Abstract
Objective: To observe the effects of tauroursodeoxycholic acid (TUDCA) on nerve function after acute spinal cord injury (SCI) in rats, observe its effect on neuronal apoptosis and caspase-12 expression levels, and investigate the underlying mechanism. Methods: We used a modified Allen’s weight-drop trauma method to establish a rat acute SCI model. The rats were randomly divided into three groups: group A (sham surgery group), group B (DMSO control group) and group C (TUDCA treatment group), with 36 rats in each group. At one minute and at 24 hours after successfully establishing the model, rats in group C received an intraperitoneal injection of TUDCA (200 mg/kg), while rats in group B received an equal amount of DMSO at the same time points. At 24 hours, three days, and five days after injury, a modified Tarlov scoring method and Rivlin’s oblique plate test were used to evaluate rat spinal cord nerve function recovery. Animals were sacrificed at 24 hours, three days, and five days after injury. Specimens were obtained from the center of the injury sites; the pathological changes in spinal cord tissue were observed after hematoxylin-eosin (HE) staining; apoptosis was detected using the TUNEL method, and the expression of caspase-12 was measured at the protein level using immunohistochemistry and Western blots. Results: Group C differed significantly from group B in Tarlov scores and the oblique table test as early as 24 hours after the injury (P < 0.05). The TUNEL assay test results showed that neurons underwent apoptosis after SCI, which peaked at 24 hours. The ratios of apoptotic cells in group C were significantly lower than those in group B at 24 hours, three days, and five days after injury (P < 0.01). The immunohistochemistry and Western blot results showed that the caspase-12 expression levels of group C were lower than those of group B at 24 hours, three days, and five days after injury (P < 0.05). Conclusion: TUDCA can inhibit the expression of caspase-12 in rat neurons after SCI, reduce cell apoptosis, and exert neuroprotective effects on rat secondary nerve injuries after SCI.
Keywords: Spinal cord injury, tauroursodeoxycholic acid, neuroprotection, apoptosis, caspase-12
Introduction
Spinal cord injury (SCI) is a type of severe trauma with poor reversibility and high disability rates. Its treatment has always been a difficult problem in the medical field. Growing amounts of clinical and experimental evidence suggest that apoptosis is an important pathological change after SCI [1]. Recent studies show that spinal cord cells can undergo endoplasmic reticulum stress (ERS) after SCI, activating Caspase-12 on the endoplasmic reticulum (ER) membrane. Activated Caspase-12 is released into the cytoplasm, leading to further activation of Caspase-9 and Caspase-3, thereby causing apoptosis [2]. This process offers new information for clinical researchers to identify drugs for the treatment of secondary nerve damage after SCI. Tauroursodeoxycholic acid (TUDCA) is the main bile acid in bear bile and is a type of ursodeoxycholic acid conjugated derivative. Studies have shown that TUDCA has anti-apoptotic effects in the treatment of various diseases, which may be related to ERS [3,4]. Recent studies have shown that TUDCA can also have a neuroprotective effect during SCI, although its mechanism is not clear [5,6]. This study was conducted to observe the effect of TUDCA on hindlimb motor function after SCI, examine the conditions of neuronal apoptosis in an animal model, detect the effect of TUDCA on caspase-12 protein expression, and explore the mechanism underlying the neuroprotective effect of TUDCA.
Materials and methods
Main reagents
The rabbit anti-mouse Caspase-12 polyclonal antibody was purchased from Abcam Co. The goat anti-rabbit secondary antibody was purchased from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., and the TUNEL assay kit was purchased from Roche Co. Tauroursodeoxycholic acid (99% purity) was purchased from Chengdu Chemical Reagent Company, and dimethyl sulfoxide (DMSO) was purchased from Sigma Co.
Animals
A total of 108 male clean-grade Sprague Dawley (SD) rats that were approximately six to eight weeks old and 190-220 g were provided by the Experimental Animal Center of Ningxia Medical University. The rats were housed in a temperature controlled (22 ± 2°C) animal facility with a light:dark cycle of 12:12 h. The SD rats were randomly divided into group A (sham surgery group, n = 36), group B (injury group, n = 36) and group C (TUDCA treatment group, n = 36).
