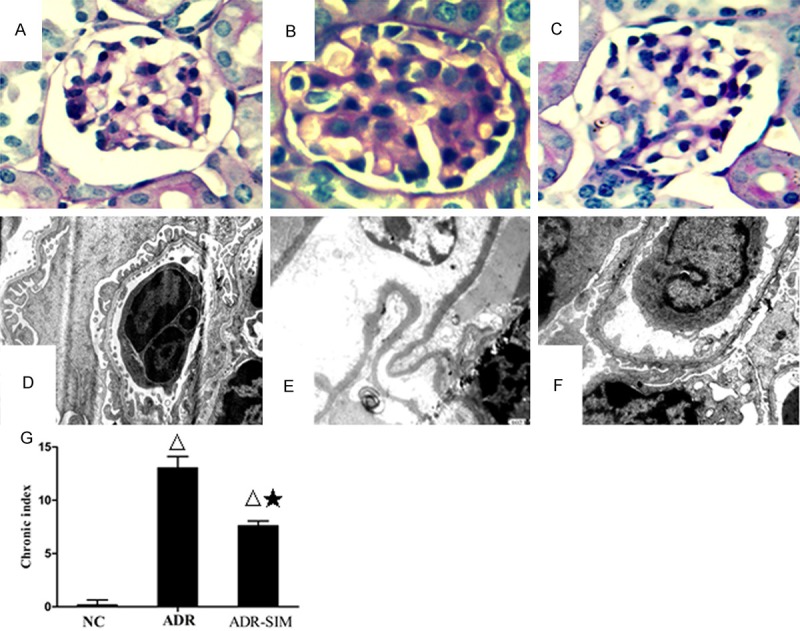
Renal pathological changes under light microscopy (×400) and electron microscopy (×10000). Renal pathological changes in the NC group (A), ADR group (B) and ADR-SIM group (C) (D) No thickening of GBM or fusion of foot processes in the NC group. (E) Marked thickening of GBM with the proliferation of mesangial matrix and mesangial interposition and extensive fusion of foot processes in the ADR group. (F) Thinning of thickened GBM and partial fusion of foot processes in the ADR-SIM group. (G) CI levels in different groups. Data represent mean ± S. D. P < 0.05 vs. NC group (Δ), P < 0.05 vs. ADR group (★).
The results from electron microscopy showed no thickening of the glomerular basement membrane (GBM) and no foot process fusion in the NC group (Figure 2E). Compared with the NC group, the GBM was pervasively thickened, the foot processes were widely fused, and some foot processes were effaced in the ADR group (Figure 2F). Compared with the ADR group, podocyte injury was markedly relieved, mesangial proliferation and thickening of the GBM in the ADR-SIM group were significantly reduced yet did not completely restore to a normal level (Figure 2G).
Effects of simvastatin on the expression of CXCL16 in the glomerulus
CXCL16 protein expression in the kidneys of mice from different groups was measured using Western blotting (Figure 3A). The expression of CXCL16 in the ADR group was significantly elevated compared to NC and ADR-SIM groups (P < 0.05), which suggested that treatment with simvastatin inhibited CXCL16 expression in the glomerulus (Figure 3B). To investigate the location of CXCL16 in the glomerulus, double immunofluorescence analysis of CXCL16 and synaptopodin (an actin-binding protein of the podocyte) expression in the kidney of the experimental animal was performed. Figure 3C illustrates that CXCL16 was located in the cytomembrane of podocytes.
Figure 3.
