Abstract
Traumatic injuries to the brain and spinal cord affect a large percentage of the world’s population. However, there are currently no effective treatments for these central nervous system (CNS) injuries. In our study, we evaluated the neuroprotective role of functionalized multi-walled carbon nanotubes (MWCNTs) carrying brain derived neurotrophic factor (BNDF), nogo-66 receptor (NgR) and Ras homolog gene family member A (RhoA) in spinal cord injury (SCI). Our results showed that transfection into rat cortical neurons with BDNF-DNA significantly elevated the expression of BDNF both in vitro and in vivo. Meanwhile, transfection with NgR-siRNA and RhoA-siRNA resulted in an obvious down-regulation of NgR and RhoA in neuron cells and in injured spinal cords. In addition, the functionalized MWCNTs carrying BDNF-DNA, NgR-siRNA and RhoA-siRNA exhibited remarkable therapeutic effects on injured spinal cord. Taken together, our study demonstrates that functionalized MWCNTs have a potential therapeutic application on repair and regeneration of the CNS.
Keywords: Spinal cord injury, multi-walled carbon nanotubes, BDNF, NgR, RhoA
Introduction
Spinal cord injury (SCI) is one of the most common causes of long-term disability among young adults worldwide [1]. Such nervous tissue lesions pose a serious social concern, as they associate with high sanitary costs and affect an increasing number of people [2]. Unfortunately, there are currently no effective treatments to improve functional outcomes after SCI due to its functional and structural complexity and the hematoencephalic barrier [3,4]. Thus, new treatment options are urgently needed for effective treatment of SCI.
Nanoparticles (NP), the small objects with < 100 nanometers in diameter, exhibit an extremely high surface to volume ratio [5]. These unique properties account for their abilities to enter into organisms and attribute to the carbon nanotubes (CNTs) with numerous applications including drug delivery systems [6-8]. Importantly, CNTs have demonstrated not to interfere with neuronal functionality [9], suggesting the potential application of CNTs on traumatic injury in central nervous system (CNS) and peripheral nervous system.
Gene therapies hold great promise for the treatment of many traumatic injuries and neurodegenerative disorders in the CNS [10]. During SCI, various signaling pathways affect the ability of axons to functionally regenerate in the spinal cord and genetic regulation of these pathways has been proven to be a valuable tool for clinical benefits [11,12]. Three biomolecular targets including brain derived neurotrophic factor (BNDF), nogo-66 receptor, (NgR) and Ras homolog gene family member A (RhoA) have been identified to be involved in neuronal functional recovery [13-15]. Herein, we combined genetic manipulation of these three molecules and multi-walled CNTs (MWCNTs) to investigate their potential neuroprotective effects using a rat SCI model.
Materials and methods
Cell culture
Embryonic neural cells were obtained from cerebral cortices of E16 SD rat embryos as previously described [16]. To be brief, cerebral cortices were cleaned from meningeal membranes and cut in small pieces (< 2 mm in diameter) in Hank’s Balanced Salt Solution (HBSS). Then, tissue pieces were digested with trypsin and DNAse at 37°C for 30 min. After neutralization with serum-containing media, cell suspension was passed through Pasteur pipettes for further tissue disaggregation. Isolated cells were then centrifuged at 300 g for 4 min and confirmed for adequate cell viability by trypan blue staining.
Gene synthesis and transfection
Rat BDNF coding sequence (gb|M61178.1) was amplified with the primers as follows. Forward 5’-TCCAGGATCCATGACCATCCTTTTCCTT-3’; reverse 5’-TCCAGAATTCCTATCTTCCCCTTTTAATGG-3’. Synthesized siRNA sequences of NgR and RhoA are as shown in Table 1. These synthetic genes were transfected into rat cortical neurons with Lipofectamine 2000 according to the manufacture’s protocol (Invitrogen, USA).
Table 1.
