Abstract
Objectives: The efficacy of gene overexpression of CASP5, a caspase family member, in angiogenesis in vitro and its mechanisms were clarified. Methods: Human full-length CASP5 gene was delivered into human microvascular endothelial HMEC-1 cells by recombinant lentivirus. The infection was estimated by green fluorescent protein. MTT method was used to analyze the efficacy of gene overexpression in cell proliferation ability, and Matrigel was used to estimate its effects in angiogenesis ability of cells. Meanwhile, Western blot was used to analyze the effects of CASP5 gene overexpression on the expression levels of angpt-1, angpt-2, Tie2 and VEGF-1 in the cells, which were signaling pathway factors related to angiogenesis. Results: Recombinant lentivirus containing human full-length CASP5 gene was packed and purified successfully, with virus titer of 1×108 TU/ml. The recombinant lentivirus was used to infect HMEC-1 cells with MOI of 1, leading to a cell infection rate of 100%. There were no significant effects of CASP5 gene overexpression on both cell proliferation ability and the expression level of angpt-1. Meanwhile, expressions of angpt-2 and VEGF-1 were both enhanced, while Tie2 expression was inhibited. Results indicated that CASP5 gene overexpression promoted angiogenesis of HMEC-1 cells. Conclusion: CASP5 gene overexpression significantly promoted angiogenesis ability of HMEC-1 cells, which was probably achieved by inhibiting angpt-1/Tie2 and promoting VEGF-1 signal pathway.
Keywords: CASP5 gene, caspase, HMEC-1 cells, angiogenesis
Introduction
Angiogenesis is a physiological phenomenon involved in the process of organism growth and development, especially under the conditions of trauma repair, ischemia hypoxia and inflammatory. It is closely related with the occurrence, development and prognosis of many diseases [1]. The mechanism of angiogenesis early started with cell phenotype and functional status in alveolar wall vessels. Then it turned into research about the regulation of growth factors and extracellular matrix to vascular wall cells. Vascular endothelial growth factor (VEGF) is the most critical antigenic stimulator we have already known and its core effects of inducing endothelial cell proliferation is mainly achieved by inhibiting endothelial cell apoptosis [2,3]. Recently, increasing attention is paid to mechanisms of extracellular matrix degradation by proteolytic enzyme system involved in angiogenesis [4-6]. The reciprocity between apoptosis-related factors and angiogenesis-related factors results in the regulation of the signal transduction pathways of the latter one and thereby causes cell angiogenesis [7].
CASP5 is a member of the caspase family comprising 418 amino acids, and its molecular weight is 47 kDa. After being activated, CASP5 produces heterodimers with activity and acts as the terminal effector in the apoptosis transduction pathway [8]. CASP5 overexpression in cells may influence the level of angiogenesis-related signal factors and then cell angiogenesis ability. In order to enhance the transduction efficiency of the exogenous gene, we used recombinant lentivirus to deliver CASP5 gene into HMEC-1 cells. The efficacy of the exogenous gene expression in cell proliferation and angiogenesis abilities was observed and the involved mechanisms were also discussed.
Materials and methods
Cell lines and culture conditions
Human microvascular endothelial cell line HMEC-1 and human embryonic kidney cell line 293T was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). HMEC-1 cells were cultured in 1640 medium containing 1% antibiotics (Penicillin/Streptomycin, Gibco) and 10% FBS (Gibco), while DMEM (Gibco) complete medium was used for 293T cell culture. Both cells were cultured under 37°C, 5% CO2 and saturated humidity.
