Abstract
Lymphangiogenesis has received considerable attention and become a new research hotspot of tumor metastasis. Recently, C-C chemokine receptor 7 (CCR7) is known to promote metastasis of non-small cell lung cancer (NSCLC) cells into lymph nodes. In this study, we investigated the relationship between CCL21/CCR7 and the lymphangiogenic factor vascular endothelial growth factor (VEGF)-D in human lung cancer cells and its impact on patients’ prognosis. We found that CCL21/CCR7 increase the expression of VEGF-D in NSCLC Cell Lines through induced ERK1/2 and Akt phosphorylation. In addition, our study found that the expression levels of CCR7 and CCL21 were correlated with VEGF-D, lymphatic vessels density (LVD), clinical stages, lymph node metastasis, and patient Survival in 90 human non-small cell lung cancer (NSCLC) specimens. Taken together, our results provide evidence that CCL21/CCR7 induce VEGF-D up-regulation and promote lymphangiogenesis via ERK/Akt pathway in lung cancer.
Keywords: CCR7, VEGF-D, NSCLC, lymphangiogenesis
Introduction
Tumor invasion and metastasis are the key malignant characteristics of human cancers. Mortality of tumor patients principally resulted from cancer cells spread to distant organs [1]. Lymph node involvement was one of the important features of metastatic cancers. Recent researches strongly implicated that lymphatic vessels played an important role in tumor cells dissemination. So to clarify the mechanism of the cancer cells lymphatic metastasis was a challenging and important subject.
VEGF-C and VEGF-D was two major lymph-angiogenic factors, which had been linked to induce lymph-angiogenesis in animal models [2,3]. The binding of VEGF receptor (VEGFR)-3 and VEGF-C or VEGF-D stimulated the proliferation and migration of lymphatic endothelial cells [4], which following increased the numbers of new lymphatic vessels. The high expression of VEGF-D strongly correlated with metastases in regional lymph nodes in human bladder cancer, papillary thyroid, gastric, colorectal, prostate, breast, ovarian, and lung carcinomas [5-12]. Immunohistochemical staining demonstrated that VEGF-D was significantly elevated within many tumor cells [13], suggesting that tumor cells played a critical role in generating VEGF-D and activating the lymph-angiogenesis. But the mechanism of VEGF-D up-regulated expression in tumor cells is poorly understood.
CCR7 is a chemokine receptor, and is expressed on naive T and B-cells, some central memory T-cells and mature dendritic cells (DCs). The interactions between CCR7 and its ligands (chemokines CCL19 and CCL21) play a central role in lymphocyte trafficking and homing to lymph nodes during immune and inflammatory reactions [14,15]. Recently, CCR7 expression in several different types of cancer was confirmed including breast cancer, head and neck squamous cell carcinoma, esophageal squamous cell carcinoma, lung cancer, melanoma [16-22]. Some mechanisms of CCR7-mediated metastasis have been proposed, Cuesta-Mateos C et al. reported that CCL19/21 was markedly reduced by inhibitors of PI3K and the Rho effect or molecule and MAPKs signal transduction pathways, which could mediate B-Chronic Lymphocytic Leukemia cells migration and survival [23]. Wang J et al. had reported that CCR7 could activate phosphoinositide-3 kinase and mediated invasion in head and neck cancer cells [24]. Recently, a critical role of CCR7 in lymph-angiogenesis has been reported in SW620 human colon cancer cells nude mice xenograft model [20]. However, the precise mechanisms of CCR7 in lymph-angiogenesis remain to be determined.
In this study, we discovered that VEGF-D was one of the major downstream genes of CCR7 in human lung cancer cells. The up-regulation of VEGF-D by CCR7 was mediated by the ERK1/2 and Akt pathway. CCR7-induced VEGF-D up-regulation correlated with clinical stages, the lymph node metastasis and prognosis in NSCLC patients. Our findings open newer therapeutic modalities against lymphatic metastasis by targeting CCR7.
Material and methods
Antibodies and reagents
Polyclonal anti-CCR7, VEGF-D, Akt, ERK1/2, and p-ERK1/2 antibodies and monoclonal anti-β-actin antibody were purchased from Santa Cruz Biotechnology(USA). Polyclonal p-akt antibody was purchased from Cell signaling (USA). Monoclonal D2-40 (ready to use) antibody and 3, 3’-diaminobenzidine tetrahydrochloride (DAB) were purchased from MaiXin Biotechnology (China). P-Akt inhibitor (LY294002) and p-ERK1/2 inhibitor (PD98059) were purchased from Cell signaling (USA).
Cell culture
Human lung cancer cell lines A549 (pulmonary adenocarcinoma cell), NCI-H460 (pulmonary large carcinoma cell), SPC-A1 (pulmonary adenocarcinoma cell), BE1 (pulmonary large carcinoma cell), LTE (pulmonary adenocarcinoma cell), were maintained in RPMI1640 supplemented 10% fetal bovine serum (Gibco, USA) with 100 units/ml streptomycin, and 100 units/ml penicillin in a humidified 5% CO2 atmosphere until 75% confluent. Cells were incubated with RPMI1640 including respectively CCL19 (100 ng/ml), anti-CCR7 antibody (50 µg/ml), p-ERK1/2 inhibitor PD98059 (50 µmol/ml), p-Akt inhibitor LY294002 (50 µmol/m).