Synthesized siRNA sequences
| Gene name | Sense (5’-3’) | Anti-sense (5’-3’) |
|---|---|---|
| Ng R1 | AAUCAGCUCACUGAUGAGGAGCCUGUCUC | CUCCUCAUCAGUGAGCUGAUUCCUGUCUC |
| Ng R2 | AAAUGCACUCAAGGGACGUGUCCUGUCUC | ACACGUCCCUUGAGUGCAUUUCCUGUCUC |
| Ng R3 | AAUGACUCUCCAUUUGGGACUCCUGUCUC | AGUCCCAAAUGGAGAGUCAUUCCUGUCUC |
| Rho A1 | AAGGCGGGAGUUAGCCAAAAUCCUGUCUC | AUUUUGGCUAACUCCCGCCUUCCUGUCUC |
| Rho A2 | AAUGAAGCAGGAGCCGGUAAACCUGUCUC | UUUACCGGCUCCUGCUUCAUUCCUGUCUC |
| Rho A3 | AAAGACCAAAGACGGAGUGAGCCUGUCUC | CUCACUCCGUCUUUGGUCUUUCCUGUCUC |
Generation of MWCNTs
MWCNTs were dispersed in sulfuric acid/nitric acid mixed solution, and ultrasonicated for 4 h, 8 h, and 16 h. Transmission electron microscopy (TEM) and fourier transform infrared spectrophotometer (FTIR) were used to determine the length and morphology of MWCNTs. 0.5 mg of carboxylated MWCNTs were added into 0.1% polyethyleneimine (PEI) solution and ultrasonicated for 15 min. After ultrosonication, MWCNTs were filtered and thoroughly rinsed with double distilled water, and the functionalized MWCNTs were re-dispersed in water to a final concentration of 0.1 mg/mL. Then, 25 μl MWCNTs-PEI was placed in 1.5 ml EP tube; and mixed with three kinds of synthetic therapeutic gene for 3 h. Finally, MWCNTs combined with BDNF-DNA, NgR-siRNA and RhoA-siRNA were synthesized at the concentrations of 1.25 mg/L for MWCNTs-PEI and 150 nM for genes.
Real time PCR
Total RNA was extracted by using TRIzol reagent (Invitrogen) and complementary DNA was synthesized using reverse transcription Kit (Promega) according to the manufacturer’s protocol. The sequences of primers used were as follows: BDNF-F: 5’-CACACCAAGTGGTGGGCGATCC-3’; BDNF-R: 5’-AGGCCAAGTTGCCTTGTCCGTG-3’; NgR-F: 5’-GGAGCTGGGGCCTGGCCTAT-3’; NgR-R: 5’-GCAAGCCACGGAAAGCGTGC-3’; RhoA-F: 5’-AAGTGGACGGGAAGCAGGTAGAGT-3’; RhoA-R: 5’-CCCAACTAGGATGATGGGCACATTT-3’; GAPDH-F: 5’-GGGGCTCTCTGCTCCTCCCTG-3’; GAPDH-R: 5’-CCAGGCGTCCGATACGGCCA-3’.
PCR was performed according to the instruction by the manufacturer using the SYBR Green PCR Master Mix (Toyobo, Japan). Amplification protocols were followed: 95°C for 10 min; 40 cycles of 95°C/15 s, 55°C/60 s and 72°C/30 s. The transcript levels of interest genes were normalized to the GAPDH and were calculated with 2-ΔΔCt method.
MTT assay
Cells were seeded in a 96-well plate at a concentration of 5×104 cells/well for 24 hours. Then the cultured medium was replaced by conditional medium, which was added with indicated doses of MWCNTs. 12 h, 20 μl MTT was added and continually cultured for 4 h. Then the optical density was determined the values at 490 nm using microplate reader.
Western blot
Rat cortical neurons were harvested, washed twice with PBS, and lysed in RIPA lysis buffer. The cell lysates were centrifuged at 12,000×g for 5 min at 4°C. Samples with equal amounts of protein were separated by SDS-PAGE, and then the proteins were transferred onto a nitrocellulose membrane. The membrane was blocked with 5% not-fat milk in TBST and incubated with primary antibodies including BDNF, NgR and RhoA. The membrane was washed with TBST for three times and incubated with secondary antibodies in TBST at room temperature for 1 h. The proteins were detected using enhanced chemiluminescence (ECL) detection reagents (Roche, Switzerland).
