Abstract
The Hippo pathway is a highly conserved potent regulator of cell growth and apoptosis including large tumor suppressor (LATS) and Yes-associated protein (YAP). LATS has been regarded as a tumor suppressor gene and YAP as either of a tumor suppressor gene or an oncogene. We investigated their expression in lung adenocarcinoma. YAP and LATS protein expression was assessed in 167 surgically resected lung adenocarcinomas and compared with clinicopathologic factors. Disease free survival and overall survival were also evaluated. YAP expression was noted in cytoplasm (48 cases; 28.7%), nuclear (34; 20.4%) and both locations (4; 2.4%). The nuclear expression was typically observed in well differentiated adenocarcinoma. LATS was expressed in cytoplasm when its signal is weak. Perinuclear expression of LATS was observed when it is strongly expressed. While cytoplasmic and nuclear YAP expressions were inversely related. In well differentiated adenocarcinoma patients, YAP nuclear expression was related with more frequent relapse. Both of nuclear YAP and LATS expression were more frequently observed in well differentiated adenocarcinoma. Furthermore, YAP expression exhibited more frequent relapse in well differentiated adenocarcinoma group. We suggest that YAP may act as an oncogene and predict poorer prognosis in well differentiated lung adenocarcinoma.
Keywords: Lung, adenocarcinoma, hippo pathway, YAP, LATS
Introduction
Lung cancer is one of the leading causes of cancer related death thorough out the world [1]. Recently, adenocarcinoma became the most common lung cancer [1]. The most important reason why the prognosis is lung cancer is not much improved even with developments of new therapies is that lung adenoccarcinoma characteristically develop metastases or recurrences within months of diagnosis [2]. How we can predict early metastasis or recurrence is still to be discovered.
Hippo signal transduction pathway was first discovered in Drosophila melanogaster, regulating tissue growth and development [3,4]. Evolutionally conserved, it is also discovered in human. Hippo pathway consists of mammalian STE20 like protein kinase (MST), large tumor suppressor (LATS), and adaptor proteins Salvador homologue (SAV), MOB kinase activator. These proteins exert various effects through final transcription coactivators Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ) which are negatively regulated by LATS. YAP has been suggested as tumor suppressor gene or oncogene in many cancers [3]. In hepatocellular carcinoma, it was known to take a role of tumor suppressor [5] while it plays as an oncogene in breast cancer [3]. Although there are a few studies of YAP protein expression in lung cancer, the data are still limited and those about LATS are even rarer [6,7]. In the present study, we investigated the expression of YAP and LATS proteins in adenocarcinoma of lung.
Materials and methods
Patients and specimen
All adenocarcinoma patients who underwent wedge resection, lobectomy or pneumonectomy at Pusan National University Hospital, and Pusan National University Yang-San Hospital from 2008 to 2012 were selected for this study. After exclusion of cases in which there were insufficient pathological materials remaining for further study, a total of 167 cases were enrolled and representative formalin fixed paraffin tissue blocks were collected. Tumors were staged according to the 7th edition of the American Joint Committee on Cancer (AJCC) Staging Manual after review of the clinical, radiological, and pathological data [8]. Other clinical information was extracted from medical records. Histological classification was according to the IASLC/ATS/ERS classification of lung adenocarcinomas [9]. Histologic grades were divided into well and poorly differentiated groups. Well differentiated group was defined as the major portion of tumor consists of lepdic, acinar, or papillary growth, while poorly differentiated group include micropapillary, or solid growth [10]. This study was approved by the institutional review board of Pusan National University Yangsan Hospital.
Immunohistochemistry
Sections were transferred to poly-L-lysine-coated glass slides. They were dewaxed in xylene, rehydrated in ethanol. Staining was performed using the BondMax autostainer and reagents (Vision Biosystems). Deparaffinization was performed automatically in the autostainer with BondWash solution at 72°C for 30 minutes. Slides were then incubated with Epitope Retrieval Solution 1 (Leica Microsystems, Wetzlar, Germany) for 20 minutes at 100°C, peroxide block for 5 minutes, primary monoclonal antibody for 15 minutes, post primary reagent for 8 minutes, and polymer for 8 minutes. YAP (polyclonal, Santa Cruz Biotechnology, Santa Cruz, CA), and LATS1 (polyclonal, Abcam, Cambridge, MA) were used as primary antibodies.
