Abstract
Bile acid plays an important role in regulating blood glucose, lipid and energy metabolism. The present study was implemented to determine the effect of duodenal-jejunal bypass (DJB) on FXR, TGR-5expression in terminal ileum and its bile acid-related mechanism on glucose and lipid metabolism. Immunohistochemistry was used to detect relative gene or protein expression in liver and intestine. Firstly, we found that expression of FXR in liver and terminal ileum of DJB group was significantly higher than that in S-DJB group (P<0.05). In addition, DJB dramatically increased the activation of TGR-5 in the liver of rats. Furthermore, PEPCK, G6Pase, FBPase 1 and GLP-1 were up-regulated by DJB. In conclusion, these results showed that bile acid ameliorated glucose and lipid metabolism through bile acid-FXR and bile acid- TGR-5 signaling pathway.
Keywords: Duodenal-jejunal bypass, bile acid, FXR, TGR-5
Introduction
It is known that bile acid plays a role in regulating blood glucose, lipid and energy metabolism. In the past, bile acid was only considered as an amphipathic molecule that came from liver, which promoted the absorption of cholesterol, fat-soluble vitamins and lipid [1]. Bile acid and its semi synthetic derivatives promote glucagonlike peptide-1 (GLP-1) secretion through activating G-protein-coupled receptor for bile acids (TGR5) in ileal L cells, and finally up-regulate insulin to boost proliferation and inhibit apoptosis of beta cell [2]. The mechanism underlying the effect of bile acid is that it increases the levels of intracellular cyclic adenosine monophosphate (cAMP) and changes the ATP/ADP ratio, which ultimately leads to the calcium influx [3].
As an important receptor for bile acid, Famesoid X Receptor (FXR), mainly expressed in liver and intestinal, is widely involved in the regulation of carbohydrate and lipid metabolism. FXR is an orphan nuclear receptor with typical nuclear receptor structure including amino terminal highly conserved DNA binding domain (DBD), carboxy terminal ligand binding domain (LBD), amino terminal ligand-independent transcription activation region (AF-1), carboxy terminal-dependent activation region (AF-2), hinge region etc. [4,5]. Reports have identified that human FXR gene is located on chromosome 12 (12q23.1) and FXR gene in mice is composed of 76997 base pairs containing 11 exons and 10 introns [6]. FXR-α is mainly expressed in liver, intestine, kidney and adrenal cortex, and its expressions in liver and small intestine are consistent. Enterohepatic circulation of bile acid and feedback regulation of bile acid synthesis are significantly regulated by FXR-α [7]. In tissue, FXR-α activates the toxic accumulation of bile acids to protect the body [8]. Meanwhile, FXR-α in liver leads to an increase binding of bile acids that secreted into bile canaliculus by hepatocytes, and finally promotes the outflow of bile [9]. In addition, activation of FXR-α in intestine increases the expression of ileal bile acid binding protein (I-BABP), bile acid transporter protein (OST), grown factors and fibroblast grown factor 19 (FGF-19, FGF-15 in mouse) [10]. Furthermore, FXR-α in liver and intestine could even induced the expression of short heterodimer complex (SHP), a typical nuclear receptor that does not bind to DNA and suppresses the activity of several other nuclear receptors [11]. CYP7A1 is a regulator of liver receptor homolog-1 (LRH-1) and hepatic nuclear factor-4a (HNF-4a). SHP can inhibit CYP7A1 negative feedback regulation of bile acid biosynthesis [12]. However, it should be emphasized that FXR-α-dependent signaling pathway in vivo is not limited to the liver and small intestine. Dramatic activity of FXR-α in kidney and adrenal gland of rats can be detected, indicating that FXR-α signaling in these tissues are reserved [13].