Human CASP5 recombinant lentivirus package
CASP5 homo gene (NCBI Genbank accession No. NM_001136109) obtained by gene synthesis was used to construct pLV5 shuttle plasmid by clone. Packaging plasmids of pGag/Pol, pRev and pVSV-G (Western Technology Inc.), along with the shuttle plasmid were used to transform competent Escherichia coli DH5α cells. Plasmids were then extracted by High-purity, Endotoxin-free Plasmid Midi Preparation Kit (NucleoBond Xtra Midi Plus, Macherey-Nagel). 293T cells were seeded in 15 cm culture dishes and cultured overnight to gain 80 to 90% confluence. Shuttle plasmid and package plasmid were mixed proportionally and diluted in 1.5 ml serum-free DMEM medium, while 300 μl RNAi-Mate (Western Technology Inc.) was diluted in another 1.5 ml serum-free DMEM medium. After placed at room temperature for 5 min, the 2 mixtures above were mixed and placed for another 20-25 min. Culture medium in 293T cell was changed to serum-free medium, and the transfection mixture was added dropwise. After incubated for 4-6 h in an incubator, cells were placed onto complete medium and cultured for 72 h before supernatant was collected.
Human CASP5 recombinant lentivirus purification
The supernatant of culture medium in transfected cells was collected under 4°C, centrifuged at 4000 rpm for 4min and filtrated with a 0.45 μm filter. Every 100 ml filtered supernatant was added with 50 ml 20% PEG8000 solution containing 2.5 M NaCl and an ice bath of 1 h aimed to precipitate virus. After centrifugation at 4°C, 12000 rpm for 20 min, the supernatant was abandoned and the precipitate was suspended in 10 ml 1.10 g/ml CsCl solution, solvent of which was 20 mM Tris-HCl (pH=8.0). Over speed centrifugal tube was added with 5 ml virus suspension, 3 ml CsCl solution (1.30 g/ml) and 2 m CsCl solution (1.40 g/ml) from top to bottom and centrifuged at 20000 rpm for 2 h under room temperature. Virus band between 1.30-1.40 g/ml was collected into a dialysis bag and dialyzed overnight with dialysis buffer of 10 mM Tris-HCl and 2 mM MgCl2 (pH 8.0). The dialyzed virus was collected, subpackaged and preserved at -80°C.
Titration of human CASP5 recombinant lentivirus
293T cells were seeded at a concentration of 1×105 cells/well in 96-well culture plates and cultivated in the incubator for 24 h. The purified recombinant lentivirus was tenfold diluted in DMEM complete medium. Each well was added with 100 μl diluted virus (10-2-10-6). Blank control group was also established and both groups were incubated for 24-72 h. Positive cells were counted with an inverted fluorescence microscope, and virus titer was calculated combined with the dilution ratio (Transducing units per ml, TU/ml).
Infection of HMEC-1 cells by recombinant lentivirus
HMEC-1 cells were seeded at a concentration of 5×104 cells/well in 24-well culture plates and cultivated in the incubator for 24 h to reach confluence of approximately 70%. DMEM complete medium containing 5 μg/ml polybrene (Sigma) was used to dilute the recombinant lentivirus, which was used to infect HMEC-1 cells with multiple of infection (MOI) of 15. The infected cells were cultivated for another 72 h and inverted fluorescence microscope was used to analyze efficiency of infection.
Cell proliferation detection by MTT method
HMEC-1 cells were seeded at a concentration of 1×104 cells/well in 96-well culture plates with 3 parallel wells and cultivated in the incubator for 24 h. Each well was added with 20 μl 5 mg/ml MTT (Sigma) solution and incubated for 4-6 h. Then medium in each well was discarded and replaced by 150 μl DMSO. The plate was placed on the shaker under room temperature, and shake for 10 min. Light absorption under 490 nm wave length was detected with a microplate reader, while blank control group was also set up.
In vitro angiogenesis assay
Matrigel (BD) liquefaction was made under 4°C for 12 h. Each well of a 24-well culture plate was added with 200 μl Matrigel and placed in an incubator for 30 min to solidify Matrigel. HMEC-1 cells were seeded at a concentration of 4×104 cells/well in a plate with 3 parallel samples and cultivated in an incubator for 12 h. After that, formation of close tubular structure was observed under an inverted microscope.