Establishment of the animal model
Rats in group A were anesthetized via intraperitoneal injection of 30 g/L chloral hydrate (40 mg/kg). For rats in group A, a midline incision was made in the lower back under sterile conditions, and tissues were dissected layer by layer to reveal the T8-T10 vertebra. A T9 total laminectomy was then conducted to expose the dura. While maintaining the integrity of the dura, the incision was sutured layer by layer using No. 0 sutures. The dura of the rats in group B and group C was exposed as described above, and the spinous processes of the T8 and T10 vertebra were fixed by clamps. A stainless steel rod with a diameter of 2.5 mm and a weight of 10.0 g was dropped vertically from a height of 25 mm along a tube with graduations and hit a plastic bar that had a concave bottom and a diameter of 3 mm, causing incomplete SCI in rats. After generating the injury, the weight was quickly removed, and the tissue was sutured using No. 0 sutures. The criteria for successful model establishment included post weight-drop trauma, hemorrhage and edema shown by the spinal cord at the injury site, tail wagging reflection, retraction-like flutter shown in both lower extremities and the torso, and flaccid paralysis shown in the lower extremities after awakening from anesthesia. The rats were kept warm and housed separately, with free access to food. On each day at 8 am and 8 pm, bladder massage was conducted to assist urination until the establishment of a reflex to empty the bladder. After modeling, all groups were randomly divided into three subgroups corresponding to different time points after SCI (24 hours, three days, and five days), with 12 rats for each time point. All surgeries were performed by the same person.
Drug delivery method. Rats in group A did not receive any treatment. Rats in group C received intraperitoneal injections of 200 mg/kg TUDCA at one minute and 24 hours after successfully establishing the model, and rats in group B received intraperitoneal injections of the same dose of DMSO at the same time points [6].
Spinal cord nerve function evaluation
At 24 hours, three days, and five days after the surgery, the modified Tarlov score and Rivlin’s oblique plate test were used to conduct neurological evaluations of all animals. All observations were performed at 8 pm by two investigators who were not aware of the experimental details but were familiar with the scoring standards. Each person conducted the evaluations three times, and the scores were then averaged. Prior to evaluation, the bladder was emptied so that the results would not be affected. The modified Tarlov scores were obtained first according to the scoring criteria, and Rivlin’s oblique plate test was performed 30 minutes later. The modified Tarlov score was used to classify the hindlimb motor function of SCI animals into grades 0-5 [7]: grade 0, no spontaneous movement; grade 1, movement limited to non-reflective movements of the hip and knee, without ankle movement; grade 2, movements of the limbs and the three major joints of the hip, knee, and ankle; grade 3, active weight support and uncoordinated gaits or occasional coordinated gaits; grade 4, gaits with coordinated forelimbs and hindlimbs and interphalangeal joint movements while walking; and grade 5, normal gaits. The modified Rivlin’s oblique plate test [8] used a custom-made oblique plate (the test surface consisted of a rubber surface with shallow trenches) to measure the ability of rats to grip and maintain posture, as well as the maximum angle that rats could sustain for five seconds without sliding down the oblique plate. Each rat was measured three times, and the highest score was recorded. During each measurement, we placed the animal on a fixed spot in the middle of the oblique plate, with the body axis of the rat perpendicular to the longitudinal axis of the oblique plate. The oblique plate was lifted by 5° in each trial, and the maximum angle that the rat could sustain for five seconds without sliding down the plate was recorded as the functional value.
HE and immunohistochemical staining
At appropriate time points, six rats from each group were given an overdose of anesthesia. After cannulation of the left ventricle-ascending aorta, rapid perfusion with ice-cold normal saline was performed. When the efflux became clear, the rats were perfused using 4% paraformaldehyde/phosphate buffered saline (PBS) for 45 minutes. The spinal cords were exposed from the original incisions in the back. Centered at the injury site, a segment of spinal cord approximately 1.5 cm in length was dissected, fixed overnight in 4% paraformaldehyde/PBS, subjected to gradient dehydration, and paraffin-embedded (two tissue blocks were generated for each animal). Sagittal serial sections with a slice thickness of 5 μm were generated, and six serial sections were randomly selected from each tissue block and subjected to hematoxylin-eosin (HE) staining, terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL) detection (according to the kit instructions), and immunohistochemistry. After HE staining, fields were selected using the most severely injured spinal cord segment as the center, and the morphological changes were observed under a light microscope. After TUNEL and immunohistochemical staining, image data were measured automatically by the true color multi-function cell image analysis management system (Image-Pro Plus, Media Cybernetics, USA). The five fields with the strongest positive expression were selected to calculate the number of positive cells and the staining intensity of the positive cells, thereby obtaining the immunohistochemical score (IHS) (IHS = number of positive cells × positive cell staining intensity).