Stable transfection of CCR7
The plasmid pLNCX2-CCR7 was kindly provided by Dr. Sam T. Hwang (National Institutes of Health USA). A549 cells were stably transfected pLNCX2-CCR7 in the presence of Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions and placed under G418 (500 µg/ml) selection for 4 weeks. Surviving colonies were isolated and expanded.
Western blot analysis
The protein was extracted with lysis buffer (150 mM NaCl, 1% NP-40, 0.1% SDS, 2 μg/ml aprotinin, 1 mM PMSF) for 1 h at 4°C. The supernatants were centrifuged at 12000×g for 30 min at 4°C. The supernatants containing total protein were harvested. Aliquots containing 80 μg of proteins were separated on a 10% SDS-polyacrylamide gel and transferred to polyvinylidene fluoride (PVDF) membranes. After blocking, blots were respectively incubated with different primary antibodies and followed by each corresponding second antibody at room temperature for 2 h. The EC3 Imaging system (UVP Inc.) was used to catch up the specific bands, and the optical density of each band was measured using the Image J software.
RNA isolation and reverse transcriptase-PCR
Total RNA was isolated using TRIZOL (Invitrogen USA) according to the manufacturer’s instructions. The primer sequences are: CCR7, 5’-GAG GCT ATT GTC CCC TAA ACC-3’ (forward) and 5’-TGG AGG ACA GTG AAG AAA ACG-3’ (reverse). VEGF-D, 5’-TCA GCA TCC CAT CGG TCC ACT AG-3’ (forward) and 5’-CAA CAG CCA CCA CAT CGG AAC AC-3’ (reverse) and β-actin, 5’-AAA TCG TGC GTG ACA TTA A-3’ (forward) and 5’-CTC GTC ATA CTC CTG CTT G-3’ (reverse). Reaction mixture was first denatured at 95°C for 10 min. The PCR condition was 95°C for 1 min, 59°C for 1 min, and 72°C for 1 min for 30 cycles, followed by 72°C for 5 min. PCR products were visualized by ethidium bromide staining after agarose gel electrophoresis.
siRNA treatment of cells
Design of siRNA and Transfection of A549 cells. Small interference RNA (siRNA) specific to CCR7 was designed as follow: sense 5’-GGA CGT GCG GAA CTT TAA AdTdT-3’; antisense 5’-TTT AAA GTT CCG CAC GTC CdTdT-3’. Control scrambled siRNA sequences: 5’-GAC TTC ATA AGG CGC ATG CdTdT-3’; antisense 5’-GCATGCGCCTTATGAAGTCdTdT-3’. Cells were plated onto 10-cm2 cell culture dishes and grown to 30-50% confluence before transfection. The duplexes were diluted to give a final concentration of 30 nM. Transfections were performed with Lipofectamine 2000 (Invitrogen) and by following the manufacturer’s instructions.
Patients and specimens
A total of 90 cases of NSCLC were obtained from the 1st January 1980 to the 31st December 2005 at the First Affiliated Hospital of China Medical University. The tumor tissues in this study were from patients who had NSCLC proved by pathological diagnosis without distant metastasis. None of the 90 cases had received radiation therapy or chemotherapy before surgery. The NSCLC stage classification was based on the TNM staging system of the UICC. The survival time was calculated from the operation day to death until the last follow-up date (December 2006). The following-up of the surviving patients averaged 32.55 months and ranged from 1 to 96 months. Clinicopathological information of the patients about age, tumor size, clinical stage, histological type, differentiation, and lymph node metastasis was summarized in Table 2. This study has been approved by hospital ethical committee.
Table 2.