Expression of angpt-1, angpt-2, Tie2 and VEGF-1
Digested HMEC-1 cells were collected by centrifugation, rinsed with PBS and added with appropriate amount of RIPA lysis solution. Mixture above was suspended by Vortex, and reaction was carried out in ice bath for 5 min. Then supernatant was collected after centrifugation of 12000 g at 4°C and used for SDS-PAGE and Western blot. The obtained film was scanned and the gray value of targeted band was analyzed by Labworks 4.6 of the UVP gel image processing system.
Statistical analysis
Each experiment was set up with 3 parallel samples and analyzed by SPSS 10.0 software. T-test was used for average compare of two samples.
Results
Recombinant lentivirus titration and its infection of HMEC-1 cells
Results of packaged and purified human CASP5 recombinant lentivirus titration shown in Figure 1 indicated that the virus titer was 1×108 TU/ml. Lentivirus used in the negative control group, which did not contain exogenous gene, was prepared by Western Technology Inc. in the same way with virus titer of 5×108 TU/ml. Both types of lentivirus were used to infect HMEC-1 cells with the MOI of 15. As shown in Figure 2, the infection rates of both groups were 100% after 96 h.
Figure 1.
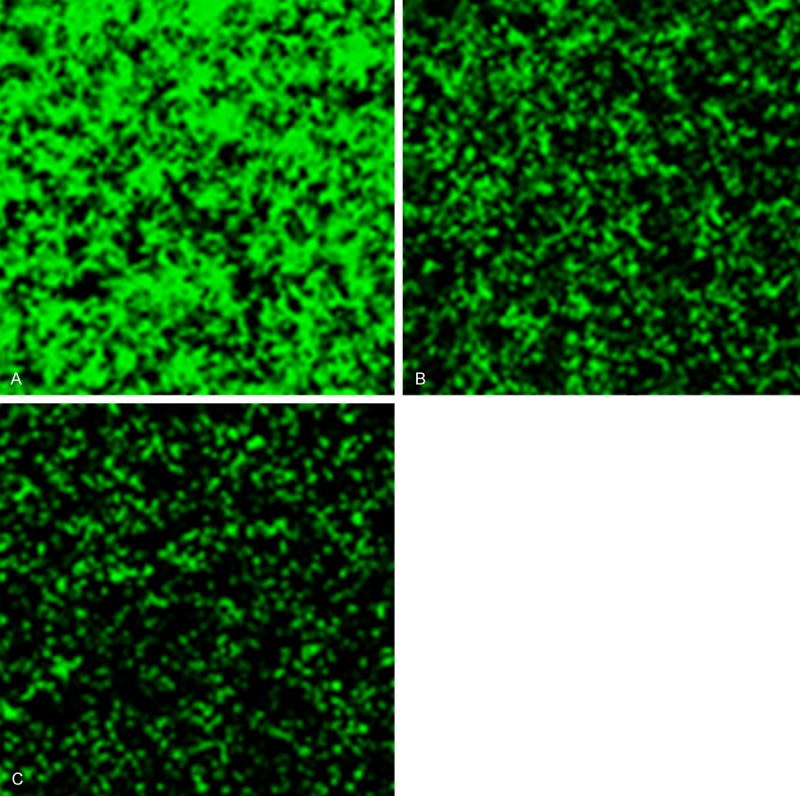
Results of human CASP5 recombinant lentivirus titration (A: 10-2, B: 10-3, C: 10-4).
Figure 2.
