Abstract
The purpose of this study was to investigate the expression of transforming growth factor beta-activated kinase 1 (TAK1) and its activation ligand, TAK1-binding protein 1 (TAB1), in non-small cell lung carcinoma (NSCLC) and adjacent normal tissues and to analyze the relevance between TAK1 and TAB1 protein expression and the pathological features of NSCLC patients. Surgical resection NSCLC specimens were collected from 74 patients undergoing surgery in our hospital from September 2003 to July 2008; tumor-adjacent normal tissue specimens were collected as controls. All cases were pathologically confirmed after surgery, and pathological data were complete for all patients. The expression of TAK1/TAB1 proteins in NSCLC and adjacent cancer tissues was detected by immunohistochemical analysis. The correlation between TAK1/TAB1 protein expression and the clinicopathological features and outcome of NSCLC was assessed. The positive expression ratio of TAK1 in NSCLC tissue was 63.5%, which was significantly higher than that in tumor-adjacent normal tissue (31.1%). The positive expression ratio of TAB1 in NSCLC tissue was 51.4%, which was significantly higher than that in tumor-adjacent normal tissue (24.3%). Further analysis showed that positive protein expression of TAK1 and TAB1 was unrelated to patient gender, age, tumor size, degree of differentiation, and history of smoking (P>0.05) but was significantly related to clinical stage and lymph node metastasis (P<0.05). Additionally, the expression of TAK1 as well as TAB1 was negatively related to NSCLC patient prognosis, and patients with positive protein expression had a significantly lower 5-year survival rate than those with negative protein expression (P<0.05). TAK1/TAB1 expression in NSCLC tissue is significantly increased and closely associated with patient clinical prognosis. These two proteins are likely to become new therapeutic targets for the treatment of NSCLC.
Keywords: Non-small cell lung carcinoma, TAK1, TAB1
Introduction
Non-small cell lung carcinoma (NSCLC) accounts for more than 85% of all lung cancers and is a malignant tumor that seriously threatens human life and health. Although new methods for NSCLC diagnosis and treatment have continuously emerged, and the overall therapeutic effect on NSCLC has improved, the 5-year survival rate of NSCLC is still approximately 15% [1]. Therefore, improving patient prognosis through an in-depth understanding of the exact molecular mechanisms of NSCLC development and progression and identifying specific targets for medication treatment is critical. Transforming growth factor beta-activated kinase 1 (TAK1) shows high expression in a variety of tumor tissues, and this protein is closely related to the development, progression, and invasion of various tumors [2]. The present study investigated the expression of TAK1 and its activation ligand, TAK1-binding protein 1 (TAB1), in NSCLC tissue. We further analyzed the relevance of TAK1/TAB1 expression and the clinicopathological features and prognosis of NSCLC patients to determine new areas of interest for NSCLC treatment.
Materials and methods
Specimens
The study enrolled 74 NSCLC patients who underwent surgery in our hospital from September 2003 to July 2008. Surgical section specimens of NSCLC and adjacent normal tissues were collected for testing. Prior to surgery, none of the patients underwent comprehensive therapy, such as radiotherapy, chemotherapy, and biological therapy. All cases were confirmed by pathological diagnosis after surgery. Clinical data were complete for all patients, and all subjects signed an informed consent form.
Immunohistochemical and hematoxylin-eosin staining
All antibodies used for immunohistochemical staining were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Tissue specimens from surgical resection were immediately immersed in neutral formalin and kept at 4°C for 1 d. The fixed specimens were embedded in paraffin and then sliced into 5 µm tissue sections using a microtome. Section specimens were deparaffinized with xylene and hydrated with graded ethanol, followed by antigen retrieval using antigen retrieval solution. Thereafter, the specimens were blocked with rabbit immune sera and incubated with TAK1 and TAB1 antibodies in a 37°C incubator for 2 h. After thoroughly washing with phosphate-buffered saline (PBS), the specimens were incubated with the corresponding secondary antibodies for 1 h, thoroughly washed with PBS on a shaker, and incubated with horseradish peroxidase-labeled streptavidin. After 15 min of reaction, the specimens were washed with PBS followed by coloration with diaminobenzidine, counter-staining with hematoxylin, and microscopic examination. The negative control was prepared with PBS replacing primary antibodies.