Correlation of clinicopathologic parameters with CCR7 and VEGF-D expressions in NSCLCs
| Factor | No. of cases | CCR7 Expression | P value | VEGF-D Expression | P value | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Lower | Higher | Lower | Higher | ||||
| LVD | 20.58±7.6 | 28.09±10.57 | 0.001 | 19.38±7.22 | 30.14±9.87 | < 0.001 | |
| Age | |||||||
| ≤ 50 | 50 | 13 | 37 | 20 | 30 | ||
| > 50 | 40 | 14 | 26 | 0.355 | 16 | 24 | 1.000 |
| Tumor size | |||||||
| T1+T2 | 38 | 12 | 26 | 19 | 19 | ||
| T3+T4 | 52 | 15 | 37 | 0.78 | 17 | 35 | 0.098 |
| Histology | |||||||
| Sq | 55 | 17 | 38 | 23 | 32 | ||
| Ad | 35 | 10 | 25 | 0.813 | 13 | 22 | 0.659 |
| Differentiation | |||||||
| Well | 14 | 4 | 10 | 4 | 10 | ||
| Poor-moderate | 76 | 23 | 53 | 0.899 | 32 | 44 | 0.342 |
| Stage | |||||||
| I+II | 39 | 18 | 21 | 22 | 17 | ||
| III+IV | 51 | 9 | 42 | 0.003 | 14 | 37 | 0.005 |
| Lymph node status | |||||||
| Negative | 36 | 17 | 19 | 22 | 14 | ||
| Positive | 54 | 10 | 44 | 0.004 | 14 | 40 | 0.001 |
Immunohistochemistry and quantitation of lymphatic vessel densities
4 μm sections were prepared from the paraffin-embedded tissues. Immunostaining was performed by the streptavidin-peroxidase (S-P) method (UltrasensitiveTM MaiXin, Fuzhou, China). The primary antibodies were anti-D2-40 antibody, anti-CCR7 (1:200) and anti-VEGF-D antibody (1:150). The peroxidase reaction was developed with 3, 3’-diaminobenzidine tetrahydrochloride (DAB, MaiXin, Fuzhou, China), the primary antibodies were replaced by non-immune serum for negative control. The percentage of positive tumor cells (0% = negative, 1-50% = 1, 51-75% = 2, ≥ 76% = 3) and the intensity of CCR7 or VEGF-D immunostaining (1 = weak, 2 = moderate, and 3 = intense) were assessed in at least 5 high power fields (×400 magnification). The scores of each tumorous sample were multiplied to give a final score of 0, 1, 2, 3, 4, 6 or 9, and the tumors were finally determined as low expression: score ≤ 4; or high expression: score ≥ 6. If the difference between the numbers counted by two pathologists was more than 10%, the lumens were recounted and a consensus between each observer was reached. We identified the area containing the most D2-40-positive vessels (hot spot) for determination of lymphatic vessel density by scanning the sections at low magnification. Lymphatic vessel density was then determined by counted the number of D2-40-positive vessels in a blinded manner from three areas of the highest vessel density/section at high-power fields. Three sections/tumors were analyzed. Each stained lumen was regarded as a single countable microvessel. All the samples were evaluated by 2 independent pathologists.
Statistical analysis
The statistical package SPSS16.0 (SPSS incorporated, Chicago) was used for all analysis. All values were expressed as mean ± SD. The Chi-square test was used to determine the correlation between CCR7 expression and clinicopathological factors; Kaplan-Meier curves were used for survival analysis, and log-rank was determined based on the differences. Other results were analyzed using the t-test. Values of P < 0.05 were considered statistically significant.
Results
CCR7 up-regulated VEGF-D in NSCLC Cell Lines
To study whether the lymphangiogenic factor VEGF-D was the downstream effectors gene of CCR7 in human lung cancer, we developed an CCR7 over-expressed cell clone (BE1-CCR7 cells) through transducing retroviral vectors carrying CCR7 gene to BE1 cells (human big cell lung cancer) and observed the transfection efficiency by RT-PCR and western blot. Meanwhile, we analyzed the expression of VEGF-D by RT-PCR and western blotting. The results showed that CCR7 was over-expressed in BE1-CCR7 cells than control cells both at mRNA and protein levels (Figure 1A), simultaneously the expression of VEGF-D mRNA and protein in BE1 cells were increased after CCR7 over-expression (Figure 1B).
Figure 1.
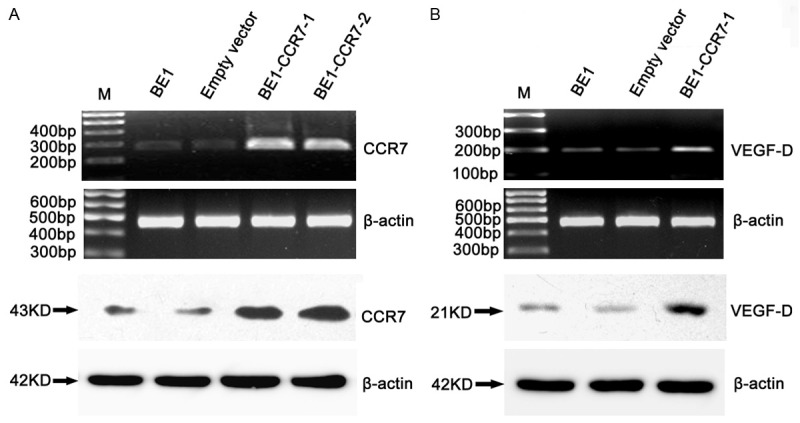
CCR7 up-regulates VEGF-D in NSCLC cells. CCR7-overexpressed cells (BE1-CCR7-1, BE1-CCR7-2), and the vector control cells were obtained as described under “Materials and Methods.” A. The expressions of CCR7 mRNA (top) and protein (bottom) were increased by RT-PCR and western blotting. B. RT-PCR and western blotting analyses of VEGF-D and β-actin mRNA (top) and protein (bottom) in CCR7 tansfected cells (BE1-CCR7-1).