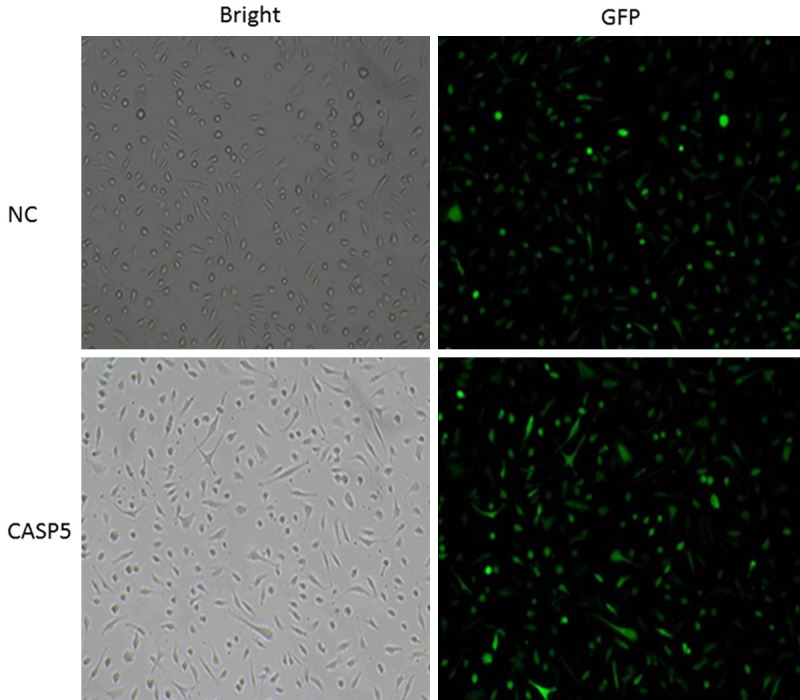
Human CASP5 recombinant lentivirus infections of HMEC-1 cells.
Effects of the CASP5 gene overexpression on HMEC-1 cell proliferation
Different groups were set up, which were CASP5 recombinant lentivirus infected cells group, control lentivirus infected cells group and normal cells group, respectively. MTT results at different time points shown in Figure 3 indicated that the OD490 value of HMEC-1 cells with overexpressed CASP5 gene (the experimental group) was 0.178±0.002, while that of HMEC-1 cells infected with empty virus (the control group) and normal HMEC-1 cells (the blank control group) was 0.207±0.017 and 0.209±0.020, respectively. There was no significant difference between the experiment group and the two control groups in cell proliferation.
Figure 3.
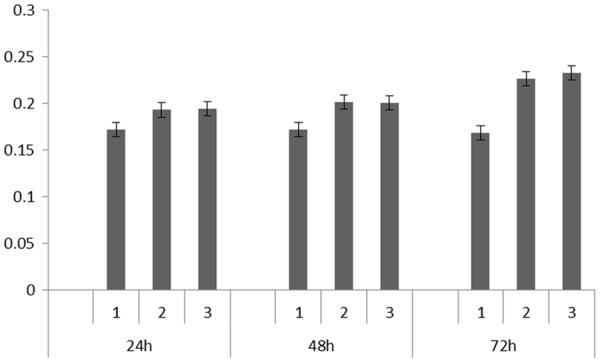
MTT results of HMEC-1 cells with different treatment at 24 h, 48 h, and 72 h. (1: Blank control group; 2: Experimental group; 3: Control group).
Effects of CASP5 gene overexpression on the angiogenesis ability of HMEC-1 cells
Under the effects of CASP5 gene overexpression, HMEC-1 cells gradually stretched, connected with each other and formed a streak and reticulate structure, with tubular structure of different sizes and shapes (Figure 4). The results of tubular structure counting showed that HMEC-1 cells with overexpressed CASP5 gene (experimental group) had significantly more tubular structures (48.2±4.6) than cells infected with empty lentivirus in the control group (24.4±3.0) (P<0.05); Similarly, experimental group had significantly more tubular structures than the blank control group (22.6±2.6) (P<0.05).
Figure 4.