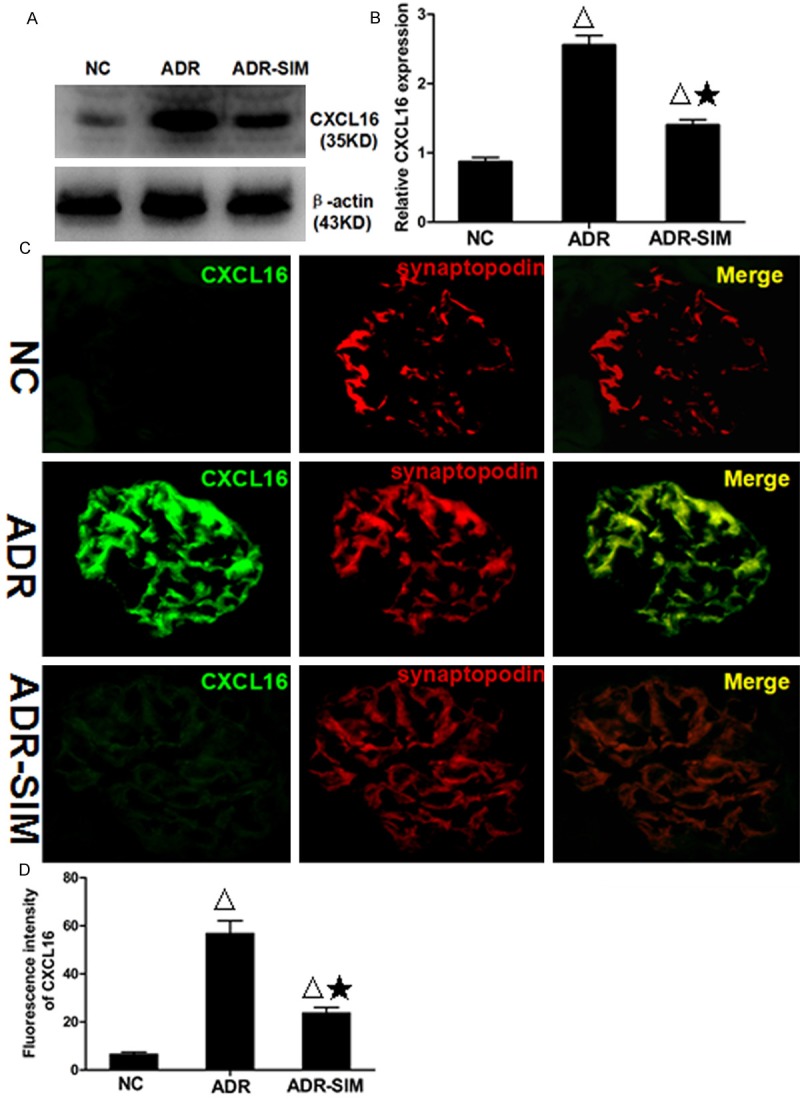
Expression of renal CXCL16 in the glomerulus of different groups by Western blotting and double immunofluorescence analysis. A. Levels of CXCL16 protein in the NC group, ADR nephropathy group and ADR-SIM group as assessed by Western blot. B. CXCL16 protein levels from Western blotting relative to the housekeeping gene protein, β-actin. C. Double immunofluorescence staining of glomeruli for CXCL16 and synaptopodin protein expression in different groups (×400). D. Immunofluorescence intensity of CXCL16 expression using Image-Pro Plus 6.0 software. Data represent mean ± S.D. P < 0.05 vs. control group (Δ), P < 0.05 vs. ADR group (★).
Effects of simvastatin on the expression of ox-LDL in the glomerulus
In the NC group glomerulus, little to no ox-LDL expression was present. However, in the ADR group, ox-LDL was significantly increased (P < 0.05) (Figure 4D), and following treatment with simvastatin, the level of ox-LDL was significantly decreased (P < 0.05) (Figure 4D). To investigate the location of ox-LDL expression in the glomerulus, double immunofluorescence analysis of ox-LDL and synaptopodin expression in the kidney of the experimental animal was performed. The expression of ox-LDL was mainly located in the cytomembrane and cytoplasm of podocytes (Figure 4A-C).
Figure 4.