We additionally examined whether CCR7 regulation of the VEGF-D gene in other five human lung cancer cell lines. We found a correlation between the expression levels of CCR7 and VEGF-D mRNA (Figure 2A). Consistent to mRNA levels, western blot showed that high expression levels of CCR7 protein also displayed greater abundance of VEGF-D protein (Figure 2B). Treatment of A549 cells with CCR7 antibody could significantly attenuate the endogenous VEGF-D protein level (Figure 2C). Genetic inhibition of CCR7 by siRNA method significantly decreased VEGF-D expression in A549 cells (Figure 2D). These data demonstrated that CCR7 could up-regulate VEGF-D expression in the NSCLC cells.
Figure 2.
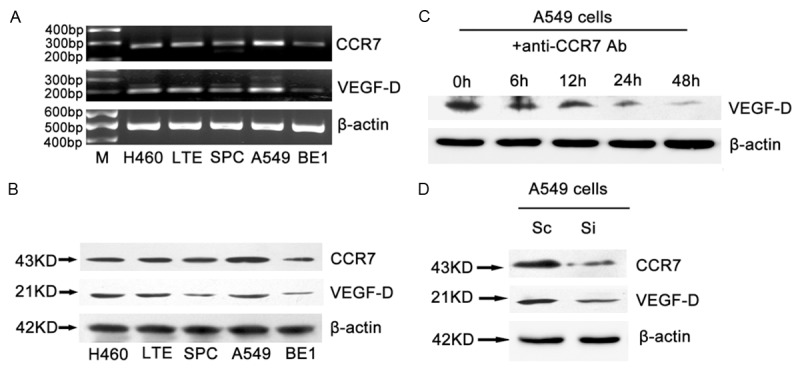
VEGF-D expression was correlated with CCR7 in different NSCLC cell lines and reduced by CCR7 antibody. The correlation between the VEGF-D and CCR7 in mRNA (A) and protein (B) levels in NSCLC cell lines. Cells were cultured in the same condition and analysis the protein and mRNA by Western blot and RT-PCR. (C) CCR7 antibody reduced the expression of VEGF-D protein in A549 cells. A549 cells were serum-starved and treated with CCR7 antibody for indicated times. (D) A549 cells were transfected with scrambled siRNA sequence (Sc) or siRNA for CCR7 (Si) followed by incubation for 48 h. Total protein was isolated and expression of CCR7 and VEGF-D was analyzed by Western blot.
CCR7 induced ERK1/2 and Akt phosphorylation in BE1 cells
To investigate CCR7 how to regulate VEGF-D, we detected p-ERK1/2, ERK1/2, p-Akt and Akt protein expressions in CCR7 gene over-expressed cell clone (BE1-CCR7 cells). Western blotting showed that the expressions of p-ERK1/2 and p-Akt were increased in BE1-CCR7 cells, while exhibiting no effect on the total protein levels of ERK1/2 or Akt (Figure 3A). Furthermore, Specific blocking CCR7 expression by siRNA method inhibited the expression of phosphorylation of ERK1/2 and Akt were determined by western blot analysis (Figure 3B).
Figure 3.
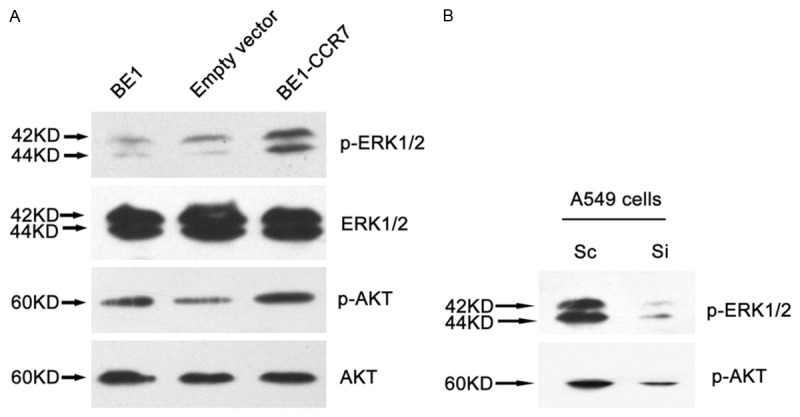
CCR7 induced ERK1/2 and Akt phosphorylation in lung cancer cells. The expression of p-ERK1/2, ERK1/2, p-Akt, Akt protein were determined by western blotting. A. The expressions of p-ERK1/2 and p-Akt were increased in BE1-CCR7 cells, while exhibiting no effect on the total protein levels of ERK1/2 or Akt. B. A549 cells were transfected with scrambled siRNA sequence (Sc) or siRNA for CCR7 (Si) followed by incubation for 48 h. Total protein was isolated and expression of phosphorylation of ERK1/2 or Akt was determined by western blot analysis.