Statistical analysis
Data were expressed as mean ± SD and statistical comparisons were performed using one-way analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
Results
Up-regulation of BDNF and down-regulation of NgR and RhoA
Firstly, we constructed the plasmid encoding BDNF and synthesized NgR-siRNA and RhoA-siRNA. As shown in Figure 1A, BDNF-DNA transfection into neurons significantly increased the mRNA expression of BDNF. Transfection with NgR-siRNA and RhoA-siRNA, especially siRNA-3, inhibited the mRNA expression of NgR and RhoA in neurons (Figure 1B and 1C).
Figure 1.
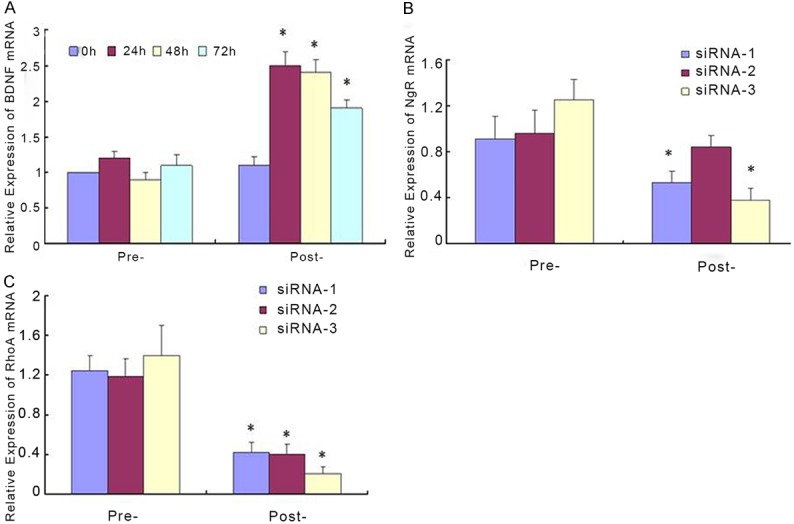
Up-regulation of BDNF and down-regulation of NgR and RhoA. Neurons were transfected with BDNF-DNA (A), NgR-siRNA (B) and RhoA-siRNA (C). Real time PCR was performed to measure the mRNA expression of BDNF, NgR and RhoA. *P < 0.05.
Characterization of MWCNTs
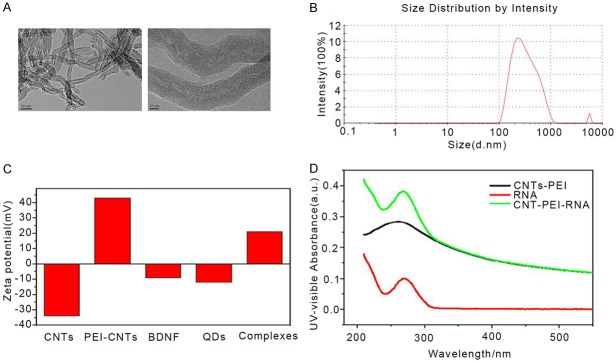
TEM and dynamic light scattering were used to measure the structure and size distribution of MWCNTs and functionalized MWCNTs. Results showed that MWCNTs exhibited tubular nanostructures with a length of 300-500 nm (Figure 2A and 2B). Particularly, there was a significant difference in the electronic property on the surface of functionalized MWCNTs and MWCNTs. The Zeta potential changed from -34.8 mV for MWCNTs to +43.6 mV for PEI-modified MWCNTs (Figure 2C). UV-Vis spectra MWCNTs-siRNA complex displayed characteristic absorption peaks at 260 nm (Figure 2D). Taken together, these results indicated that BNDF and NgR-siRNA and RhoA-siRNA successfully incorporated to the surface of functionalized MWCNTs.
Figure 2.