Bile acid-FXR signaling promotes glycogen synthesis and inhibits gluconeogenesis. Zhang et al. found decreased phosphoenolpyruvate carboxylase kinase and glucose-6-phosphatase gene in diabetic mice given GW4064 (a farnesoid X receptor agonist) [14]. What’s more, Yamagata et al. found that increased concentration of bile acid inhibited the expression of gluconeogenesis related genes through bile acid- FXR-SHP pathway [15]. Increased SHP evoked by bile acid competitively occupied Foxol and HNF-1 binding sites that normally occupied by CREB binding protein (CBP), which up-regulated PEPCK, G6Pase and fructose 1, 6-bisphosphatase (FBPase-1) gene, and finally inhibited hepatic gluconeogenesis. Dong et al. reported that glycogen deposition in liver of IRS-1 and IRS-2 knockout mice was not completely affected. They supposed that some other transduction pathways, different form insulin signaling pathway, may regulate glycogen synthesis in vivo.
TGR5 is a specific G-protein coupled receptor of bile acid. Recently, TGR5 has been determined to be a bile acid activated membrane receptor [16]. TGR5, initially considered to be an isolated G-protein-coupled receptor, is a G-protein coupled receptors (A) subfamily. Until recently, it has been re-classified as a downstream factor of G-protein coupled receptor of bile acid. The cDNA of TGR5 was dependently cloned by Kawamata et al. It is encoded by an exon of a gene encoding and locates in human 2q35 chromosome or mice 1c3 chromosome.
Bile acid-TGR5 signaling promotes glycogen synthesis and inhibits gluconeogenesis. Bile acid maintains metabolic balance through activating TGR5-cAMP signaling. When ligand binds to TGR5 on cell membrane, released TGR5 endocytosis GαS subunit and activated adenylate cyclase induce intracellular cAMP production and protein kinase A (PKA) activation, and finally regulate cell and gene expression [17]. In recent years, clinical and animal studies showed that gastric bypass surgery significantly increased serum bile acid concentration and dramatically changed the proportion of each component of bile acids [18]. When recognized by TGR5 and FXR-α receptor in liver, bile acids induces liver glycogen synthesis, inhibits gluconeogenesis, ameliorates body’s insulin sensitivity and controls glucose metabolism. However, it is not clear that whether bile acid could affect glucose and lipid metabolism through TGR5 and FXR after duodenal-jejunal bypass (DJB). In the present study, we detected levels of TGR5 and FXR-α in liver and ileum after DJB to elucidate this unknown mechanism.
Methods and materials
Materials
30% Acr-Bis (29:1), N,N,N’,N’-Tetramethylethylenediamine, and primary antibody dilution buffer were obtained from Beyotime Biotechnology Corporation (Shanghai, China). PEPCK (H-130) antibody (1:200, No. sc-13063) was purchased from Santa Cruz Biotechnology, Inc. (Delaware, USA). G6Pase (C-16) PAK, FBPase Goat-anti-Mouse antibody were obtained from Abcam (Cambridge, MA, USA). Horseradish peroxidase-conjugated AffiniPure Goat Anti-Rabbit IgG was from Bioworld Technology, Inc. (St. Paul, MN, USA). Super UltraSensitiveTM SP (Rabbit) IHC Kit was from Maixin Bio. (Fuzhou, China).
Animals
The induced T2DM sprague-dawley rats (SD rats) were randomly divided into 3 groups as follows: (1) DJB group: Rats were im-plemented with DJB, with same method described above. (2) Sham operation (S-DJB) group: Rats were implemented with sham operation, with same method described above. (3) Control group: Rats were only given liquid diet without operation.
Total protein extract
Total Protein Extraction Kit (BestBio Inc., Shanghai, China) was used according to the operation manual and incubated on ice for further use. 100 mg tissue sample was cut (1 mm*1 mm) with ophthalmic scissors and mixed with total protein extracts. Then, the mixture was milled with a tissue homogenizer or cracked with ultrasonic cracking apparatus until no visible tissue solids. After cracked on ice for 20 min, the mixture was centrifuged (1200 rpm, 5 min). The supernatant was sucked into a pre-chilled tube for further use or storage in 80°C.
Western blot
0.8 ml protein solution was added to the standard configuration (20 mg BSA) and sufficiently dissolved to formulate the protein standard (25 mg/ml). The procedure was implemented sequentially: SDS electrophoresis, block, transfer, primary and secondary antibody (as describe in 1.1) incubation and blot.