Involvement of ERK1/2 and Akt in CCR7-mediated VEGF-D up-regulation
To confirm whether CCR7 up-regulate VEGF-D by p-ERK1/2 and p-Akt, we inhibit the activity of p-ERK1/2 and p-Akt with their inhibitors PD98059 and LY294002 respectively. RT-PCR and western blotting results suggested that the VEGF-D mRNA and protein expression in BE1-CCR7 cells were decreased significantly after incubation with PD98059 or LY294002, respectively (Figure 4A). Furthermore, in BE1-CCR7 cells, PD98059 did not affect phosphorylation of Akt, and LY294002 did not inhibit ERK1/2 phosphorylation under basal condition (Figure 4B), indicating that PI3K/Akt and ERK1/2 pathways are independently involved in CCR7-mediated VEGF-D up-regulation The results suggested that ERK1/2 and Akt signal pathways were independent of each other during CCR7 up-regulate VEGF-D.
Figure 4.
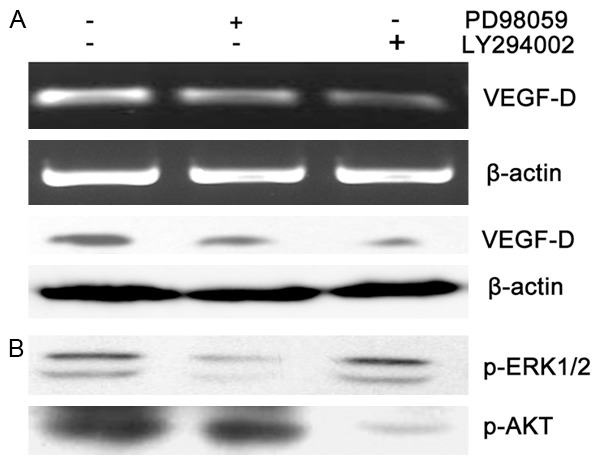
Involvement of ERK1/2 and Akt in CCR7-mediated VEGF-D up-regulation. Serum-starved BE1-CCR7 cells were treated with ERK1/2 inhibitor PD98059 (50 µm) or AKT inhibitor LY294002 (50 µm) followed by incubation for 24 h. A. Total protein and RNA were isolated and subjected to RT-PCR and Western blotting to analysis the expression of VEGF-D. The results showed that VEGF-D mRNA (top) and protein (bottom) were reduced after incubation with PD98059 or LY294002, which was more significant in LY294002 incubated cells. B. The expression of p-ERK1/2 and p-Akt were decreased significantly by PD98059 or LY294002 respectively, while exhibiting no effect on each other.
CCR7 expression correlates with VEGF-D level, lymphatic vessel density, clinical stages, lymph node metastasis, and patient survival in NSCLC
Immunohistochemical analysis revealed that the VEGF-D expression was significantly associated with CCR7 expression in 90 NSCLC specimens (r = 0.455, P < 0.001, Table 1). We then quantified the number of lymphatic vessels in NSCLC with CCR7 and VEGF-D high and low expression NSCLC using antibodies against D2-40. Quantification of the D2-40-positive vessels in the tumors indicated that tumor with high expression of CCR7 had a higher LVD than in low CCR7 expressing tumors (28.09±10.57 for CCR7 high expression tumors, n = 63, and 20.58±7.6 for CCR7 low expression tumors, n = 27, P = 0.001, t test). Tumors with VEGF-D high expression had a higher LVD than in low VEGF-D expressing tumors 30.14±9.87 for VEGF-D high expression tumors, n = 54, and 19.38±7.22 for VEGF-D low expression tumors, n = 36, P < 0.001, t test). These results indicated that the expression patterns of CCR7, VEGF-D associated well with the expression of LVD in NSCLC tissues (Figure 5).
Table 1.
Correlation between levels of chemokine receptor 7 (CCR7) and vascular endothelial growth factor-D (VEGF-D) expression in human lung cancer
| VEGF-D | CCR7 | r | P-value | |
|---|---|---|---|---|
|
| ||||
| Lower | Higher | |||
| Lower | 20 | 16 | ||
| Higher | 7 | 47 | 0.455 | P < 0.001 |
Figure 5.