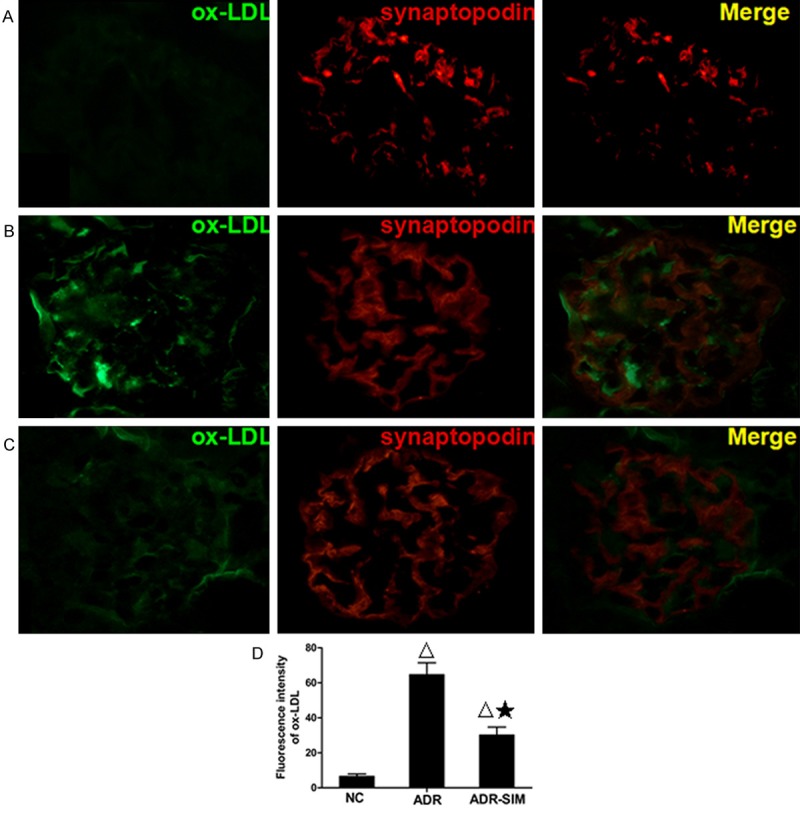
Expression of renal ox-LDL in the glomerulus of different groups by double immunofluorescence analysis (×400). A. Renal tissue of normal group with double immunofluorescence staining of ox-LDL (green fluorescence) and synaptopodin (red fluorescence) in glomerulus. B. Renal tissue of ADR group with double immunofluorescence staining of ox-LDL (green fluorescence) and synaptopodin (red fluorescence) in glomerulus. C. Renal tissue of ADR-SIM group with double immunofluorescence staining of ox-LDL (green fluorescence) and synaptopodin (red fluorescence) in glomerulus. D. Immunofluorescence intensity of ox-LDL expression using the Image-Pro Plus 6.0 software. Data represent mean ± S.D. P < 0.05 vs. NC group (Δ), P < 0.05 vs. ADR group (★).
Effects of simvastatin on the expression of NF-κB in the glomerulus
To evaluate NF-κB activity, we examined NF-κB protein expression in the glomerulus by Western blot and immunohistochemical analysis (Figure 5). The level of NF-κB in the ADR group was significantly higher than that in the NC and ADR-SIM groups (P < 0.05). In other words, treatment with simvastatin significantly decreased the level of NF-κB in the glomerulus (P < 0.05).
Figure 5.
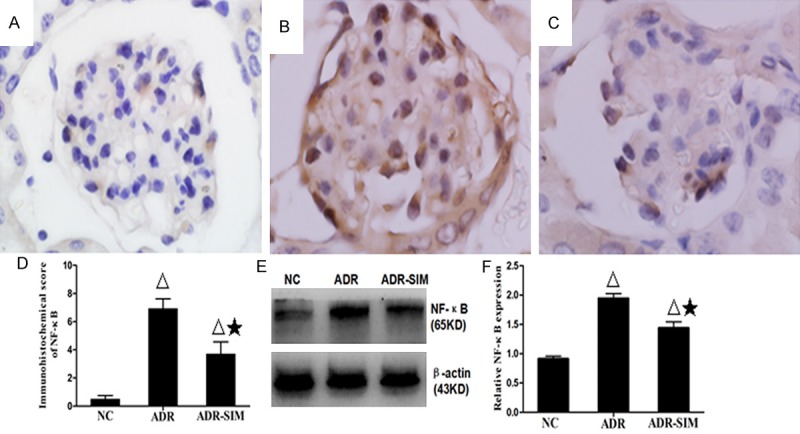
Expression of renal NF-κB expression in different groups. Immunohistochemical analysis of NF-κB expression in tissue sections of the NC group (A), ADR group (B) and ADR-SIM group (C) (×1000). (D) Immunohistochemical score of NF-κB expression in tissue sections. (E, F) Western blot analysis of renal NF-κB in different groups. Data represent mean ± S.D. P < 0.05 vs. NC group (Δ), P < 0.05 vs. ADR group (★).