The criteria for interpreting the results of immune staining were based on the literature [3]: TAK1 and TAB1 expression was scored positive based on the presence of brownish-yellow precipitates in the cytoplasm; two experienced pathologists interpreted the results using a double-blind method; each section was examined at high magnification (400×) to count cells in 15 fields of view; the ratio of positive cells was calculated from the mean number of TAK1/TAB1-positive cells in 100 cells; the mean value from 15 fields of view was taken as the final result of each pathological section; four section specimens were selected from each patient, and the mean value of the four sections was taken as the final result of the patien (Figure 1). Further, immunohistochemical data were interpreted according to previous reports, mainly based on the following two points: 1) the percentage of positive cells with <1% for 0 points; 1-10% for 1 point, 10-30% for 2 points, 30-60% for 3 points, and >60% for 4 points; and 2) the staining intensity of positive cells with negative for 0 points, light yellow for 1 point, yellow for 2 points, and brownish yellow for 3 points. The final result was interpreted as 0-2 points for negative and 3-7 points for positive [4].
Figure 1.
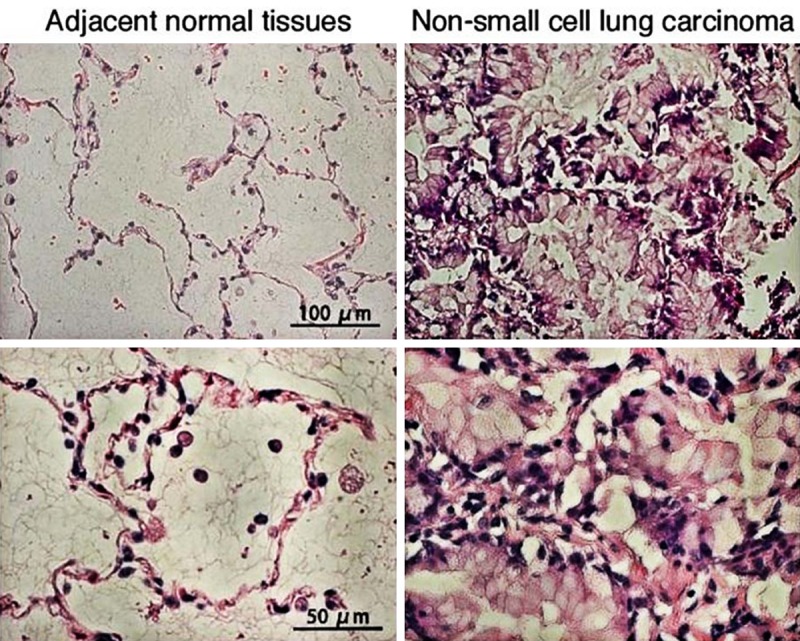
Microphotographs of hematoxylin-eosin-stained non-small cell lung carcinoma and adjacent normal tissues.
Statistical analyses
Statistical analyses were performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). The count data were analyzed using χ2 test. Survival analysis was performed using the Kaplan-Meier method. P<0.05 was considered significant.
Results
TAK1 and TAB1 protein expression in NSCLC and adjacent normal tissues
Positive expression of TAK1 (Figure 2) and TAB1 (Figure 3) in NSCLC tissue was reflected by the presence of brownish-yellow granules, mainly distributed in the cytoplasm. By comparison, the positive expression of TAK1/TAB1 was significantly reduced in tumor-adjacent normal tissue. Statistical analysis showed that the positive expression ratios of TAK1 were 63.5% in NSCLC tissue and 31.1% in tumor-adjacent normal tissue, showing a significant difference between groups (Table 1, P<0.05). TAB1 had a positive expression ratio of 51.4% in NSCLC tissue, which was significantly higher than that in tumor-adjacent normal tissue, 24.3% (Table 1, P<0.05).