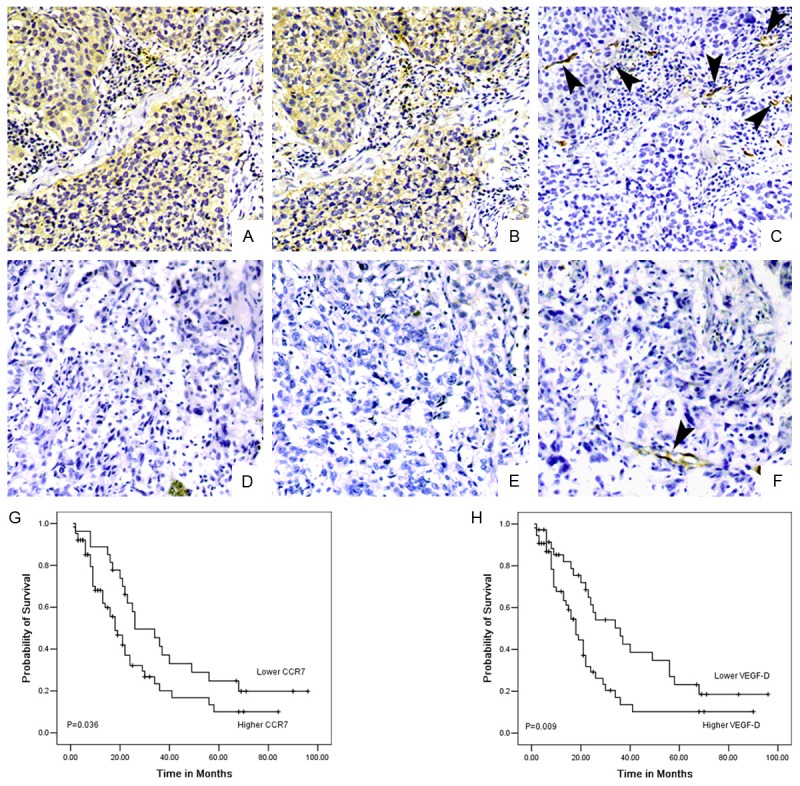
CCR7 expression correlates with mediated VEGF-D protein levels, lymphatic vessel density, and prognosis of patients with lung cancer. CCR7 immunostaining with (A) and without (D) positive nodes. VEGF-D expression in serial sections with (B) and without (E) node metastasis. D2-40 expression in serial sections with (C) and without (F) node metastasis. It was shown that CCR7 and VEGF-D expression was correlated with lymph node status; identical coexpression and localization of CCR7 and VEGF-D; D2-40 expression was correlated with CCR7 and VEGF-D expression. Original magnification: all ×200. Kaplan-Meier survival plots for patients grouped according to CCR7 (G) and VEGF-D (H) protein expression. It was statistically significant between overall survival of patients with CCR7 expression (P = 0.036) and VEGF-D expression (P = 0.009).
Patients with high expression of CCR7 were more likely to have advanced disease (P = 0.003) and lymph node metastasis (P = 0.004) than that of low expression patients. High expression of VEGF-D have advanced disease (P = 0.005) and lymph node metastasis (P = 0.001) than that of low expression patients. In addition, Patients who low expression CCR7 had a statistically significantly longer survival than high expression patients (P = 0.036; Figure 5G). The median survival time for patients with high VEGF-D was 24.64±3.9 months; 95% confidence interval 16.97-32.31 months, which was significantly shorter than patients with low VEGF-D expression (P = 0.009, Figure 5H). These results provide a possible explanation that CCR7 high expression patients were more likely to have poor prognosis, possibly resulting from CCR7-mediated lymphangiogenesis via up-regulation of VEGF-D in human NSCLC.
Discussion
CCR7 is expressed by many immune cells and interacts with its ligand CCL21/19 may regulate directionally migration. Previous studies have demonstrated that CCR7 expression correlated with lymph node metastasis in several different types of cancers [7,22-26]. Furthermore a significant correlation between lymphangiogenesis and lymph node metastasis has been shown in human breast cancer [27,28], suggesting a possible role of CCR7 in tumor lymphangiogenesis. Recently, a critical role of CCR7 in lymphangiogenesis has been reported in SW620 colon cancer cells [24], but the mechanism had not been published up to now.
Studies have demonstrated that VEGF-D strongly correlated with lymph node metastases of various human cancers and has been identified as a lymphangiogenic growth factor. We therefore examined the effect of CCR7 on the expression of VEGF-D in lung cancer cells. In our studies, we found that the expression of VEGF-D mRNA and protein in BE1 cells were greatly increased after CCR7 over-expression. In contrast, VEGF-D expression level was decreased when CCR7 was blocked with antibody or specific siRNA. Furthermore CCR7 tightly regulates VEGF-D gene expression, was commonly observed in the different human lung cancer cell lines. These data supported that VEGF-D is a downstream gene of CCR7 in lung cancer.
The human VEGF-D promoter contains one optimal binding site for transcription factor AP-1 [29]. AP-1 is a different member of Fos and Jun protein family members which is mainly regulated via the activation of ERK1/2. It therefore might be anticipated that ERK1/2 is involved in VEGF-D up-regulation in response to CCR7 in lung cancer cells. In our studies, we found that the expression of p-ERK1/2 was increased after CCR7 over expression. However specific blocking CCR7 expression by siRNA method inhibited ERK1/2 phosphorylation. Furthermore, CCR7-induced VEGF-D up-regulation was decreased significantly after blocking phosphorylation of ERK1/2 with PD98059. The data presented here suggest that activation of ERK1/2 plays an important role in CCR7-induced VEGF-D up-regulation.
PKC plays an important role in lymphangiogenesis. Here, we found that p-Akt was increased after CCR7 over expression; using Akt inhibitor LY294002 significantly suppressed CCR7-induced VEGF-D up-regulation in protein level. These data strongly suggest that Akt is a critical mediator to transducer the signaling of VEGF-D expression.
Various isoforms of PKC differentially modulate MAPK signaling pathways [30,31]. In contrast, studies been shown to activate MAPK (ERK1/2) via PKC-independent pathways [32]. In this study, we showed that inhibit Akt did not affect ERK1/2 phosphorylation, in contrast, inhibit ERK1/2 did not affect phosphorylation of Akt. These data supporting the possibility that MAPKs and Akt were independent of each other.