Characterization of MWCNTs. A. Electron microscope analysis for MWCNT and functionalized MWCNT. B. Representative image of the size distribution of MWCNT. C. Zeta potentials of MWCNT, functionalized-MWCNT, BNDF, and functionalized-MWCNT-BNDF complexes. D. Normalized UV-visible spectra of RNA, CNTs-PEI and CNTs-PEI-RNA complex.
Cytotoxic effect of MWCNTs
Nest we measured the cytotoxic effects of MWCNTs by MTT assay. Neurons were incubated with different concentrations of MWCNTs for 48 h. Results showed that the cytotoxic effects of functionalized MWCNTs decreased with increasing dilution (100-5000) of MWCNTs (Figure 3A-H).
Figure 3.
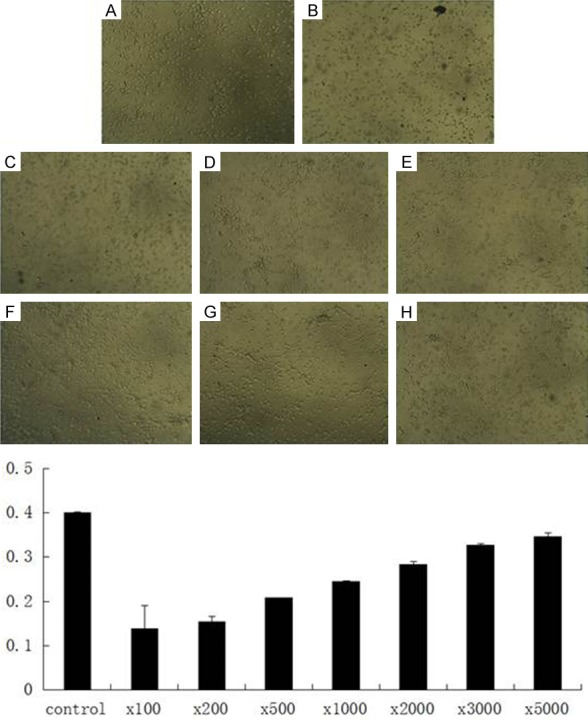
Cytotoxic effects of MWCNTs. Cytotoxicities of the differently diluted solutions of the functionalized MWCNTs on neurons were detected by MTT assay.
Expression of BDNF, NgR and RhoA in vivo
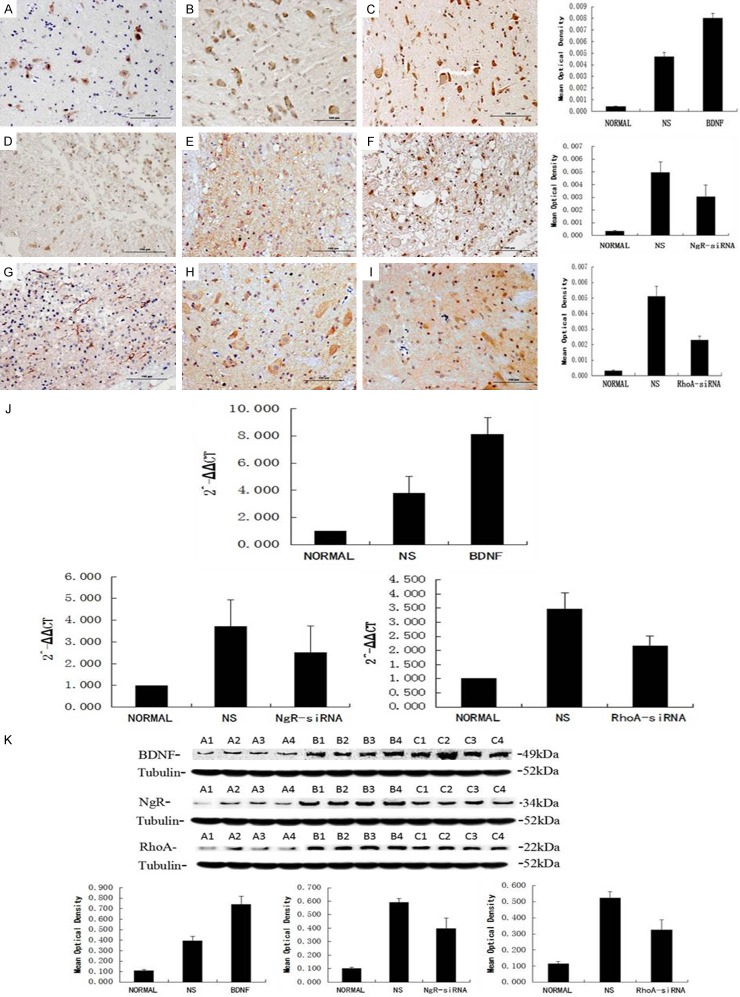
After successful establishment of functionalized MWCNTs composite carrying BDNF-DNA, NgR-siRNA and RhoA-siRNA, we further evaluated the expression of BDNF, NgR and RhoA in vivo using a rat SCI model. Following cortex injection with MWCNTs, the expression of BDNF (Figure 4A-C) was significantly increased compared with the control group. Meanwhile, SCI-induced up-regulation of NgR (Figure 4D-F) and RhoA (Figure 4G-I) was decreased in the spinal cord after treatment with functionalized MWCNTs. In addition, real time PCR and western blot analysis also showed the up-regulation of BDNF and down-regulation of NgR and RhoA in the spinal cord in MWCNTs treated rats (Figure 4J and 4K).