Figure 2.
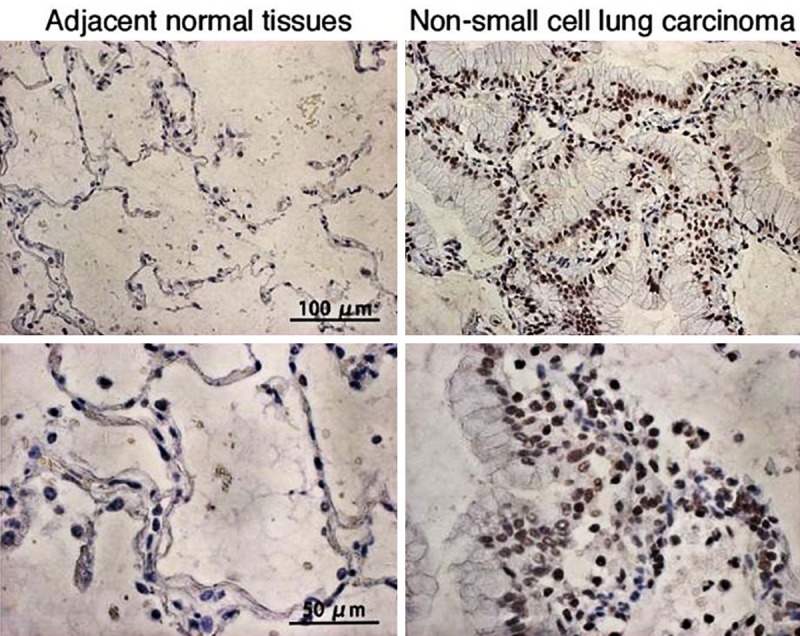
Representative microphotographs of immunohistochemical staining of TAK1 in non-small cell lung carcinoma and adjacent normal tissues.
Figure 3.
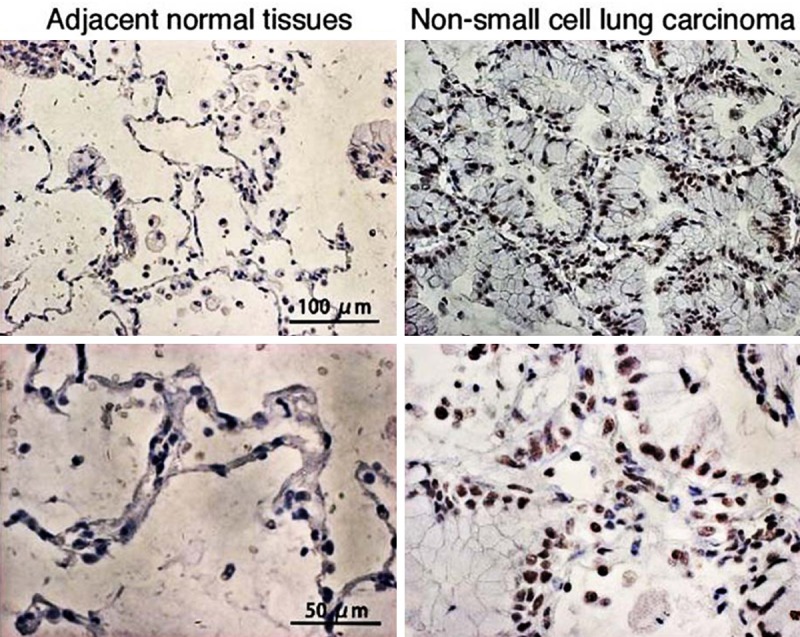
Representative microphotographs of immunohistochemical staining of TAB1 in non-small cell lung carcinoma and adjacent normal tissues.
Table 1.