Immunohistochemistry scoring
Slides were evaluated by two independent observers using light microscopy in a blinded fashion by two pathologists (YKK and SDH). Discordant cases were re-evaluated on a multi-headed microscope to achieve a consensus. For both of YAP and LATS, grading was done according to previous studies with our modification [6,11]. Weak cytoplasmic reactivity or strong cytoplasmic reactivity in less than 50% of cells was scored as low. Strong cytoplasmic reactivity in over 50% of cells was designated as high. For nuclear staining, it was scored as low when the reactivity is observed in less than 10% of the cells and regarded as high when the reactivity is observed in over 10% of cells.
Statistical analyses
Pearson Chi-square test or Fisher’s exact test was used to evaluate the statistical significance of immunohistochemical results related to clinicopathological parameters. Mann-Whitney test was used for continuous variables. Follow-up information was also obtained for survival analysis. Patient survival was calculated as the time between operation and death. Patients who were still alive at the time of data collection were censored in the statistical analyses. The cases lost to follow-up or deaths from any other cause were defined as censored data for the analysis of survival rates. The survival curves were plotted using the Kaplan-Meier method, and p values were calculated using the log-rank test. All statistical analyses were performed on a personal computer with the SPSS version 15.0 statistical package (SPSS, Chicago, IL, USA). P value less than 0.05 was regarded as statistically significant.
Results
Patient characteristics
A series of 167 adenocarcinomas has been retrieved for the study. They consist of 79 males and 88 females, ranging from 37 to 85 years old. Mean and median ages were 66.43 and 66, respectively. Among them, 103 cases were T1 stage. T2 and T3 were 57 and 6 cases. T4 was 1 case. One hundred seven patients had no lymph node metastasis while 40 patients had metastatic lymph nodes. Sixteen patients had metastatic disease in sites other than lymph node at the time of diagnosis and regarded as stage 4. The patient characteristics are summarized in Table 1.
Table 1.
Summary of cases in this study
| YAP expression | LATS expression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Cytoplasm | Nuclear | |||||||||
|
|
||||||||||
| No. | Low | High | P | Low | High | P | Low | High | P | |
| Age | ||||||||||
| No. | 167 | 119 | 48 | 0.59 | 133 | 34 | 0.81 | 149 | 18 | 0.38 |
| Mean | 64.5 | 64.2 | 54.2 | 65.5 | 64.3 | 65.6 | ||||
| Sex | ||||||||||
| Male | 79 | 58 | 21 | 0.61 | 67 | 12 | 0.13 | 74 | 5 | 0.88 |