Figure 4.
Expression of BDNF, NgR and RhoA in vivo. Immunohistochemical staining (A-I), real time PCR (J) and western blot (K) analysis for the BDNF, NgR and RhoA proteins in normal and injured spinal cords after the treatment.
Neuroprotective effects of MWCNTs composite on SCI
Finally we assessed the neuroprotective effects of functionalized MWCNTs carrying BDNF-DNA, NgR-siRNA and RhoA-siRNA, HE staining showed that SCI was alleviated in the therapeutic group (Figure 5A-E). Functional scores demonstrated that the BBB scores is therapeutic group was significantly elevated compared with the control (Figure 5F). These results revealed the neuroprotective role of MWCNTs composite in SCI.
Figure 5.
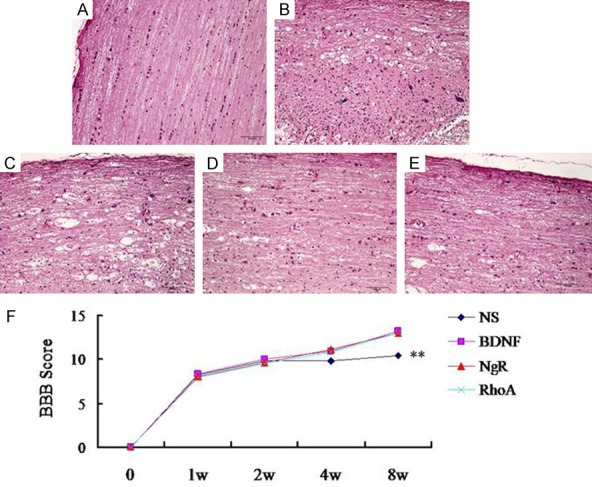
Neuroprotective effects of MWCNTs composite on SCI. HE staining for the rat spinal cords (A-E, A: control, B: SCI, C: BDNF-DNA, D: NgR-siRNA, E: RhoA-siRNA). Magnification × 200. (F) Time course of the BBB score changes in different groups.
Discussion
Nervous tissue lesions such as SCI are an important social concern with serious consequences. Nevertheless, treatment strategy for CNS injury still limited due to its complex structure and hematoencephalic barrier [3]. In the current study, we demonstrated that functionalized MWCNTs incorporated with therapeutic genes including BNDF, NgR and RhoA has obvious neuroprotective effects in injured spinal cords.