Data analysis of TAK1 and TAB1 expression in non-small cell lung carcinoma (NSCLC) and adjacent normal tissues
| Group | Total cases | TAK1 | P | TAB1 | P | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Positive cases (%) | Negative cases (%) | Positive cases (%) | Negative cases (%) | ||||
| NSCLC | 74 | 47 (63.5) | 27 (36.5) | <0.001 | 38 (51.4) | 47 (63.5) | 0.0007 |
| Adjacent normal tissue | 74 | 23 (31.1) | 51 (68.9) | 18 (24.3) | 23 (31.1) | ||
TAK1: transforming growth factor beta-activated kinase 1; TAB1: TAK1-binding protein 1; NSCLC: Non-small cell lung carcinoma.
Correlation of TAK1/TAB1 expression and clinicopathological features of NSCLC
TAK1 expression was unrelated to patient gender, age, tumor size, degree of differentiation, and smoking history (P>0.05) but was positively related to clinical stage and lymph node metastasis (P<0.05, Table 2). Similarly, positive TAB1 expression was unrelated to patient gender, age, tumor size, degree of differentiation, and history of smoking (P>0.05) but was closely related to clinical stage and lymph node metastasis (P<0.05, Table 2). The correlation of TAK1 and TAB1 expression to the clinicopathological features of NSCLC patients was consistent.
Table 2.
Correlation between TAK1/TAB1 expression and clinicopathological grading of non-small cell lung carcinoma
| Group | Total cases | TAK1 | P | TAB1 | P | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Positive cases | Negative cases | Positive cases | Negative cases | ||||
| Gender | 0.21 | 0.88 | |||||
| Male | 54 | 32 | 22 | 28 | 26 | ||
| Female | 20 | 15 | 5 | 10 | 10 | ||
| Age (year) | 0.37 | 0.24 | |||||
| <65 | 38 | 26 | 12 | 17 | 21 | ||
| ≥65 | 36 | 21 | 15 | 21 | 15 | ||
| Degree of differentiation | 0.61 | 0.79 | |||||
| Low | 18 | 13 | 5 | 10 | 8 | ||
| Moderate | 29 | 20 | 9 | 16 | 13 | ||
| High | 17 | 14 | 3 | 11 | 6 | ||
| Clinical stage | 0.0002 | <0.0001 | |||||
| I, II | 39 | 17 | 22 | 11 | 28 | ||
| III, IV | 35 | 30 | 5 | 27 | 8 | ||
| Lymph node metastasis | 0.005 | 0.001 | |||||
| Yes | 43 | 33 | 10 | 29 | 14 | ||
| No | 31 | 14 | 17 | 9 | 22 | ||
| Smoking | 0.32 | 0.97 | |||||
| Yes | 41 | 24 | 17 | 21 | 20 | ||
| No | 33 | 23 | 10 | 17 | 16 | ||
| Tumor size (cm) | 0.58 | 0.24 | |||||
| <3 | 38 | 23 | 15 | 17 | 21 | ||
| ≥3 | 36 | 24 | 12 | 21 | 15 | ||
TAK1: transforming growth factor beta-activated kinase 1; TAB1: TAK1-binding protein 1.
Correlation between TAK1/TAB1 expression and clinical outcome of NSCLC
The results showed that patients with positive TAK1 expression had a significantly lower 5-year survival rate than those with negative TAK1 expression (Figure 4, P<0.05). Similarly, patients associated with positive TAB1 expression had a significantly lower 5-year survival rate than those with negative TAB1 expression (Figure 5, P<0.05).
Figure 4.
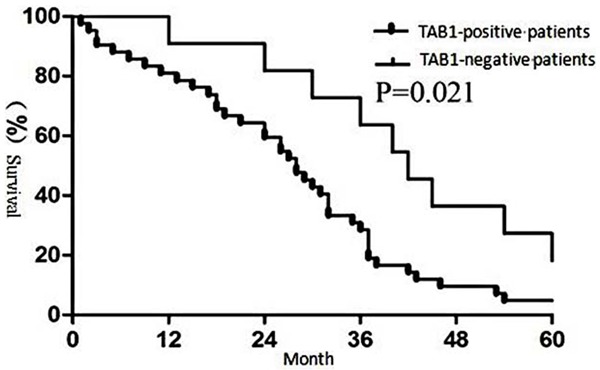
Correlation between TAK1 expression and clinical outcome of non-small cell lung carcinoma.