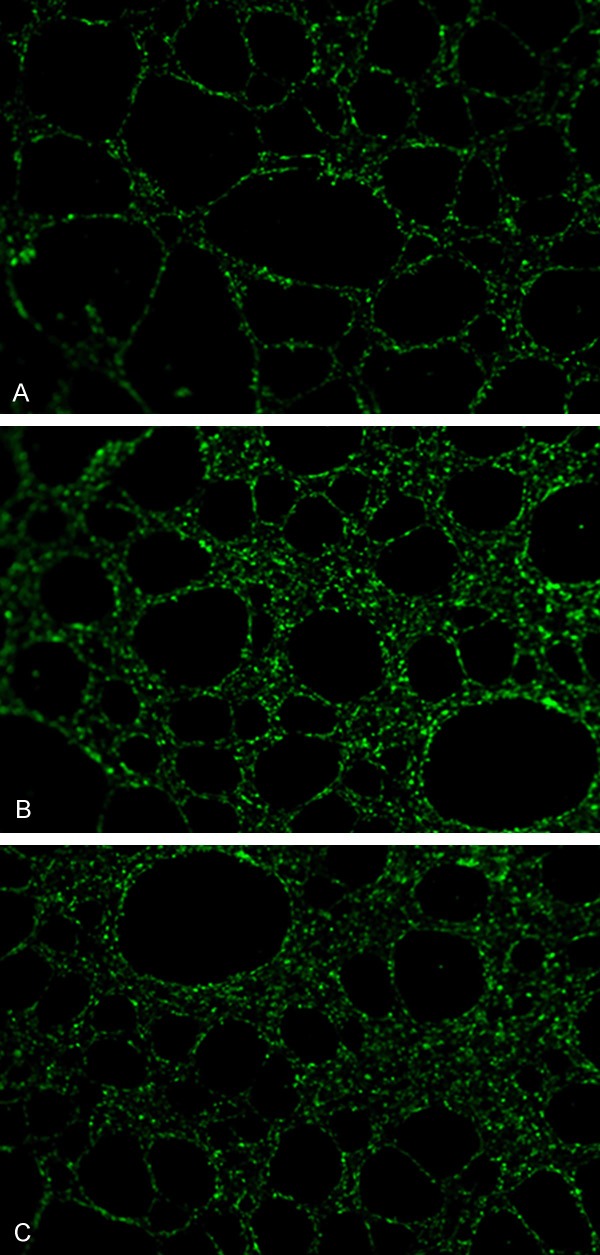
Angiogenesis ability of HMEC-1 cells with different treatment. A: Blank control group; B: Experimental group; C: Control group.
Efficacy of CASP5 gene overexpression in angpt-1, angpt-2, Tie2 and VEGF-1 expression in HMEC-1 cells
The results of Western blot and gray value scanning analysis showed that angpt-1 expression did not change significantly among the experimental, control and blank control groups. As shown in Figure 5, angpt-2 and VEGF-1 expression of cells in experimental group were obviously enhanced, while expression level of Tie2 was obviously inhibited.
Figure 5.
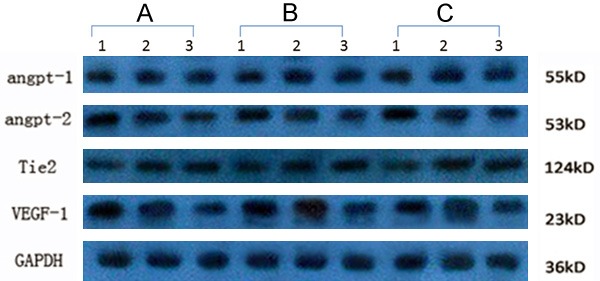
Detection of Angpt-1, angpt-2, Tie2 and VEGF-1 expression level in HMEC-1 cells with different treatment. A: Experimental group; B: Blank control groups; C: Control group.