The repair and regeneration of CNS is a complex process which is involved with signaling transduction, protein transport, cytoskeleton synthesis and cell adhesion [17]. Among them, nerve growth factor deficiency, neurite growth inhibitory microenvironment and glial scar formation greatly inhibits the CNS repair and regeneration [18]. BDNF, widely distributed in CNS, plays diverse roles in neuron growth, development, differentiation under physiological conditions, and contributes to injured neurons repair and regeneration in pathological conditions [19]. In addition, BDNF production alleviates inflammatory response and promotes the functional recovery in acute spinal cord injury [20]. On the other hand, some factors have been identified to inhibit nerve regeneration such as Nogo, Ompg and MAG. Nogo is one of the most important neurite outgrowth inhibitory proteins with three isoforms Nogo A, B, C. Nogo receptor mediates axonal growth inhibition and plays a role in regulating axonal regeneration and plasticity in the adult central nervous system [21,22]. RhoA is a small GTPase protein known to regulate the actin cytoskeleton, transcription, cell cycle progression and cell transformation [23]. Recent study suggest that Nogo, Ompg and MAG. exhibit the neurite inhibitory function through regulation of RhoA, indicating the key role of RhoA in nerve system [24]. In our study, BDNF-DNA, NgR-siRNA and RhoA-siRNA transfection into neurons significantly increased the expression of BDNF and inhibited the expression of NgR and RhoA.
CNTs are being extensively in nerve regeneration, drug delivery, gene therapy and tumor targeted medicine for their unique properties including electrical conductivity, mechanical reinforcement, and high stability in biological environments [25,26]. However, there are several studies indicating that CNTs could induce undesirable effects on human health including cell apoptosis, DNA damage, and oxidative stress [27,28]. We established the functionalized MWCNTs by incorporating BDNF-DNA, NgR-siRNA and RhoA-siRNA into the surface of MWCNTs. The BDNF level was obviously increased while the expression of NgR and RhoA was decreased in the cerebral cortex in the therapeutic group. Although the slight cytotoxicity of MWCNTs in neurons, we observed that treatment with functionalized MWCNTs alleviated the spinal cord injury and promoted the functional recover in SCI mice.
In conclusion, the present study demonstrates the functionalized MWCNTs-delivered BDNF-DNA, NgR-siRNA, and RhoA-siRNA has a neuroprotective effect on spinal cord injury in rats. These findings provide that MWCNTs-gene compound may serve as a potential therapeutic tool in CNS injury.
Disclosure of conflict of interest
None.
References
- 1.Lipinski MM, Wu J. Modification of autophagy-lysosomal pathway as a neuroprotective treatment for spinal cord injury. Neural Regen Res. 2015;10:892–893. doi: 10.4103/1673-5374.158344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belkas JS, Shoichet MS, Midha R. Peripheral nerve regeneration through guidance tubes. Neurol Res. 2004;26:151–160. doi: 10.1179/016164104225013798. [DOI] [PubMed] [Google Scholar]
- 3.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience. 2002;111:761–773. doi: 10.1016/s0306-4522(02)00025-8. [DOI] [PubMed] [Google Scholar]
- 5.Linkov I, Satterstrom FK, Corey LM. Nanotoxicology and nanomedicine: making hard decisions. Nanomedicine. 2008;4:167–171. doi: 10.1016/j.nano.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Agasti SS, Rana S, Park MH, Kim CK, You CC, Rotello VM. Nanoparticles for detection and diagnosis. Adv Drug Deliv Rev. 2010;62:316–328. doi: 10.1016/j.addr.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyes WK, Chen R, Chen C, Yokel RA. The neurotoxic potential of engineered nanomaterials. Neurotoxicology. 2012;33:902–910. doi: 10.1016/j.neuro.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol. 2005;9:674–679. doi: 10.1016/j.cbpa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Gaillard GC, Li S, Toma FM, Dumortier H, Spaliuto G, et al. Carbon nanotubes carrying cell-adhesion peptides do not interfere with neuronal functionality. Adv Mater. 2009:2903–2908. [Google Scholar]