Figure 5.
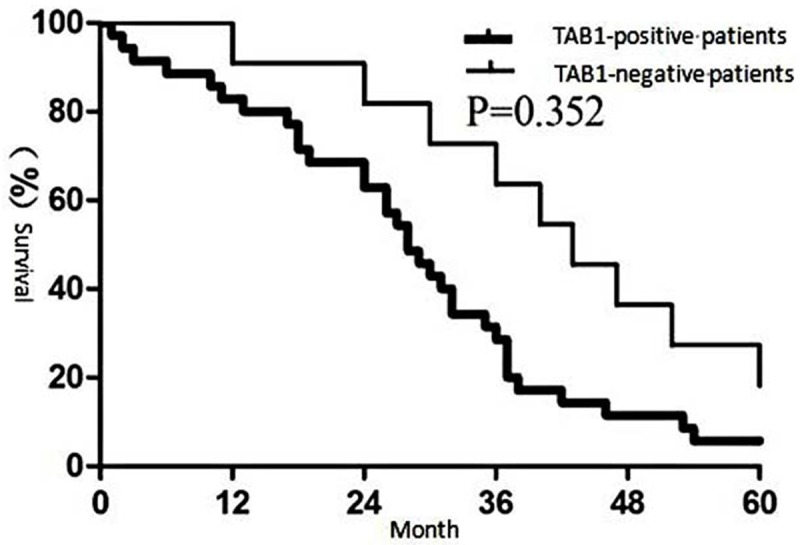
Correlation between TAB1 expression and clinical outcome of non-small cell lung carcinoma.
Discussion
TAK1 is a serine/threonine protein kinase belonging to the mitogen-activated protein kinase (MAPK) kinase (MAPKKK) protein family. Early studies have shown that TAK1 plays an important role in signal transduction of TGF-β and bone morphogenetic proteins. Recently, numerous studies have shown that the TAK1-mediated signal transduction pathway plays a critical role in tumor development and progression [5]. In the TLR, TCR, BCR, and ILR signal transduction cascade, TAK1 is located at a key node that regulates the activities of nuclear factor-kappa beta (NF-κB) and MAPK. After the binding of IL-1β to the IL-1 receptor (IL-1R), the activated IL-1R activates IL-1R-associated kinases through an adaptor protein, myeloid differentiation factor 88, while the latter further activates TNF-α receptor-associated factor 6. The activated TNF-α receptor-associated factor 6 recruits and activates TAK1 and TAB1, and the activated TAK1 further activates NF-κB-inducing kinase and IκB kinase, as well as p38 MAPK, c-Jun N-terminal kinase (JNK), and extracellular-signal-regulated kinase (ERK) [6]. The present study showed that the expression of TAK1 and its desired activation ligand, TAB1, was significantly increased in NSCLC tissue. The expression of these two proteins was closely related to tumor stage and metastasis, and their high expression status severely affected NSCLC patient prognosis. These results suggest that TAK1 is an important participant in NSCLC development and progression. Additionally, this study found that the positive expression rate of TAK1 was higher than that of TAB1 in NSCLC tissue. A possible reason is that TAB1 is not the only activation ligand of TAK1; the other activation ligands include TAB2 and TAB3.
Previous studies have found that members of the MAPK family are important components involved in NSCLC development, progression, and invasion. It has been confirmed that an increase in ERK activity upregulates the expression of matrix metalloproteinases 2 and 9, thereby promoting NSCLC cell invasion and proliferation [7]. Additionally, ERK can synergize with vascular endothelial growth factor, and inhibition of ERK activity downregulates the expression of vascular endothelial growth factor, thereby inhibiting tumor angiogenesis [7,8]. Recent studies have also found that inhibition of ERK activity enhances the chemosensitivity of NSCLC [9], whereas inhibition of JNK activity inhibits cap-dependent translation in tumor cells [10]. Moreover, p38 expression is significantly higher in lung cancer tissue, and inhibition of p38 activity significantly inhibits the proliferation of tumor cells [11].