Western blots
At appropriate time points, the remaining six rats in each group were given an overdose of anesthesia, and the spinal cords were exposed from the original incisions in the back. Centered at the injury site, a segment of spinal cord of approximately 1.5 cm in length was dissected and stored in a -80°C refrigerator. The spinal cord tissue was removed, weighed, and ground, and the protein concentration was determined using the bicinchoninic acid (BCA) method in strict accordance with the instructions of the protein extraction kit and quantification kit. The protein samples were mixed with 5 × SDS gel loading buffer, denatured at 100°C for five minutes, electrophoresed, and transferred to a PVDF membrane at 400 mA for 30 minutes. The membrane was blocked in Tris-buffered saline-Tween (TBS-T) containing 5% skim milk at room temperature for one hour, and Caspase-12 antibody (1:1000) was added, followed by overnight incubation at 4°C and subsequent TBST washes. The secondary antibody (1:3000) was then added to the membrane for one hour at room temperature. The PVDF membrane treated with enhanced chemiluminescence (ECL) reagent was placed on a gel imager. The resulting images were then processed by GraphPad Prism 5 to obtain the gray values of each sample band, using β-actin as an internal reference.
Statistical analysis
Experimental data were statistically analyzed using the SPSS17.0 statistical package. Measurement data are expressed as the means ± standard deviation (x̅ ± S). Data from repeated measures were analyzed using univariate repeated measures analysis of variance, and when the difference between groups was statistically significant, the least significant difference (LSD) method was used to conduct comparisons between two groups. The false positive level was α = 0.05, and a value of P< 0.05 was considered to be statistically significant.
Results
Results of spinal cord nerve function evaluation
At 24 hours, three days, and five days after SCI, the modified Tarlov scores of group A differed significantly from group B and group C (P < 0.05), and the scores of group B differed significantly from group C (P < 0.05) (Table 1). At 24 hours, three days, and five days after SCI, the scores of group A differed significantly from group B and group C in Rivlin’s oblique plate test (P < 0.015), and group B differed significantly from group C (P < 0.015) (Table 2).
Table 1.
Modified Tarlov score
| Groups | N | 24 h | 3 d | 5 d |
|---|---|---|---|---|
| Group A | 12 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 |
| Group B | 12 | 1.31 ± 0.26 | 1.50 ± 0.26 | 1.83 ± 0.34 |
| Group C | 12 | 1.39 ± 0.26 | 1.79 ± 0.24 | 2.37 ± 0.29 |
Note: Fgroups = 1189.442, P = 0.000; Ftime = 107.098, P = 0.000; Ftime*groups = 34.083, P = 0.000.
Table 2.
Rivlin’s oblique plate test results
| Groups | N | 24 h | 3 d | 5 d |
|---|---|---|---|---|
| Group A | 12 | 63.33 ± 0.79 | 64.79 ± 1.43 | 66.25 ± 1.93 |
| Group B | 12 | 39.24 ± 3.54 | 45.21 ± 5.07 | 48.40 ± 5.15 |
| Group C | 12 | 41.53 ± 3.31 | 45.49 ± 1.79 | 53.47 ± 2.75 |
Note: Fgroups = 175.037, P = 0.000; Ftime = 219.377, P = 0.000; Ftime*groups = 28.788, P = 0.000. When compared using the LSD, there were significant differences between group B and group C.
Spinal cord morphological examination results
Group A showed normal spinal cord tissue morphology. At 24 hours after SCI, the spinal cord tissues in the injured segment of group B showed large areas of hemorrhage, edema, gray matter structural damage, vacuolar degeneration, death of large amounts of neurons, and pyknosis in some surviving neurons, while group C did not significantly differ from group B regarding the morphology (Figure 1A). At three days after SCI, the injuries in the center of the trauma and adjacent segments had deteriorated in group B, with obvious structural damage in the gray and white matter and morphological changes in some cells. In contrast, the center of trauma and adjacent segments of group C showed milder injuries. At five days after SCI, group B showed defined areas of damage; the injured segments showed reduced diameters with the formation of cavities. There were relatively fewer remaining neurons in the gray matter, the white matter showed demyelination, and surrounding areas showed infiltration by a small number of inflammatory cells and proliferation of a large number of glial cells. Group C had more remaining neurons and showed milder inflammation (Figure 1B).