In 90 NSCLC tissues, we confirmed that CCR7 highly expressed. The high expression level of CCR7 correlated well with VEGF-D high expression, clinical stages, more lymph node metastasis, higher lymphatic vessel density, and shorter survival rate of patients. Together with our studies in lung cancer cells, we speculated that CCR7 promote lymphangiogenesis via VEGF-D and may induce lymph node metastasis in NSCLC tissues.
In conclusion, our study demonstrated that, CCR7 could induce VEGF-D gene expression via ERK1/2 and Akt signaling pathway in NSCLC cells. Overexpression of CCR7 and VEGF-D play an important role in lung cancer lymphangiogenesis. Thus, targeting CCR7 may potentiate new therapeutic strategy against lymphangiogenesis and lymphatic node metastasis.
Acknowledgements
This work was supported by Program of Science and Technology Department of Liaoning Province (No. 2013225021) and Program for Liaoning Innovative Research Team in University (No. LC2015029) and Natural Science Foundation of Liaoning Province of China (No. L2015598).
Disclosure of conflict of interest
None.
References
- 1.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stacker SA, Baldwin ME, Achen MG. The role of tumor lymphangiogenesis in metastatic spread. FASEB J. 2002;16:922–934. doi: 10.1096/fj.01-0945rev. [DOI] [PubMed] [Google Scholar]
- 3.Yonemura Y, Endo Y, Tabata K, Kawamura T, Yun HY, Bandou E, Sasaki T, Miura M. Role of VEGF-C and VEGF-D in lymphangiogenesis in gastric cancer. Int J Clin Oncol. 2005;10:318–27. doi: 10.1007/s10147-005-0508-7. [DOI] [PubMed] [Google Scholar]
- 4.van Iterson V, Leidenius M, von Smitten K, Bono P, Heikkilä P. VEGF-D in association with VEGFR-3 promotes nodal metastasis in human invasive lobular breast cancer. Am J Clin Pathol. 2007;128:759–66. doi: 10.1309/7FXVRMXF58PVRJUH. [DOI] [PubMed] [Google Scholar]
- 5.Mashino K, Sadanaga N, Yamaguchi H, Tanaka F, Ohta M, Shibuta K, Inoue H, Mori M. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002;15:2937–41. [PubMed] [Google Scholar]
- 6.Takanami I. Overexpression of CCR7 mRNA in nonsmall cell lung cancer: correlation with lymph node metastasis. Int J Cancer. 2003;105:186–9. doi: 10.1002/ijc.11063. [DOI] [PubMed] [Google Scholar]
- 7.Ding Y, Shimada Y, Maeda M, Kawabe A, Kaganoi J, Komoto I, Hashimoto Y, Miyake M, Hashida H, Imamura M. Association of CC chemokine receptor 7 with lymph node metastasis of esophageal squamous cell carcinoma. Clin Cancer Res. 2003;9:3406–12. [PubMed] [Google Scholar]
- 8.Schimanski CC, Schwald S, Simiantonaki N, Jayasinghe C, Gönner U, Wilsberg V, Junginger T, Berger MR, Galle PR, Moehler M. Effect of Chemokine Receptors CXCR4 and CCR7 on the Metastatic Behavior of Human Colorectal Cancer. Clin Cancer Res. 2005;11:1743–50. doi: 10.1158/1078-0432.CCR-04-1195. [DOI] [PubMed] [Google Scholar]
- 9.Gao F, Lu YM, Cao ML, Liu YW, He YQ, Wang Y. Expression and quantification of LYVE-1 in human colorectal cancer. Clin Exp Med. 2006;6:65–71. doi: 10.1007/s10238-006-0097-4. [DOI] [PubMed] [Google Scholar]
- 10.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 11.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 12.Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrmann E, Eltze E, Bierer S, Köpke T, Görge T, Neumann J, Hertle L, Wülfing C. VEGF-C, VEGF-D and Flt-4 in transitional bladder cancer: relationships to clinicopathological parameters and long-term survival. Anticancer Res. 2007;27:3127–33. [PubMed] [Google Scholar]
- 14.Yan C, Zhu ZG, Yu YY, Ji J, Zhang Y, Ji YB, Yan M, Chen J, Liu BY, Yin HR, Lin YZ. Expression of vascular endothelial growth factor C and chemokine receptor CCR7 in gastric carcinoma and their values in predicting lymph node metastasis. World J Gastroenterol. 2004;10:783–90. doi: 10.3748/wjg.v10.i6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Günther K, Leier J, Henning G, Dimmler A, Weissbach R, Hohenberger W, Förster R. Prediction of lymph node metastasis in colorectal carcinoma by expression of chemokine receptor CCR7. Int J Cancer. 2005;116:726–33. doi: 10.1002/ijc.21123. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Q, Sun L, Yin L, Ming J, Zhang S, Luo W, Qiu X. CCL19/CCR7 upregulates heparanase via specificity protein-1 (Sp1) to promote invasion of cell in lung cancer. Tumour Biol. 2013;34:2703–2708. doi: 10.1007/s13277-013-0822-z. [DOI] [PubMed] [Google Scholar]