The transcription factor NF-κB also plays an important role in NSCLC development and progression, and inhibition of NF-κB activity suppresses the development and growth of tumor tissue. TAK1 simultaneously regulates the two signaling pathways in a variety of tumor cells. High TAK1 expression has been demonstrated in various tumor tissues and is closely associated with tumor cell proliferation and invasion. Inhibition of TAK1 activity significantly increases apoptosis and suppresses proliferation of tumor cells. Given the pivotal role of TAK1 in the MAPK and NF-κB signaling pathways, and taking into account the results from the present study, we predict that TAK1 is likely to become a new therapeutic target for the treatment of NSCLC. This study provides a new area of interest for NSCLC treatment, which needs to be verified in future studies through in vitro cell culture and animal experiments to lay a solid foundation for guiding clinical therapy.
In conclusion, TAK1 and TAB1 protein expression is significantly increased in NSCLC tissue. TAK1/TAB1-targeted therapy may have great implications for the treatment of lung cancer.
Disclosure of conflict of interest
None.
References
- 1.D’Arcangelo M, Hirsch FR. Clinical and comparative utility of afatinib in non-small cell lung cancer. Biologics. 2014;8:183–192. doi: 10.2147/BTT.S40567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai H. Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol Sci. 2012;33:522–530. doi: 10.1016/j.tips.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Marsit CJ, Zheng S, Aldape K, Hinds PW, Nelson HH, Wiencke JK, Kelsey KT. PTEN expression in non-small-cell lung cancer: evaluating its relation to tumor characteristics, allelic loss, and epigenetic alteration. Hum Pathol. 2005;36:768–776. doi: 10.1016/j.humpath.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Tang JM, He QY, Guo RX, Chang XJ. Phosphorylated Akt overexpression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung Cancer. 2006;51:181–191. doi: 10.1016/j.lungcan.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Sakurai H. Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol Sci. 2012;33:522–530. doi: 10.1016/j.tips.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Ajibade AA, Wang HY, Wang RF. Cell type-specific function of TAK1 in innate immune signaling. Trends Immunol. 2013;34:307–316. doi: 10.1016/j.it.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Buonato JM, Lazzara MJ. ERK1/2 blockade prevents epithelial-mesenchymal transition in lung cancer cells and promotes their sensitivity to EGFR inhibition. Cancer Res. 2014;74:309–319. doi: 10.1158/0008-5472.CAN-12-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vicent S, Lopez-Picazo JM, Toledo G, Lozano MD, Torre W, Garcia-Corchón C, Quero C, Soria JC, Martín-Algarra S, Manzano RG, Montuenga LM. ERK1/2 is activated in non-small-cell lung cancer and associated with advanced tumours. Br J Cancer. 2004;90:1047–1052. doi: 10.1038/sj.bjc.6601644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Schmid-Bindert G, Wang D, Zhao Y, Yang X, Su B, Zhou C. Blocking the PI3K/AKT and MEK/ERK signaling pathways can overcome gefitinib-resistance in non-small cell lung cancer cell lines. Adv Med Sci. 2011;56:275–284. doi: 10.2478/v10039-011-0043-x. [DOI] [PubMed] [Google Scholar]
- 10.Song W, Ma Y, Wang J, Brantley-Sieders D, Chen J. JNK signaling mediates EPHA2-dependent tumor cell proliferation, motility, and cancer stem cell-like properties in non-small cell lung cancer. Cancer Res. 2014;74:2444–54. doi: 10.1158/0008-5472.CAN-13-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg AK, Basu S, Hu J, Yie TA, Tchou-Wong KM, Rom WN, Lee TC. Selective p38 activation in human non-small cell lung cancer. Am J Respir Cell Mol Biol. 2002;26:558–564. doi: 10.1165/ajrcmb.26.5.4689. [DOI] [PubMed] [Google Scholar]