Figure 1.
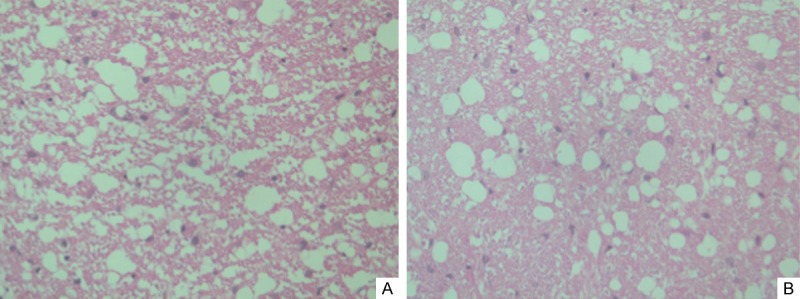
A. Photomicrograph of spinal cord tissue obtained 24 h after spinal cord injury. B. Photomicrograph of spinal cord tissue obtained 5 days after injury showing more remaining neurons and milder inflammation in TUDCA-treated mice (H&E stain, original magnification ×40).
TUNEL assay results
After SCI, TUNEL-positive cells were not detected in group A. TUNEL-positive cells could be detected in both groups B and C, with typical apoptotic changes. Normal neurons showed clear and intact structures, and the nuclear membrane was intact. In contrast, apoptotic cells showed chromatin condensation, with chromatin gathered in patches near the periphery of the nuclear membrane, and the cytoplasm was stained heavily, while necrotic cells had fuzzy structures, nucleus dissolution, and disintegration of the nuclear membrane and intracellular membrane. At 24 hours after SCI, the injured segment of group B showed a significantly increased number of positive cells, most of which were glial cells, and the same phenomenon also appeared in the white matter (Figure 2A). At three days and five days after SCI, the numbers of positive glial cells in the white matter and positive cells in the adjacent segments were slightly decreased but remained at high levels. Compared to group B, the apoptotic cell counts of group C showed that the numbers of TUNEL-positive cells at the corresponding time points were significantly reduced, and the difference in the apoptotic index between the two groups was significant (P < 0.05) (Figure 2B). The apoptotic cell scores (IHS) of spinal cord tissue in injured segments at different time points are shown in Table 3.
Figure 2.
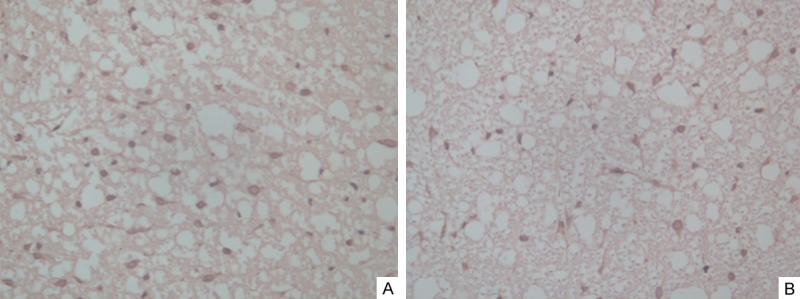
Spinal cord samples obtained after injury and stained with TUNEL. A. Photograph of a spinal cord sample obtained from the injured rats showed multiple TUNEL-positive cells in the white matter in rats of group B. B. Photograph of a spinal cord sample obtained from rats treated with TUDCA showing fewer TUNEL-positive cells and more healthy cells than for animals in group B (TUNEL stain, original magnification ×40).
Table 3.
Comparison of apoptosis (IHSs) between groups B and C measured at different time points
| Groups | N | 24 h | 3 d | 5 d |
|---|---|---|---|---|
| Group B | 6 | 164.33 ± 36.20 | 145.67 ± 24.25 | 139.83 ± 18.65 |
| Group C | 6 | 102.83 ± 13.98 | 117.83 ± 23.58 | 118.50 ± 14.54 |
Fgroups = 15.921, P = 0.003.