Discussion
Angpt-1 and angpt-2/Tie2 signal transduction pathway, a new signal transduction pathway besides the VEGF pathway, plays important roles in the process of human physiological and pathological angiogenesis [9,10]. Angpt-1 does not cause spurred endothelial cell proliferation, which is often found in the anti-apoptotic process of VEGF or other cytokines. It protects cell vitality to inhibit apoptosis through activation of serine-threonine protease pathway [11,12]. The amino acid sequence homology between angpt-1 and angpt-2 is 60%. Tie2 is activated by binding of Angpt-1 and its receptor, as a result, angiogenesis is promoted by inhibiting endothelial cells apoptosis, promoting sprouting of endothelial cell migration, stabilizing blood vessels, reducing vascular permeability and other ways. When VEGF exists, angpt-2 combines with Tie2 and competitively inhibits the pro-Tie2 tyrosine kinase phosphorylation of angpt-1, leading to vascular sprouting. And the absence of VEGF is beneficial for vascular regression [13-16].
CASP5 mainly participates in cell growth, apoptosis and other physiological process. Its family member CASP3, CASP8 and CASP9 can interact with angpt-2 and regulate cell proliferation and apoptosis [17,18].
In order to improve the efficiency of gene transduction, we delivered the full-length CASP5 gene into human microvascular endothelial HMEC-1 cells by recombinant lentivirus. GFP was used to detect infection and expression efficiency of exogenous gene. Results showed that the CASP5 gene overexpression in HMEC-1 cells did not affect the expression level of angpt-1 and cell proliferation ability, while it did promote angpt-2 expression and inhibit Tie2 expression. Meanwhile, CASP5 overexpression promoted the expression level of VEGF-1 significantly. Based on the results of Matrigel observation, angiogenesis ability of HMEC-1 cells was obviously enhanced owing to CASP5 gene expression. All the results above indicated that CASP5 overexpression can promote the angiogenesis ability of HMEC-1 cells significantly, possibly by inhibiting the angpt-1/Tie2 pathway and promoting VEGF-1 pathway. However, further study is required to verify the specific mode of its action, the key factors and dose effects.
Disclosure of conflict of interest
None.
References
- 1.Orbay H, Hong H, Zhang Y, Cai W. PET/SPECT imaging of hindlimb ischemia: focusing on angiogenesis and blood flow. Angiogenesis. 2013;16:279–87. doi: 10.1007/s10456-012-9319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Yang C, Gu Q, Sims M, Gu W, Pfeffer LM, Yue J. KLF4 Promotes Angiogenesis by Activating VEGF Signaling in Human Retinal Microvascular Endothelial Cells. PLoS One. 2015;10:e0130341. doi: 10.1371/journal.pone.0130341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen HM, Tsai CH, Hung WC. Foretinib inhibits angiogenesis, lymphangiogenesis and tumor growth of pancreatic cancer in vivo by decreasing VEGFR-2/3 and TIE-2 signaling. Oncotarget. 2015;6:14940–52. doi: 10.18632/oncotarget.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colorado PC, Torre A, Kamphaus G, Maeshima Y, Hopfer H, Takahashi K, Volk R, Zamborsky ED, Herman S, Sarkar PK, Ericksen MB, Dhanabal M, Simons M, Post M, Kufe DW, Weichselbaum RR, Sukhatme VP, Kalluri R. Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res. 2000;60:2520–6. [PubMed] [Google Scholar]