Discussion
In children with primary NS, the MCNS histological subtype is one of the most common. Most children with MCNS are sensitive to steroid therapy and have a good prognosis once started on treatment. However, those with steroid-dependent or steroid-resistant NS may gradually progress to focal segmental glomerular nephritis and eventually to glomerulosclerosis leading to end-stage renal disease (ESRD) [21]. ADR-induced nephropathy in mice, which simulates the pathological characteristics of human NS, is a classic experimental model of kidney disease. The early pathological phenotype of ADR nephropathy is minimal change disease followed by focal segmental glomerulosclerosis, ultimately developing into global sclerosis and interstitial fibrosis [8,9]. In the present study, the Balb/c mice (an ADR-susceptible strain) were used to establish this ADR-induced nephropathy model. Following ADR injection and simvastatin treatment, both the ADR (untreated) and ADR-SIM (simvastatin-treated) groups displayed massive proteinuria, hypoalbuminemia and hyperlipidemia. Furthermore, histological analysis of the kidneys revealed distinct proliferation of mesangial cells, marked expansion of the mesangial matrix, glomerular tufts adhering to Bowman’s space and focal segmental sclerosis. CI values of the ADR and ADR-SIM groups were significantly increased, and the results from electron microscopy suggested that the GBM was pervasively thickened, and foot processes were widely fused. Collectively, these data indicated that the ADR-induced nephropathy model was successfully established.
Hyperlipidemia, which is characterized by increasing levels of total cholesterol and LDL in plasma, is an important pathophysiological change in children with primary NS. We documented that the level of serum oxidized LDL (ox-LDL) in children with active simple-type NS was significantly higher than that in remissive NS and control groups [22]. This data suggested that, compared to the control group, the levels of ox-LDL in the serum and kidneys of ADR nephropathy subjects were significantly increased. Overall, the data suggest that ox-LDL is involved in the development of ADR nephropathy.
Chemokines are a class of small secreted proteins involved in inflammation and the immune response. The interaction of chemokines and their receptors is a key mediator of inflammation and arteriosclerosis. CXCL16 exists in both transmembrane-bound and soluble forms [23,24]. Transmembrane-bound CXCL16 acts as both a cell surface adhesion molecule and a novel scavenger receptor and may be released to its soluble form upon digestion by a disintegrin and metalloproteinase (ADAM) protein, specifically ADAM10 and ADAM17. Soluble CXCL16 can recruit activated immune cells that express CXCR6, the receptor of CXCL16, and mediate immune response-related inflammation [10,25-27]. Recently, CXCL16 was found to participate in the development of atherosclerosis [10,11]. However, the relationship between the occurrence and development of renal inflammation and CXCL16, especially in its role as the scavenger receptor of ox-LDL during podocyte injury, is not yet well understood. Zhao et al. [13] showed that the levels of LDL cholesterol and CXCL16 in serum were significantly increased in diabetic nephropathy subjects compared with healthy and type 2 diabetes mellitus (T2DM) subjects, indicating that serum CXCL16 may be a sign of renal injury in subjects with T2DM. Moreover, in the presence or absence of a broad spectrum metalloproteinase inhibitor, treatment of human podocytes with IFN-γ promoted the uptake of ox-LDL, and the application of a CXCL16 blocking antibody strongly reduced the uptake of ox-LDL in human podocytes, suggesting that CXCL16 could also act as a scavenger receptor for ox-LDL in podocytes. Notably, glomerular ox-LDL and CXCL16 levels were significantly higher in subjects with membranous nephropathy than in healthy subjects [11]. However, few studies have focused on the association of CXCL16 alteration in children with primary NS and ADR nephropathy in mice. We have documented that the level of serum CXCL16 in children with active NS was significantly higher than that in remissive NS and control groups. Correlation analysis showed that serum CXCL16 was positively correlated with blood lipids and 24-h urine protein but negatively correlated with albumin in active NS patients, which indicated that CXCL16 and ox-LDL are involved in the occurrence of NS in children and are related with the activity of the disease [22]. Our previous in vitro study indicated that the presence of ox-LDL at a certain concentration and for a certain duration with cultured podocytes induced lipid accumulation and expression of CXCL16 in the podocytes. When CXCL16 on the cell surface was blocked by anti-CXCL16 monoclonal antibodies, the uptake of ox-LDL by podocytes was significantly decreased, suggesting that CXCL16 plays an essential role as a scavenger receptor in the uptake of ox-LDL by podocytes [28].