Immunohistochemistry results
Expression of activated caspase-12 was not detected in group A. The cytosol of cells with positive caspase-12 expression contained brown granules. Group B showed large quantities of positive cells 24 hours after SCI (Figure 3A), and the number of positive cells showed a slight decline at three days and five days after SCI but remained at a high level. Caspase-12 was mainly present in the white matter; its distribution and expression time frames were consistent with those of TUNEL-labeled apoptotic cells. Compared to group B, the apoptotic cell counts of group C showed significantly reduced numbers of caspase-12-positive cells at corresponding time points (Figure 3B), and the difference in the apoptosis index between the two groups was significant (P < 0.05) (Table 4).
Figure 3.
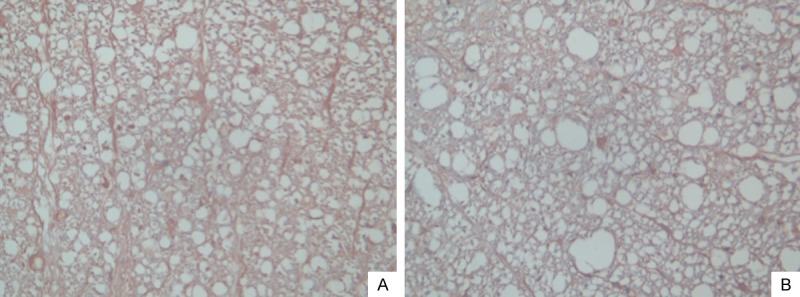
Immunochemical analysis of caspase-12 in spinal cord samples after injury. A. Multiple caspase 12-positive cells were seen in the white matter in rats of group B after SCI. B. The number of caspase-12-positive cell was reduced in TUDCA-treated rats compared to group B (Immunochemical stain, original magnification ×40).
Table 4.
Comparison of CASPASE-12 content (IHSs) between groups B and C measured at different time points
| Groups | N | 24 h | 3 d | 5 d |
|---|---|---|---|---|
| Group B | 6 | 146.67 ± 28.92 | 137.00 ± 5.25 | 122.50 ± 14.12 |
| Group C | 6 | 99.17 ± 13.00 | 95.67 ± 15.41 | 112.67 ± 16.97 |
Note: Fgroups = 39.095, P = 0.000.
Western blot results
Caspase-12 expression was not detected in group A. Compared to group B, the caspase-12 levels of group C were significantly reduced at the corresponding time points (Figure 4), and the differences were significant (P < 0.05) (Table 5).
Figure 4.
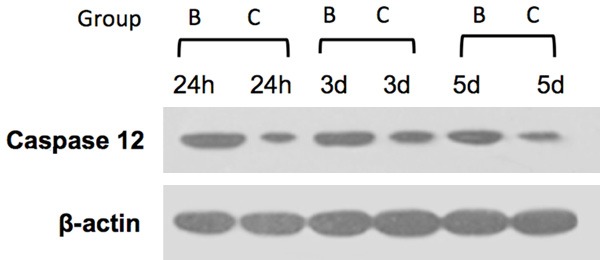
Caspase 12 expressions in spinal cord samples at different time points (24 h, 3 days and 5 days) after spinal cord injury. At all the time points, there were statistically significant differences from DMSO-treated rats and those from rats treated with TUDCA (P < 0.05).
Table 5.
Comparison of WB results of groups B and C at different time points
| Groups | N | 24 h | 3 d | 5 d |
|---|---|---|---|---|
| Group B | 6 | 0.57960 ± 0.06092 | 0.55835 ± 0.04243 | 0.46510 ± 0.00896 |
| Group C | 6 | 0.15190 ± 0.01399 | 0.14434 ± 0.00889 | 0.14703 ± 0.02823 |
Fgroups = 1098.257, P = 0.000.
Discussion
The results of this study showed that rat neurons underwent apoptosis, and the caspase-12 expression level of the rats was increased after SCI. TUDCA inhibited the expression of caspase-12 in rats after SCI, promoted rat neurological recovery after SCI, and had neuroprotective effects.
The spinal cord is the central pathway that mediates nerve function. SCI can lead to loss of nerve function, causing tissue and organ dysfunction. Loss of neurons and glial cells is the main cause of permanent impairment of spinal cord function after SCI [9,10]. Since Allen proposed the theory that the pathological mechanisms of SCI include both primary injury and secondary injury caused by the primary injury [11], many researchers have investigated the mechanisms of secondary injury and found considerable results. Many experimental studies have shown that neurons die via apoptosis after SCI [12-14]. The results of this study showed once again that neurons and glial cells undergo apoptosis after SCI.