| Female | 88 | 61 | 27 | 66 | 22 | 75 | 13 | |||
| Grade | ||||||||||
| Well | 110 | 82 | 28 | 0.21 | 77 | 33 | <0.01 | 94 | 16 | 0.04 |
| Poorly | 57 | 37 | 20 | 56 | 1 | 55 | 2 | |||
| T stage | ||||||||||
| 1 | 103 | 72 | 31 | 0.73 | 78 | 25 | 0.12 | 87 | 16 | 0.01 |
| 2-4 | 64 | 47 | 17 | 55 | 9 | 62 | 2 | |||
| N stage | ||||||||||
| 0 | 127 | 89 | 38 | 0.69 | 100 | 27 | 0.82 | 112 | 15 | 0.57 |
| 1-3 | 40 | 30 | 10 | 33 | 7 | 37 | 3 | |||
| M stage | ||||||||||
| 0 | 150 | 112 | 38 | <0.01 | 119 | 31 | 1.00 | 133 | 17 | 0.70 |
| 1 | 17 | 7 | 10 | 14 | 3 | 16 | 1 | |||
| Stage | ||||||||||
| I | 107 | 75 | 32 | 0.72 | 83 | 24 | 0.43 | 93 | 14 | 0.29 |
| II-IV | 60 | 44 | 16 | 50 | 10 | 56 | 4 | |||
| EGFR | ||||||||||
| Positive | 78 | 50 | 28 | 0.17 | 56 | 22 | 0.01 | 68 | 10 | 043 |
| Negative | 75 | 56 | 19 | 67 | 8 | 69 | 6 | |||
| KRAS | ||||||||||
| Positive | 22 | 15 | 7 | 1.00 | 20 | 2 | 0.25 | 17 | 5 | 0.06 |
| Negative | 130 | 90 | 40 | 102 | 28 | 119 | 11 | |||
YAP and LATS expression in pulmonary adenocarcinoma
In normal lung, YAP was expressed in type II pneumocytes infrequently and the staining was observed in nuclei (Figure 1). Bronchial epithelium didn’t express YAP. LATS was not expressed in pneumocytes and was expressed in some bronchial basal cells (Figure 1). When expressed in adenocarcinoma, cytoplasmic YAP was negative or faintly stained in cytoplasm in 119 cases and was strongly positive in cytoplasm in detected in 48 of 167 cases (Figure 2A and 2B). Nuclear YAP was observed in 34 cases (Figure 2C). Four cases showed high expression of both cytoplasmic and nuclear YAP while 44 and 30 cases exhibited high expression only for nuclei and cytoplasm, respectively. Eighty three cases showed negativity or low level expression for both locations (Table 2). The nuclear expression tended to be expressed in well differentiated adencarcinoma showing lepidic or acinar growth pattern. The cytoplasmic and nuclear expression didn’t exhibit correlation. LATS expression was mainly cytoplasmic (Figure 3A). When it is expressed weakly, it was stained diffusely across the cytoplasm. When it is strongly expressed, its location was perinuclear (Figure 3B). Fifty seven cases showed cytoplasmic positivity and only 5 cases did perinuclear positivity (Figure 3). LATS and YAP expression was not related to each other (Table 3).
Figure 1.
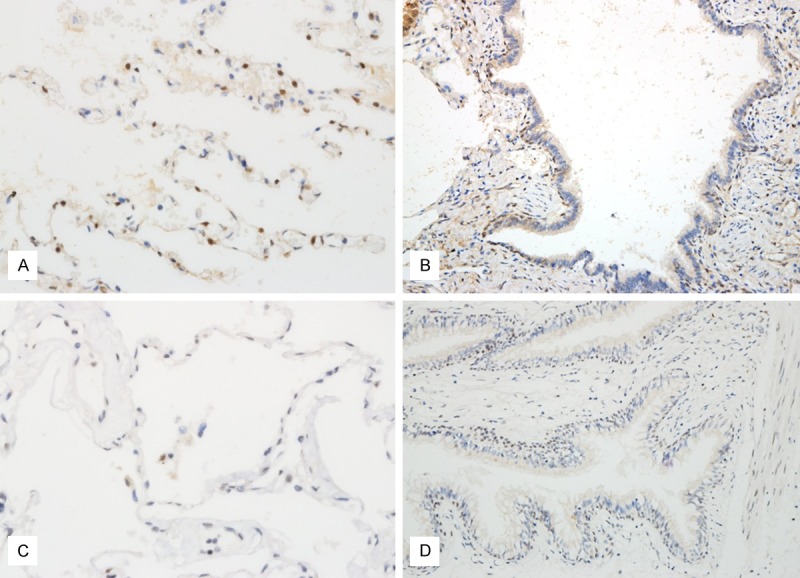
YAP and LATS expression in normal lung. A. YAP is infrequently expressed in type II pneumocytes. B. YAP is not expressed in bronchiole. C. LATS is not expressed alveolar pneumocytes. D. LATS is stained in basal cells of bronchiole.