Synaptopodin is an actin filament-associated protein located along the surface of the glomerular basement membrane and is considered the specific biomarker of podocytes [29]. In the present study, using double immunofluorescence of Synaptopodin with CXCL16 and ox-LDL respectively, we demonstrated that CXCL16 and ox-LDL were mainly expressed in the podocytes. Compared to the control group, glomerular CXCL16 and ox-LDL levels were increased in the ADR nephropathy group. These data, together with our previous studies, suggest that CXCL16 acts as a scavenger receptor for ox-LDL in podocytes and is involved in podocytes injury during the occurrence and development of ADR nephropathy. Studies in vitro have demonstrated that the major components of lipids, including LDL, ox-LDL and very low-density lipoprotein, directly stimulate the proliferation of mesangial cells and the secretion of inflammatory factors, such as IL-6, TGF-β, MCF-1, connective tissue growth factor and PDGF. LDL and ox-LDL also promote the activation of renal immune cells, subsequently upregulating NF-κB activity and hastening the release of inflammatory factors [30].
Using immunohistochemistry and Western blot analysis, we provided conclusive evidence that the level of glomerular NF-κB in the ADR nephropathy group was significantly increased compared with control group. These data indicate that the elevated CXCL16 expression can result in the increasing intake of ox-LDL in the podocytes. The upregulation of ox-LDL may be involved in the activation of the NF-κB pathway. Activation of the NF-κB pathway is often associated with increased expression of a variety of inflammatory factors that could amplify and perpetuate renal local inflammatory responses. However, the precise mechanism by which ox-LDL affects the renal local inflammatory response of ADR nephropathy mice remains unknown and should be investigated further.
Statins, competitive inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, reduce cholesterol synthesis from mevalonic acid through inhibition of HMG-CoA reductase [31]. Simvastatin is generally the most effective statin and is widely used in the treatment of hypercholesterolemia. Statins confer renal protection in a variety of renal diseases not only through their lipid-lowering effects, but also through promoting anti-proliferation, anti-inflammation, immunomodulation, reduction of urine protein and podocyte excretion in urine, reversion of podocyte injury and reduction of podocyte apoptosis [15-17,32-35]. Zhang et al. [18] proved that the beneficial effects of simvastatin on glomerulosclerosis might be mediated by the effects of anti-inflammatory action through a reduction of NF-κB activation and IL-1β and TGF-β expression in ADR nephropathy rats. Whether statins can regulate the expression of CXCL16 and ox-LDL in the kidneys of mice with ADR nephropathy to protect renal functions, however, remains unknown. The current study revealed that, compared to the ADR nephropathy group, the level of proteinuria and the quantity of renal lesions in the ADR-SIM group were markedly reduced, suggesting that simvastatin has a protective effect on renal function and structure in mice with ADR nephropathy. Moreover, our data revealed the relatively high levels of CXCL16 and ox-LDL expression in podocytes and low level of NF-κB expression in the glomerulus in the ADR-SIM group without the lowering of serum lipids. These changes suggest that simvastatin treatment might be related to the decreasing expression of CXCL16 in glomerular podocytes followed by the decreasing endocytosis of ox-LDL in podocytes and the inhibition of NF-κB pathway activation independent of its lipid-lowering effects. The precise mechanism by which simvastatin inhibits CXCL16 expression in ADR nephropathy mice remains unknown and should be investigated further.