- 17.Yu S, Duan J, Zhou Z, Pang Q, Wuyang J, Liu T, He X, Xinfa L, Chen Y. A Critical Role of CCR7 in Invasiveness and metastasis of SW620 Colon Cancer Cell in Vitro and in Vivo. Cancer Biol Ther. 2008;7:1037–43. doi: 10.4161/cbt.7.7.6065. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi H, Fujimoto A, Tanaka M, Yamano T, Hsueh E, Hoon DS. CCL21 chemokine regulates chemokine receptor CCR7 bearing malignant melanoma cells. Clin Cancer Res. 2004;10:2351–8. doi: 10.1158/1078-0432.ccr-03-0195. [DOI] [PubMed] [Google Scholar]
- 19.Mashino K, Sadanaga N, Yamaguchi H, Tanaka F, Ohta M, Shibuta K, Inoue H, Mori M. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002;62:2937–41. [PubMed] [Google Scholar]
- 20.Ishigami S, Natsugoe S, Nakajo A, Tokuda K, Uenosono Y, Arigami T, Matsumoto M, Okumura H, Hokita S, Aikou T. Prognostic value of CCR7 expression in gastric cancer. Hepatogastroenterology. 2007;54:1025–8. [PubMed] [Google Scholar]
- 21.Takeuchi H, Fujimoto A, Tanaka M, Yamano T, Hsueh E, Hoon DS. CCL21 chemokine regulates chemokine receptor CCR7 bearing malignant melanoma cells. Clin Cancer Res. 2004;10:2351–8. doi: 10.1158/1078-0432.ccr-03-0195. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Xi L, Hunt JL, Gooding W, Whiteside TL, Chen Z, Godfrey TE, Ferris RL. Expression pattern of chemokine receptor 6 (CCR6) and CCR7 in squamous cell carcinoma of the head and neck identifies a novel metastatic phenotype. Cancer Res. 2004;64:1861–6. doi: 10.1158/0008-5472.can-03-2968. [DOI] [PubMed] [Google Scholar]
- 23.Cuesta-Mateos C, López-Giral S, Alfonso-Pérez M, de Soria VG, Loscertales J, Guasch-Vidal S, Beltrán AE, Zapata JM, Muñoz-Calleja C. Analysis of migratory and prosurvival pathways induced by the homeostatic chemokines CCL19 and CCL21 in B-cell chronic lymphocytic leukemia. Exp Hematol. 2010;38:756–64. doi: 10.1016/j.exphem.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Till KJ, Lin K, Zuzel M, Cawley JC. The chemokine receptor CCR7 and alpha4 integrinare important for migration of chronic lymphocytic leukemia cells into lymph nodes. Blood. 2002;99:2977–84. doi: 10.1182/blood.v99.8.2977. [DOI] [PubMed] [Google Scholar]
- 25.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verástegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 26.Wilson JL, Burchell J, Grimshaw MJ. Endothelins induce CCR7 expression by breast tumor cells via endothelin receptor A and hypoxia-inducible factor-1. Cancer Res. 2006;66:11802–7. doi: 10.1158/0008-5472.CAN-06-1222. [DOI] [PubMed] [Google Scholar]
- 27.Zhang XH, Huang DP, Guo GL, Chen GR, Zhang HX, Wan L, Chen SY. Coexpression of VEGF-C and COX-2 and its association with lymphangiogenesis in human breast cancer. BMC Cancer. 2008;8:4. doi: 10.1186/1471-2407-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cunnick GH, Jiang WG, Douglas-Jones T, Watkins G, Gomez KF, Morgan MJ, Subramanian A, Mokbel K, Mansel RE. Lymphangiogenesis and lymph node metastasis in breast cancer. Mol Cancer. 2008;7:23. doi: 10.1186/1476-4598-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jian M, Qingfu Z, Yanduo J, Guocheng J, Xueshan Q. Anti-Lymphangiogenesis Effects of a Specific Anti-Interleukin 7 Receptor Antibody in Lung Cancer Model In vivo. Mol Carcinog. 2015;54:148–55. doi: 10.1002/mc.22082. [DOI] [PubMed] [Google Scholar]
- 30.Clerk A, Sugden PH. Untangling the Web: specific signaling from PKC isoforms to MAPK cascades. Circ Res. 2001;89:847–9. [PubMed] [Google Scholar]
- 31.Heidkamp MC, Bayer AL, Martin JL, Samarel AM. Differential activation of mitogen-activated protein kinase cascades and apoptosis by protein kinase C epsilon and delta in neonatal rat ventricular myocytes. Circ Res. 2001;89:882–890. doi: 10.1161/hh2201.099434. [DOI] [PubMed] [Google Scholar]
- 32.Lang E, Lang F. Mechanisms and pathophysiological significance of eryptosis, the suicidal erythrocyte death. Semin Cell Dev Biol. 2015;39:35–42. doi: 10.1016/j.semcdb.2015.01.009. [DOI] [PubMed] [Google Scholar]