Previous studies have suggested two main mechanisms of apoptosis after SCI: the mitochondrial-dependent pathway (extrinsic pathway) and the death receptor pathway (intrinsic pathway) [15]. Recently, ERS has been proposed as a new apoptotic pathway following the discovery of the mitochondria-dependent and death receptor pathways [16-18]. Although this pathway protects cells at early stages by temporarily suppressing intracellular protein synthesis and enabling the recovery of endoplasmic reticulum homeostasis through the activation of the unfolded protein response, it will initiate cell apoptosis in the persist presence of increased ERS-inducing factors or excessively strong ERS-inducing factors. ERS can induce apoptosis through three pathways: (1) the C/EBP homologous protein (CHOP) pathway, (2) the apoptosis signal-regulating kinase (ASK) 1/tumor necrosis factor receptor-associated factor 2 (TRAF2) pathway, and (3) the caspase-12 pathway. Caspase-12 belongs to the caspase family of enzymes and is located on the endoplasmic reticulum membrane in an inactive zymogen form under physiological conditions. When ERS occurs, the caspase-12 zymogen is cleaved, activated, and then released into the cytoplasm to activate caspase-9, and subsequently the death-execution caspase and caspase-3, to induce apoptosis [19]. It has been confirmed that caspase-3 is the most important effector proteolytic enzyme during apoptosis, and its activation is crucial for apoptosis [20].
TUDCA is the main bile acid in bear bile and is a type of ursodeoxycholic acid-conjugated derivative. It has been shown that TUDCA is one of the few non-toxic bile acids. Because it can be administered orally, intravenously, or intraperitoneally and can easily pass through the blood-brain barrier, TUDCA has broad application prospects for clinical practice, especially for nervous system protection [21]. Previous studies on TUDCA have focused on the treatment of hepatobiliary diseases, and it has been revealed that its anti-apoptotic effect in liver cells may be related to Caspase-12 [4]. Recently, some scholars have reported that TUDCA plays remarkable roles in the treatment of stroke, Huntington’s disease, and secondary injuries in SCI, but the mechanism is not clear [5].
Therefore, we speculate that TUDCA can block ERS and reduce apoptosis by inhibiting the expression of Caspase-12, thereby playing a neuroprotective role after SCI. By comparing the behavioral scores of group B and group C, we found that after TUDCA treatment, the behavioral scores of rats were significantly improved. Compared to group B, the apoptotic cell counts of group C showed that the numbers of TUNEL-positive cells at the corresponding time points were significantly reduced, indicating that TUDCA has anti-neuronal apoptosis and neural protection effects. To further confirm the changes in Caspase-12 expression before and after treatment with TUDCA, we utilized immunohistochemistry and Western blots to analyze its expression level. Our results indicated that, compared to group B, the caspase-12 levels of group C at the corresponding time points were significantly reduced. The above data confirm our hypothesis that the mechanisms by which TUDCA improves neural function and reduces apoptosis may be related to caspase-12.
In summary, our animal experiments showed that TUDCA could reduce apoptosis by inhibiting the expression of caspase-12, thus playing a protective role in secondary nerve injury after SCI in rats. This study provides a new basis for the prevention and treatment of SCI.
Acknowledgements
This work was funded by a grant from Ningxia Natural Science Foundation (NZ13136).
Disclosure of conflict of interest
None.