Immunohistochemistry
To dewax and hydrate, paraffin-section was soaked in xylene (5 min×2), absolute ethanol, 95% ethanol, 75% ethanol and PBS for 5 min respectively. To antigen retrieval, tissue sections was soaked in sodium citrate buffer (PH 6.0) and incubated in microwave with a gradient of temperature control (heated 5 min, cooled 15 min, heated 3 min and cooled 20 min accordingly). DBA staining chromogenic reagent (0.1 ml/ piece, 3-10 min) was thoroughly washed until brown precipitate was seen with microscopy. Then hematoxylin was used for counter-staining (5-30 min). Each batch of immunohistochemistry has known positive control (the use of PBS instead of antibody in blank control). Positive expression showed a yellow or brown granular distribution. Immunohistochemistry was assessed according to the cellar location of protein. Two independent experimental results were assessed to judged yin and yang pathology in immunohistochemistry. The ratio of positive cells in 100 tumor cells of three randomly selected visual field (×100) (each slice) was counted with microscope (×400). The average percentages of three fields were used for judgment. Integrated positive rate and positive cells staining intensity score were both used to determine the positive case (Sinirope criteria). Positive rate scores were as follows: 0 point (positive cells <5%), 1 point (positive cells accounted for 5% to 25%), 2 points (positive cells accounted for 25% to 75%) and 3 points (positive cells accounted for 75% to 100%). Positive staining intensity scores were as follows: 0 point (no staining), 1 point (weak staining), 2 points (moderate staining) and 3 points (strong staining).Those with positive cells <5% and any intensity score were judged as negative. Those with positive cells >5% were judged as positive. The multiplication of two score was judged as - (0 point), + (1-4 points), ++ (5-8 points) and +++ (9-12 points).
Statistical analysis
All results were expressed as mean ± SD. Data, under requirements of a normal distribution and homogeneity of variance, were converted and statistically analyzed. Statistical analyses were performed using one-way analysis of variance (ANOVA). Bonferroni analysis was used in pairwise comparisons between two groups. Bands of western blot were analyzed with Image J 1.47. IBM SPSS Statistics 20.0 was used to finish all statistical analyzes. P values less than 0.05 were considered statistically significant.
Result
Effect of DJB on FXR activity in ileum tissue of T2DM rats
In our previous study, we elucidated the mechanism of elevated serum bile acid after DJB in T2DM rats. After DJB, increased bile acid was caused by bile acid reabsorption rather than its synthesis. There were no significant changes in hepatic bile acid synthesis enzyme CYP7A1 and CYP27A1. But the expression of bile acid transporter ASBT, OST and I-BABP increased. Herein, immunohistochemical method was used to detect FRX expression in ileal tissue. 16 weeks after operation, the level of FXR in DJB group was dramatically higher than that in S-DJB group, suggesting that DJB could promote the activation of FXR in ileal tissue (Figure 1).
Figure 1.
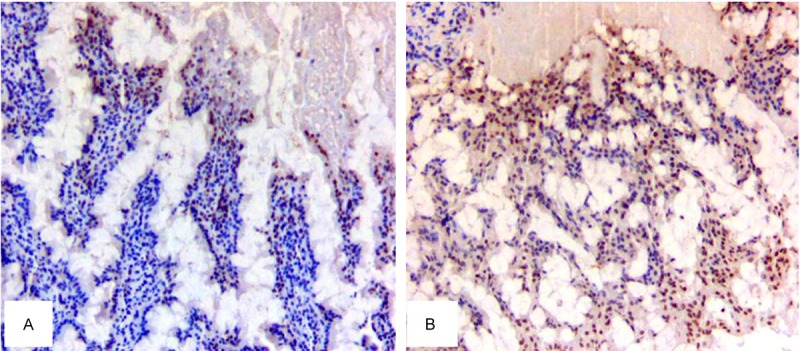
FXR expression in ileal tissue. A. FXR expression in S-DJB group. B. FXR expression in DJB group. Compared with S-DJB group, immunohistochemical staining showed a significantly increased staining area of ileal tissue FXR in DJB group 16 weeks after operation (*P<0.05).