In conclusion, this study reveals that CXCL16 and ox-LDL are involved in the occurrence and development of ADR nephropathy and are related with the severity of the disease. Furthermore, the up-regulation of local inflammatory factors in the kidney is associated with sustainable hyperlipidemia in mice with ADR nephropathy. Simvastatin was shown to have a protective effect on renal function and structure in mice with ADR nephropathy independent of its lipid-lowering effects possibly via decreasing the expression of CXCL16 in glomerular podocytes, which then reduces the uptake of ox-LDL in podocytes and inhibits the activation of the NF-κB pathway. The beneficial effects of simvastatin might therefore be associated with the decreasing of the renal local inflammatory reaction mediated by renal inflammatory factors.
Acknowledgements
This study was supported by the Shandong Province Science and Technology Development Program (2014GGH218009) and the Natural Science Foundation of Shandong Province (ZR2015HM009).
Disclosure of conflict of interest
None.
References
- 1.Moorhead JF, Chan MK, El-Nahas M, Varghese Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet. 1982;2:1309–1311. doi: 10.1016/s0140-6736(82)91513-6. [DOI] [PubMed] [Google Scholar]
- 2.Saland JM, Pierce CB, Mitsnefes MM, Flynn JT, Goebel J, Kupferman JC, Warady BA, Furth SL. Dyslipidemia in children with chronic kidney disease. Kidney Int. 2010;78:1154–1163. doi: 10.1038/ki.2010.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee HS, Song CY. Oxidized low-density lipoprotein and oxidative stress in the development of glomerulosclerosis. Am J Nephrol. 2009;29:62–70. doi: 10.1159/000151277. [DOI] [PubMed] [Google Scholar]
- 4.Garcia GE, Truong LD, Li P, Zhang P, Johnson RJ, Wilson CB, Feng L. Inhibition of CXCL16 attenuates inflammatory and progressive phases of anti-glomerular basement membrane antibody-associated glomerulonephritis. Am J Pathol. 2007;170:1485–1496. doi: 10.2353/ajpath.2007.060065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutwein P, Abdel-Bakky MS, Doberstein K, Schramme A, Beckmann J, Schaefer L, Amann K, Doller A, Kampfer-Kolb N, Abdel-Aziz AA, El Sayed el SM, Pfeilschifter J. CXCL16 and oxLDL are induced in the onset of diabetic nephropathy. J Cell Mol Med. 2009;13:3809–3825. doi: 10.1111/j.1582-4934.2009.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okamura DM, Lopez-Guisa JM, Koelsch K, Collins S, Eddy AA. Atherogenic scavenger receptor modulation in the tubulointerstitium in response to chronic renal injury. Am J Physiol Renal Physiol. 2007;293:F575–585. doi: 10.1152/ajprenal.00063.2007. [DOI] [PubMed] [Google Scholar]
- 7.Wu T, Xie C, Wang HW, Zhou XJ, Schwartz N, Calixto S, Mackay M, Aranow C, Putterman C, Mohan C. Elevated urinary VCAM-1, P-selectin, soluble TNF receptor-1, and CXC chemokine ligand 16 in multiple murine lupus strains and human lupus nephritis. J Immunol. 2007;179:7166–7175. doi: 10.4049/jimmunol.179.10.7166. [DOI] [PubMed] [Google Scholar]
- 8.Chen A, Sheu LF, Ho YS, Lin YF, Chou WY, Chou TC, Lee WH. Experimental focal segmental glomerulosclerosis in mice. Nephron. 1998;78:440–452. doi: 10.1159/000044974. [DOI] [PubMed] [Google Scholar]
- 9.Manabe N, Kinoshita A, Yamaguchi M, Furuya Y, Nagano N, Yamada-Uchio K, Akashi N, Miyamoto-Kuramitsu K, Miyamoto H. Changes in quantitative profile of extracellular matrix components in the kidneys of rats with adriamycin-induced nephropathy. J Vet Med Sci. 2001;63:125–133. doi: 10.1292/jvms.63.125. [DOI] [PubMed] [Google Scholar]