References
- 1.Emery E, Aldana P, Bunge MB, Puckett W, Srinivasan A, Keane RW, Bethea J, Levi AD. Apoptosis after traumatic human spinal cord injury. J Neurosurg. 1998;89:911–920. doi: 10.3171/jns.1998.89.6.0911. [DOI] [PubMed] [Google Scholar]
- 2.Kim EM, Shin EJ, Choi JH, Son HJ, Park IS, Joh TH, Hwang O. Matrix metalloproteinase-3 is increased and participates in neuronal apoptotic signaling downstream of caspase-12 during endoplasmic reticulum stress. J Biol Chem. 2010;285:16444–16452. doi: 10.1074/jbc.M109.093799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng P, Ma Q, Wang L, Zhang O, Han H, Liu X, Zhou Y, Zhao Y. Preconditioning with tauroursodeoxycholic acid protects against contrast-induced HK-2 cell apoptosis by inhibiting endoplasmic reticulum stress. Angiology. 2015;66:941–9. doi: 10.1177/0003319715575965. [DOI] [PubMed] [Google Scholar]
- 4.Xie Q, Khaoustov VI, Chung CC, Sohn J, Krishnan B, Lewis DE, Yoffe B. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology. 2002;36:592–601. doi: 10.1053/jhep.2002.35441. [DOI] [PubMed] [Google Scholar]
- 5.Vang S, Longley K, Steer C J, Low WC. The unexpected uses of urso- and tauroursodeoxycholic acid in the treatment of non-liver diseases. Glob Adv Health Med. 2014;3:58–69. doi: 10.7453/gahmj.2014.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colak A, Kelten B, Sağmanligil A, Akdemir O, Karaoğlan A, Sahan E, Celik O, Barut S. Tauroursodeoxycholic acid and secondary damage after spinal cord injury in rats. J Clin Neurosci. 2008;15:665–671. doi: 10.1016/j.jocn.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Rivlin AS, Tator CH. Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg. 1977;47:577–581. doi: 10.3171/jns.1977.47.4.0577. [DOI] [PubMed] [Google Scholar]
- 8.Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, Hsu CY, Choi DW. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garbossa D, Fontanella M, Fronda C, Benevello C, Muraca G, Ducati A, Vercelli A. New strategies for repairing the injured spinal cord: the role of stem cells. Neurol Res. 2006;28:500–504. doi: 10.1179/016164106X115152. [DOI] [PubMed] [Google Scholar]
- 10.Okano H, Ogawa Y, Nakamura M, Kaneko S, Iwanami A, Toyama Y. Transplantation of neural stem cells into the spinal cord after injury. Semin Cell Dev Biol. 2003;14:191–198. doi: 10.1016/s1084-9521(03)00011-9. [DOI] [PubMed] [Google Scholar]
- 11.Allen AR. Surgery for experimental lesions of spinal cord equivalent to crush injury of fracture dislocation of spinal column: a preliminary report. JAMA. 1911;57:878–880. [Google Scholar]
- 12.Lan WB, Lin JH, Chen XW, Wu CY, Zhong GX, Zhang LQ, Lin WP, Liu WN, Li X, Lin JL. Overexpressing neuroglobin improves functional recovery by inhibiting neuronal apoptosis after spinal cord injury. Brain Res. 2014;1562:100–108. doi: 10.1016/j.brainres.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Lu PG, Feng H, Yuan SJ, Zhang RW, Li M, Hu R, Liu ZS, Yin J. Effect of preconditioning with hyperbaric oxygen on neural cell apoptosis after spinal cord injury in rats. J Neurosurg Sci. 2013;57:253–258. [PubMed] [Google Scholar]
- 14.Liu G, Wang X, Shao G, Liu Q. Genetically modified Schwann cells producing glial cell linederived neurotrophic factor inhibit neuronal apoptosis in rat spinal cord injury. Mol Med Rep. 2014;9:1305–1312. doi: 10.3892/mmr.2014.1963. [DOI] [PubMed] [Google Scholar]
- 15.Nalepa G, Rolfe M, Harper JW. Drug discovery in the ubiquitin-proteasome system. Nat Rev Drug Discov. 2006;5:596–613. doi: 10.1038/nrd2056. [DOI] [PubMed] [Google Scholar]
- 16.Minamino T, Kitakaze M. ER stress in cardiovascular disease. J Mol Cell Cardio. 2010;48:1105–1110. doi: 10.1016/j.yjmcc.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic. reticulum stressinduced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scull CM, Tabas I. Mechanisms of ER stressinduced apoptosis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:2792–2797. doi: 10.1161/ATVBAHA.111.224881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim EM, Hwang O. Role of matrix metalloproteinase-3 in neurodegeneration. J Neurochem. 2011;116:22–232. doi: 10.1111/j.1471-4159.2010.07082.x. [DOI] [PubMed] [Google Scholar]
- 20.Stennicke HR, Salvesen GS. Properties of the caspases. Biochim Biophys Acta. 1998;1387:17–31. doi: 10.1016/s0167-4838(98)00133-2. [DOI] [PubMed] [Google Scholar]
- 21.Gaspar JM, Martins A, Cruz R, Rodrigues CM, Ambrósio AF, Santiago AR. Tauroursodeoxycholic acid protects retinal neural cells from cell death induced by prolonged exposure to elevated glucose. Neuroscience. 2013;253:380–388. doi: 10.1016/j.neuroscience.2013.08.053. [DOI] [PubMed] [Google Scholar]