- 10.Walthers CM, Seidlits SK. Gene delivery strategies to promote spinal cord repair. Biomark Insights. 2015;10:11–29. doi: 10.4137/BMI.S20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron AA, Smith GM, Randall DC, Brown DR, Rabchevsky AG. Genetic manipulation of intraspinal plasticity after spinal cord injury alters the severity of autonomic dysreflexia. J Neurosci. 2006;26:2923–2932. doi: 10.1523/JNEUROSCI.4390-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang BL, Low CB. Genetic manipulation of neural stem cells for transplantation into the injured spinal cord. Cell Mol Neurobiol. 2007;27:75–85. doi: 10.1007/s10571-006-9119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madura T, Yamashita T, Kubo T, Fujitani M, Hosokawa K, Tohyama M. Activation of Rho in the injured axons following spinal cord injury. EMBO Rep. 2004;5:412–417. doi: 10.1038/sj.embor.7400117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li F, Fei D, Sun L, Zhang S, Yuan Y, Zhang L, Zhao K, Li R, Yu Y. Neuroprotective effect of bone marrow stromal cell combination with atorvastatin in rat model of spinal cord injury. Int J Clin Exp Med. 2014;7:4967–4974. [PMC free article] [PubMed] [Google Scholar]
- 16.Carballo-Vila M, Moreno-Burriel B, Chinarro E, Jurado JR, Casan-Pastor N, Collazos-Castro JE. Titanium oxide as substrate for neural cell growth. J Biomed Mater Res A. 2009;90:94–105. doi: 10.1002/jbm.a.32058. [DOI] [PubMed] [Google Scholar]
- 17.Lo EH. Degeneration and repair in central nervous system disease. Nat Med. 2010;16:1205–1209. doi: 10.1038/nm.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzgerald J, Fawcett J. Repair in the central nervous system. J Bone Joint Surg Br. 2007;89:1413–1420. doi: 10.1302/0301-620X.89B11.19651. [DOI] [PubMed] [Google Scholar]
- 19.Marini AM, Jiang X, Wu X, Tian F, Zhu D, Okagaki P, Lipsky RH. Role of brain-derived neurotrophic factor and NF-kappaB in neuronal plasticity and survival: From genes to phenotype. Restor Neurol Neurosci. 2004;22:121–130. [PubMed] [Google Scholar]
- 20.Wong I, Liao H, Bai X, Zaknic A, Zhong J, Guan Y, Li HY, Wang YJ, Zhou XF. ProBDNF inhibits infiltration of ED1+ macrophages after spinal cord injury. Brain Behav Immun. 2010;24:585–597. doi: 10.1016/j.bbi.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Steward O, Sharp K, Yee KM, Hofstadter M. A re-assessment of the effects of a Nogo-66 receptor antagonist on regenerative growth of axons and locomotor recovery after spinal cord injury in mice. Exp Neurol. 2008;209:446–468. doi: 10.1016/j.expneurol.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- 23.Herzog D, Loetscher P, van Hengel J, Knusel S, Brakebusch C, Taylor V, Suter U, Relvas JB. The small GTPase RhoA is required to maintain spinal cord neuroepithelium organization and the neural stem cell pool. J Neurosci. 2011;31:5120–5130. doi: 10.1523/JNEUROSCI.4807-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- 25.Beg S, Rizwan M, Sheikh AM, Hasnain MS, Anwer K, Kohli K. Advancement in carbon nanotubes: basics, biomedical applications and toxicity. J Pharm Pharmacol. 2011;63:141–163. doi: 10.1111/j.2042-7158.2010.01167.x. [DOI] [PubMed] [Google Scholar]
- 26.Bekyarova E, Ni Y, Malarkey EB, Montana V, McWilliams JL, Haddon RC, Parpura V. Applications of Carbon Nanotubes in Biotechnology and Biomedicine. J Biomed Nanotechnol. 2005;1:3–17. doi: 10.1166/jbn.2005.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui HF, Vashist SK, Al-Rubeaan K, Luong JH, Sheu FS. Interfacing carbon nanotubes with living mammalian cells and cytotoxicity issues. Chem Res Toxicol. 2010;23:1131–1147. doi: 10.1021/tx100050h. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser JP, Wick P, Manser P, Spohn P, Bruinink A. Single walled carbon nanotubes (SWCNT) affect cell physiology and cell architecture. J Mater Sci Mater Med. 2008;19:1523–1527. doi: 10.1007/s10856-007-3296-y. [DOI] [PubMed] [Google Scholar]