- 10.Abel S, Hundhausen C, Mentlein R, Schulte A, Berkhout TA, Broadway N, Hartmann D, Sedlacek R, Dietrich S, Muetze B, Schuster B, Kallen KJ, Saftig P, Rose-John S, Ludwig A. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J Immunol. 2004;172:6362–6372. doi: 10.4049/jimmunol.172.10.6362. [DOI] [PubMed] [Google Scholar]
- 11.Gutwein P, Abdel-Bakky MS, Schramme A, Doberstein K, Kampfer-Kolb N, Amann K, Hauser IA, Obermuller N, Bartel C, Abdel-Aziz AA, El Sayed el SM, Pfeilschifter J. CXCL16 is expressed in podocytes and acts as a scavenger receptor for oxidized low-density lipoprotein. Am J Pathol. 2009;174:2061–2072. doi: 10.2353/ajpath.2009.080960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh S, Wu T, Xie C, Vanarsa K, Han J, Mahajan T, Oei HB, Ahn C, Zhou XJ, Putterman C, Saxena R, Mohan C. Urine VCAM-1 as a marker of renal pathology activity index in lupus nephritis. Arthritis Res Ther. 2012;14:R164. doi: 10.1186/ar3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao L, Wu F, Jin L, Lu T, Yang L, Pan X, Shao C, Li X, Lin Z. Serum CXCL16 as a novel marker of renal injury in type 2 diabetes mellitus. PLoS One. 2014;9:e87786. doi: 10.1371/journal.pone.0087786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Z, Gong Q, Zhou Z, Zhang W, Liao S, Liu Y, Yan X, Pan X, Lin S, Li X. Increased plasma CXCL16 levels in patients with chronic kidney diseases. Eur J Clin Invest. 2011;41:836–845. doi: 10.1111/j.1365-2362.2011.02473.x. [DOI] [PubMed] [Google Scholar]
- 15.Usui H, Shikata K, Matsuda M, Okada S, Ogawa D, Yamashita T, Hida K, Satoh M, Wada J, Makino H. HMG-CoA reductase inhibitor ameliorates diabetic nephropathy by its pleiotropic effects in rats. Nephrol Dial Transplant. 2003;18:265–272. doi: 10.1093/ndt/18.2.265. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura T, Ushiyama C, Hirokawa K, Osada S, Inoue T, Shimada N, Koide H. Effect of cerivastatin on proteinuria and urinary podocytes in patients with chronic glomerulonephritis. Nephrol Dial Transplant. 2002;17:798–802. doi: 10.1093/ndt/17.5.798. [DOI] [PubMed] [Google Scholar]
- 17.Cormack-Aboud FC, Brinkkoetter PT, Pippin JW, Shankland SJ, Durvasula RV. Rosuvastatin protects against podocyte apoptosis in vitro. Nephrol Dial Transplant. 2009;24:404–412. doi: 10.1093/ndt/gfn528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, Li Q, Wang L, Yang X. Simvastatin ameliorates glomerulosclerosis in Adriamycin-induced-nephropathy rats. Pediatr Nephrol. 2008;23:2185–2194. doi: 10.1007/s00467-008-0933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gamba G, Reyes E, Angeles A, Quintanilla L, Calva J, Pena JC. Observer agreement in the scoring of the activity and chronicity indexes of lupus nephritis. Nephron. 1991;57:75–77. doi: 10.1159/000186220. [DOI] [PubMed] [Google Scholar]
- 20.Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637–2645. doi: 10.1002/1097-0142(20001215)89:12<2637::aid-cncr17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 21.Shutto Y, Yamabe H, Shimada M, Fujita T, Nakamura N. Quality of life in patients with minimal change nephrotic syndrome. ScientificWorldJournal. 2013;2013:124315. doi: 10.1155/2013/124315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhen J, Li Q, Zhu Y, Yao X, Wang L, Zhou A, Sun S. Increased serum CXCL16 is highly correlated with blood lipids, urine protein and immune reaction in children with active nephrotic syndrome. Diagn Pathol. 2014;9:23. doi: 10.1186/1746-1596-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joles JA, Kunter U, Janssen U, Kriz W, Rabelink TJ, Koomans HA, Floege J. Early mechanisms of renal injury in hypercholesterolemic or hypertriglyceridemic rats. J Am Soc Nephrol. 2000;11:669–683. doi: 10.1681/ASN.V114669. [DOI] [PubMed] [Google Scholar]