Effect of DJB on FXR activity in liver of T2DM rats
Activation of FXR in intestine induces FGF19 (FGF15 in mouse) expression and secretion, which leads to a better control on hepatic lipid and glucose metabolism. Herein, we examined liver FXR activity and its effect on glucose metabolic pathway. Immunohistochemical staining was used to detect liver FXR expression. Compared with S-DJB group and control group, FXR in DJB group was significantly increased 16 weeks after DJB, indicating that DJB could up-regulate bioactivity of liver FXR (Figure 2).
Figure 2.

FXR expression in liver. A. FXR expression in S-DJB group. B. FXR expression in DJB group. Compared with S-DJB group, immunohistochemical staining showed a significantly increased staining area of liver FXR in DJB group 16 weeks after operation (*P<0.05).
Effect of DJB on TGR5 activity in liver of T2DM rats
We implemented immunohistochemical staining to further examine the effect of high concentration bile acid on glucose metabolic signaling. Compared with S-DJB group, TGR5 was significantly up-regulated in DJB group 16 weeks after operation. The results showed that DJB could enhance activity of liver TGR-5 to further regulate glucose metabolism (Figure 3).
Figure 3.
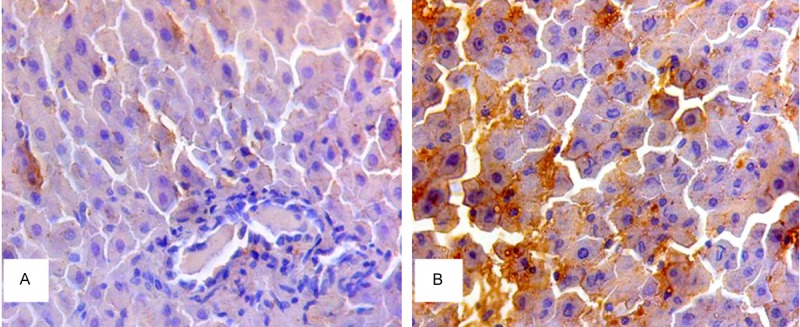
TGR-5 expression in liver. A. TGR-5 expression in S-DJB group. B. TGR-5 expression in DJB group. Compared with S-DJB group, immunohistochemical staining showed a significantly increased staining area of liver TGR-5 in DJB group 16 weeks after operation (*P<0.05).
Effect of DJB on PEEPCK, G6Gpase in liver and intestine of T2DM rats
In order to investigate the effect of DJB on the PEEPCK, G6Gpase expression in liver, the PEEPCK and G6Gpase were examined by using western blot assay. The result indicated that 16 weeks after DJB, PEPCK and G6Pase expression in DJB group were significantly increased compared to that in S-DJB group and control group (Figure 4, P<0.05). The results showed an increase activity PEPCK and G6Pase after DJB.
Figure 4.
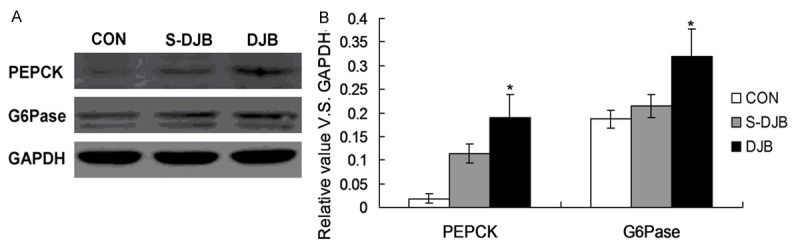
Effect of DJB on PEPCK and G6Pase expression in liver.
Effect of DJB on FBPase-1 and PAK expression in liver and intestine of T2DM rats
The FBPase-1 and PAK were also examined by using western blot assay. The result indicated that 16 weeks after DJB, PBPase and PKA expression in DJB group were also increased compared to that in S-DJB group (but with no statistically significant differences, P>0.05, Figure 5). The results showed aregulation role of DJB on expression and activity of PBPase and PKA.