- 5.Ribatti D, Nico B, Vacca A, Iurlaro M, Roncali L. Temporal expression of the matrix metalloproteinase MMP-2 correlates with fibronectin immunoreactivity during the development of the vascular system in the chick embryo chorioallantoic membrane. J Anat. 1999;195:39–44. doi: 10.1046/j.1469-7580.1999.19510039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu W H, Guo X, Villaschi S, Francesco Nicosia R. Regulation of vascular growth and regression by matrix metalloproteinases in the rat aorta model of angiogenesis. Lab Invest. 2000;80:545–55. doi: 10.1038/labinvest.3780060. [DOI] [PubMed] [Google Scholar]
- 7.Fraineau S, Monvoisin A, Clarhaut J, Talbot J, Simonneau C, Kanthou C, Kanse SM, Philippe M, Benzakour O. The vitamin K-dependent anticoagulant factor, protein S, inhibits multiple VEGF-A-induced angiogenesis events in a Merand SHP2-dependent manner. Blood. 2012;120:5073–83. doi: 10.1182/blood-2012-05-429183. [DOI] [PubMed] [Google Scholar]
- 8.Soung YH, Jeong EG, Ahn CH, Kim SS, Song SY, Yoo NJ, Lee SH. Mutational analysis of caspase 1, 4, and 5 genes in common human cancers. Hum Pathol. 2008;39:895–900. doi: 10.1016/j.humpath.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005;118:771–780. doi: 10.1242/jcs.01653. [DOI] [PubMed] [Google Scholar]
- 10.Wittig C, Scheuer C, Parakenings J, Menger MD, Laschke MW. Geraniol suppresses angiogenesis by downregulating vascular endothelial growth factor (VEGF)/VEGFR-2 signaling. PLoS One. 2015;10:e0131946. doi: 10.1371/journal.pone.0131946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang H, Bhat A, Woodnutt G, Lappe R. Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer. 2010;10:575–85. doi: 10.1038/nrc2894. [DOI] [PubMed] [Google Scholar]
- 12.Marron MB, Singh H, Tahir TA, Kavumkal J, Kim HZ, Koh GY, Brindle NP. Regulated proteolytic processing of Tie1 modulates ligand responsiveness of the receptor-tyrosine kinase Tie2. J Biol Chem. 2007;282:30509–17. doi: 10.1074/jbc.M702535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim I, Kim HG, Moon SO, Chae SW, So JN, Koh KN, Ahn BC, Koh GY. Angiopoietin-1 induces endothelial cell sprouting through the activation of focal adhesion kinase and plasmin secretion. Circ Res. 2000;86:952–9. doi: 10.1161/01.res.86.9.952. [DOI] [PubMed] [Google Scholar]
- 14.Zeng H, Li L, Chen JX. Overexpression of angiopoietin-1 increases CD133+/c-kit+ cells and reduces myocardialapoptosis in db/db mouse infarcted hearts. PLoS One. 2012;7:e35905. doi: 10.1371/journal.pone.0035905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teichert M, Stumpf C, Booken N, Wobser M, Nashan D, Hallermann C, Mogler C, Müller CS, Becker JC, Moritz RK, Andrulis M, Nicolay JP, Goerdt S, Thomas M, Klemke CD, Augustin HG, Felcht M. Aggressive primary cutaneous B-cell lymphomas show increased Angiopoietin-2-inducedangiogenesis. Exp Dermatol. 2015;24:424–9. doi: 10.1111/exd.12688. [DOI] [PubMed] [Google Scholar]
- 16.Li C, Sun CJ, Fan JC, Geng N, Li CH, Liao J, Mi K, Zhu GQ, Ma H, Song YF, Tang YL, Chen Y. Angiopoietin-2 expression is correlated with angiogenesis and overall survival in oral squamous cell carcinoma. Med Oncol. 2013;30:571. doi: 10.1007/s12032-013-0571-2. [DOI] [PubMed] [Google Scholar]
- 17.Sharma AK, Rohrer B. Calcium-induced calpain mediates apoptosis via caspase-3 in a mouse photoreceptor cell line. J Biol Chem. 2004;279:35564–72. doi: 10.1074/jbc.M401037200. [DOI] [PubMed] [Google Scholar]
- 18.Maluf DG, Mas VR, Archer KJ, Scian M, Maluf DG. Molecular pathways involved in loss of kidney graft function with tubular atrophy and interstitial fibrosis. Mol Med. 2008;14:276–85. doi: 10.2119/2007-00111.Maluf. [DOI] [PMC free article] [PubMed] [Google Scholar]