- 24.Lee HS, Jeong JY, Kim BC, Kim YS, Zhang YZ, Chung HK. Dietary antioxidant inhibits lipoprotein oxidation and renal injury in experimental focal segmental glomerulosclerosis. Kidney Int. 1997;51:1151–1159. doi: 10.1038/ki.1997.158. [DOI] [PubMed] [Google Scholar]
- 25.Gough PJ, Garton KJ, Wille PT, Rychlewski M, Dempsey PJ, Raines EW. A disintegrin and metalloproteinase 10-mediated cleavage and shedding regulates the cell surface expression of CXC chemokine ligand 16. J Immunol. 2004;172:3678–3685. doi: 10.4049/jimmunol.172.6.3678. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig A, Hundhausen C, Lambert MH, Broadway N, Andrews RC, Bickett DM, Leesnitzer MA, Becherer JD. Metalloproteinase inhibitors for the disintegrin-like metalloproteinases ADAM10 and ADAM17 that differentially block constitutive and phorbol ester-inducible shedding of cell surface molecules. Comb Chem High Throughput Screen. 2005;8:161–171. doi: 10.2174/1386207053258488. [DOI] [PubMed] [Google Scholar]
- 27.Wagsater D, Olofsson PS, Norgren L, Stenberg B, Sirsjo A. The chemokine and scavenger receptor CXCL16/SR-PSOX is expressed in human vascular smooth muscle cells and is induced by interferon gamma. Biochem Biophys Res Commun. 2004;325:1187–1193. doi: 10.1016/j.bbrc.2004.10.160. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Sun S, Zhou A, Yao X, Wang Y. oxLDL-induced lipid accumulation in glomerular podocytes: role of IFN-gamma, CXCL16, and ADAM10. Cell Biochem Biophys. 2014;70:529–538. doi: 10.1007/s12013-014-9952-1. [DOI] [PubMed] [Google Scholar]
- 29.Liao R, Liu Q, Zheng Z, Fan J, Peng W, Kong Q, He H, Yang S, Chen W, Tang X, Yu X. Tacrolimus Protects Podocytes from Injury in Lupus Nephritis Partly by Stabilizing the Cytoskeleton and Inhibiting Podocyte Apoptosis. PLoS One. 2015;10:e0132724. doi: 10.1371/journal.pone.0132724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chana RS, Wheeler DC. Fibronectin augments monocyte adhesion to low-density lipoprotein-stimulated mesangial cells. Kidney Int. 1999;55:179–188. doi: 10.1046/j.1523-1755.1999.00250.x. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 32.Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a metaanalysis. J Am Soc Nephrol. 2006;17:2006–2016. doi: 10.1681/ASN.2006010012. [DOI] [PubMed] [Google Scholar]
- 33.Bussolati B, Deregibus MC, Fonsato V, Doublier S, Spatola T, Procida S, Di Carlo F, Camussi G. Statins prevent oxidized LDL-induced injury of glomerular podocytes by activating the phosphatidylinositol 3-kinase/AKT-signaling pathway. J Am Soc Nephrol. 2005;16:1936–1947. doi: 10.1681/ASN.2004080629. [DOI] [PubMed] [Google Scholar]
- 34.Tonolo G, Velussi M, Brocco E, Abaterusso C, Carraro A, Morgia G, Satta A, Faedda R, Abhyankar A, Luthman H, Nosadini R. Simvastatin maintains steady patterns of GFR and improves AER and expression of slit diaphragm proteins in type II diabetes. Kidney Int. 2006;70:177–186. doi: 10.1038/sj.ki.5001515. [DOI] [PubMed] [Google Scholar]
- 35.Wei P, Grimm PR, Settles DC, Balwanz CR, Padanilam BJ, Sansom SC. Simvastatin reverses podocyte injury but not mesangial expansion in early stage type 2 diabetes mellitus. Ren Fail. 2009;31:503–513. doi: 10.1080/08860220902963848. [DOI] [PMC free article] [PubMed] [Google Scholar]