Figure 5.
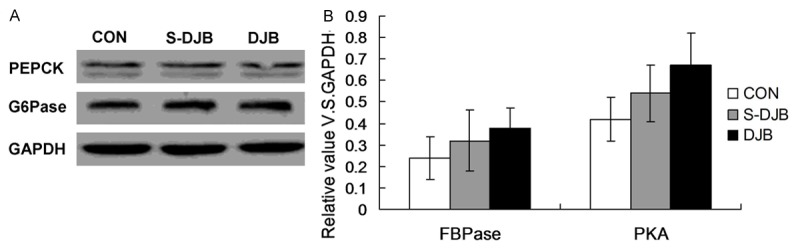
Effect of DJB on PBPase and PKA expression in liver. 16 weeks after DJB, PBPase and PKA expression in DJB group were increased and higher than that in S-DJB group (with no statistically significant differences). The results showed aregulation role of DJB on expression and activity of PBPase and PKA.
Discussion
Bile acid-FXR-TGR-5 signaling pathway could directly regulate gluconeogenesis. Recently, Cariou et al. reported impaired glucose tolerance and insulin sensitivity in FXR knockout mice [20]. In addition, they used synthetic FXR agonist or induced FXR overexpression to control blood glucose levels by suppressing hepatic gluconeogenesis and glycogen synthesis, which further determined the regulated role of FXR on glucose metabolism. Our study showed that ameliorated insulin sensitivity after DJB was tightly linked to the involvement of FXR and TGR-5 signaling pathway. As an important receptor of bile acid, FXR, mainly expresses in liver and intestine, extensively involves in the regulation of carbohydrate and lipid metabolism [21,22]. I-BABP, OST, grown factors and FGF19 in intestine can be up-regulated by FXR-α signaling [23,24]. SHP, an inhibitor to some other nuclear receptor, could directly bind to DNA after induced by FXR-α [10]. Increased bile acid concentration regulates gluconeogenesis-related gene expression through bile acid-FXR-SHP pathway. Moreover, increased SHP by bile acid competes with CREB to bind FOXO1 and HNF-4 sites [16,25]. And the resultant expression of PEPCK, G6Pase and FBPase 1 controls liver gluconeogenesis [26-28]. The negative regulative role of SHP on CYP7A1 feedback affects the biosynthesis of bile acid [31]. Meanwhile, CYP7A1 could further regulate LRH-1 and HNF-4a to inhibit the key enzyme during the synthesis of bile acid, which makes an influence on the initially bile acid synthesis [29]. Combined with our study, FXR in DJB group was increased. However, there was no significant change in levels of bile acid synthesis enzyme and transporter proteins in both DJB group and S-DJB group. The results suggested that increased blood glucose after DJB may be FXR-independent. Furthermore, FXR possibly improved FGF19 secretion and finally ameliorated insulin sensitivity. But we also found that FXR expression was increased along with increase of ABST and I-BABP, which indicated the evoked role of FXR, induced by increase bile acid reabsorption, on ileal bile acid binding protein and bile acid Transporter protein. Then the metabolism was further regulated by these up-regulated proteins. Consistent with increased TGR-5 after DJB in our study, some reports also showed that bile acid-TGR-5 pathway affected intestinal secretion of GLP-1, causing insulin secretion and pancreatic beta cells proliferation [30,31]. This conclusion supported our theory. Firstly, DJB changed the anatomy of intestine to increase reabsorption of bile acid and incite the expression of TGR-5. In addition, TGR-5 promoted the release of GLP-1 by intestinal T cells to further control glucose metabolism. GLP-1 is mainly produced and released L cells from the distal jejunum and ileal [32]. GLP-1 can stimulate glucose-dependent beta cells proliferation and insulin secretion and inhibit the apoptosis of beta cells [33]. Herein, we found that DJB could improve STZ-induced T2DM rats rapidly, effectively and persistently. Moreover, its improvement on diabetes was body weight-independent. Increased bile acid level after DJB may be the underlying mechanism to regulate glucose metabolism. Our study confirmed that bile acid-FXR and bile acid-TGR-5 signaling pathway were extremely important in ameliorating glucose metabolism. DJB could accelerate the contact between bile acid and terminal ileum to improve the early sense of bile acid change, which finally promoted the secretion of GLP-1 and FGF19/15 and ameliorated the insulin sensitivity. However, the precise mechanism remains to be further studied.
Disclosure of conflict of interest
None.
References
- 1.Ding L, Qu Z, Chi J, Shi R, Wang L, Hou L, Wang Y, Pang S. Effects of preventive application of metformin on bile acid metabolism in high fatfed/streptozotocin-diabetic rats. Int J Clin Exp Pathol. 2015;8:5450–5456. [PMC free article] [PubMed] [Google Scholar]
- 2.Pournaras DJ, Osborne A, Hawkins SC, Vincent RP, Mahon D, Ewings P, Ghatei MA, Bloom SR, Welbourn R, le Roux CW. Remission of type 2 diabetes after gastric bypass and banding: mechanisms and 2 year outcomes. Ann Surg. 2010;252:966–971. doi: 10.1097/SLA.0b013e3181efc49a. [DOI] [PubMed] [Google Scholar]
- 3.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 5.Zheng ZH, Lv GP, Si SY, Dong YS, Zhao BH, Zhang H, He JG. A cell-based high-throughput screeningassay for Farnesoid X receptor agonists. Biomed Environ Sci. 2007;20:465–469. [PubMed] [Google Scholar]
- 6.Seol W, Choi HS, Moore DD. Isolation of proteins that interact specifically with retinoid X receptor: two novel orphan receptors. Mol Endocrinol. 1995;9:72–85. doi: 10.1210/mend.9.1.7760852. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Castellani LW, Sinal CJ, Gonzalez FJ, Edwards PA. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) regulates triglyceride metabolism by activation of the nuclear receptor FXR. Genes Dev. 2004;18:157–169. doi: 10.1101/gad.1138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eloranta JJ, Kullak-Ublick GA. Coordinate transcriptional regulation of bile acid homeostasis and drug metabolism. Arch Biochem Biophys. 2005;433:397–412. doi: 10.1016/j.abb.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Houten SM, Auwerx J. The enterohepatic nuclear receptors are major regulators of the enterohepatic circulation of bile salts. Ann Med. 2004;36:482–491. doi: 10.1080/07853890410018790. [DOI] [PubMed] [Google Scholar]
- 10.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Bavner A, Sanyal S, Gustafsson JA, Treuter E. Transcriptional corepression by SHP: molecular mechanisms and physiological consequences. Trends Endocrinol Metab. 2005;16:478–488. doi: 10.1016/j.tem.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney RR, Willson TM, Kliewer SA. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1represses bile acid biosynthesis. Mol Cell. 2000;6:517–26. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 13.Houten SM, Volle DH, Cummins CL, Mangelsdorf DJ, Auwerx J. In vivo imaging of farnesoid X receptor activity reveals the ileum as the primary bile acid signaling tissue. Mol Endocrinol. 2007;21:1312–1323. doi: 10.1210/me.2007-0113. [DOI] [PubMed] [Google Scholar]
- 14.Potthoff MJ, Boney-Montnya J, Choi M, He T, Sunny NE, Satapati S, Suino-Powell K, Xu HE, Gerard RD, Finck BN, Burgess SC, Mangelsdorf DJ, Kliewer SA. FGFl5t19 regulates hepatic glucose metabolism by inhibiting the CREB-PGClalpha pathway. Cell Metab. 2011;13:729–738. doi: 10.1016/j.cmet.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamagata K, Daitoku H, Shimamoto Y, Matsuzaki H, Hirota K, Ishida J, Fukamizu A. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear faetor 4 and Fox01. J Biol Chem. 2004;279:23158–23165. doi: 10.1074/jbc.M314322200. [DOI] [PubMed] [Google Scholar]
- 16.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe M, Houten SM, Mataki C, Christo ffolete MA, Kim BW, Sato H, Messaddeg N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 18.Simonen M, Dali-Youcef N, Kaminska D, Venesmaa S, Kakela P, Paakkonen M, Hallikainen M, Kolehmainen M, Uusitupa M, Moilanen L, Laakso M, Gylling H, Patti ME, Auwerx J, Pihlajamaki J. Conjugated bile acids associate with altered rates of glucose and lipid oxidation after Roux-en-Y gastric bypass. Obes Surg. 2012;22:1473–1480. doi: 10.1007/s11695-012-0673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, Badman MK, Maratos-Flier E, Mun EC, Pihlajamaki J, Auwerx J, Goldfine AB. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009;17:1671–1677. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cariou B, Van Harmelen K, Duran-Sandoval D, van Dijk TH, Grefhorst A, Abdelkarim M, Caron S, Torpier G, Gruchart JC, Gonzalez FJ, Kuipers F, Staels B. The farnesoid X receptor modulates a(iposity and peripheral insulin sensilivity in micem. J Biol Chem. 2006;281:11039–11049. doi: 10.1074/jbc.M510258200. [DOI] [PubMed] [Google Scholar]
- 21.Kong B, Luyendyk JP, Tawfik O, Guo GL. Farnesoid X receptor deficiency induces nonalcoholic steatohepatitisinlow-density lipoprotein receptor-knockout mice fed a high-fat diet. J Pharmacol Exp Ther. 2009;328:116–122. doi: 10.1124/jpet.108.144600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu H, Liu Y, Wang H, Xu X. High-fat diet induced insulin resistance in pregnant rats through pancreatic pax6 signaling pathway. Int J Clin Exp Pathol. 2015;8:5196–5202. [PMC free article] [PubMed] [Google Scholar]
- 23.Lee H, Zhang Y, Le FY, Nelson SF, Gonzalez FJ, Edwards PA. FXR regulates organic solute transporters alpha and beta in the adrenal gland, kidney, and intestine. J Lipid Res. 2006;47:201–214. doi: 10.1194/jlr.M500417-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, Donahee M, Wang DY, Mansfield TA, Kliewer SA, Goodwin B, Jones SA. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17:1581–1591. doi: 10.1101/gad.1083503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Nakamura T, Itadani H, Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 26.Shang Q, Saumoy M, Holst JJ, Salen G, Xu G. Colesevelam improves insulin resistance in a diet-induced obesity (F-DIO) rat model by increasing the release of GLP-1. Am J Physiol Gastrointest Liver Physiol. 2010;298:G419–424. doi: 10.1152/ajpgi.00362.2009. [DOI] [PubMed] [Google Scholar]
- 27.Nauck MA. Incretin-based therapies for type 2 diabetes mellitus: properties, functions, and clinical implications. Am J Med. 2011;124(Suppl 1):S3–18. doi: 10.1016/j.amjmed.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Staels B, Handelsman Y, Fonseca V. Bile acid sequestrants for lipid and glucose control. Curr Diab Rep. 2011;11:70–77. doi: 10.1007/s11892-009-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe M, Houten SM, Mataki C, Christo ffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Castellani LW, Sinal CJ, Gonzalez FJ, Edwards PA. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) regulates triglyceride metabolism by activation of the nuclear receptor FXR. Genes Dev. 2004;18:157–169. doi: 10.1101/gad.1138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han SI, Studer E, Gupta S, Fang Y, Qiao L, Li W, Grant S, Hylemon PB, Dent P. Bile acids enhance the activity of the insulin receptor and glycogen synthase in primary rodent hepatocytes. Hepatology. 2004;39:456–463. doi: 10.1002/hep.20043. [DOI] [PubMed] [Google Scholar]
- 32.Pournaras DJ, Osborne A, Hawkins SC, Vincent RP, Mahon D, Ewings P, Ghatei MA, Bloom SR, Welbourn R, le Roux CW. Remission of type 2 diabetes after gastric bypass and banding: mechanisms and 2 year outcomes. Ann Surg. 2010;252:966–971. doi: 10.1097/SLA.0b013e3181efc49a. [DOI] [PubMed] [Google Scholar]
- 33.Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]