Figure 2.
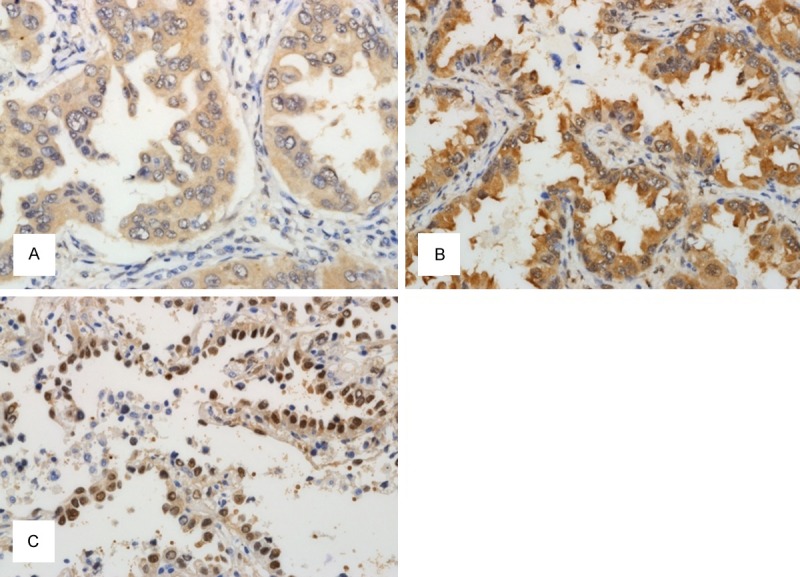
YAP expression in lung adenocarcinoma. A. YAP is weakly expressed in cytoplasm of lung adenocarcinoma. B. Strong cytoplasmic YAP expression is seen. C. Nuclear expression of YAP is observed in well differentiated adenocarcinoma.
Table 2.
Relationship of nuclear and cytoplasmic expression of YAP
| Nucleus | ||||
|---|---|---|---|---|
|
|
||||
| Low | High | |||
| Cytoplasm | Low | 89 | 30 | 0.02 |
| High | 44 | 4 | ||
Figure 3.
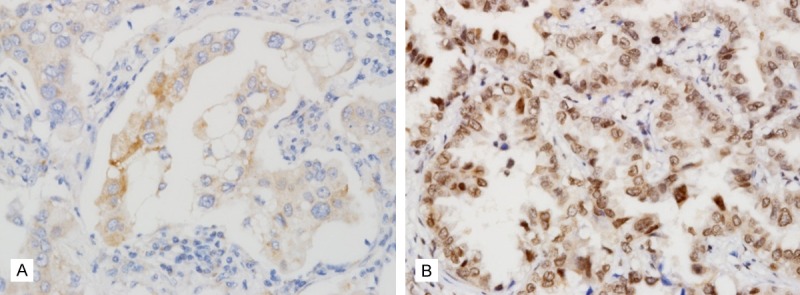
LATS expression in lung adenocarcinoma. A. LATS is weakly expressed in cytoplasm of lung adenocarcinoma. B. LATS is strongly expressed in perinuclear region.
Table 3.
Relationship of LATS and YAP expression
| YAP expression | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Cytoplasm | Nucleus | ||||||
|
|
|||||||
| No. | Low | High | P | Low | High | P | |
| LATS | |||||||
| Low | 149 | 106 | 43 | 1.00 | 120 | 29 | 0.37 |
| High | 18 | 13 | 5 | 13 | 5 | ||
Survival analysis
Among 167 patients, 13 patents died during follow up. Mean and median follow up period were 25.9 and 22.0 months. Forty patients experienced recurrence, and mean months to recur was 15.8. Histologic grade and stage were related with disease free survival and overall survival. YAP nuclear expression didn’t show statistical significance, however, when considered in well differentiated histology group, YAP nuclear expression showed more frequent relapse of cancer (Figure 4). LATS expression didn’t show any significance.
Figure 4.
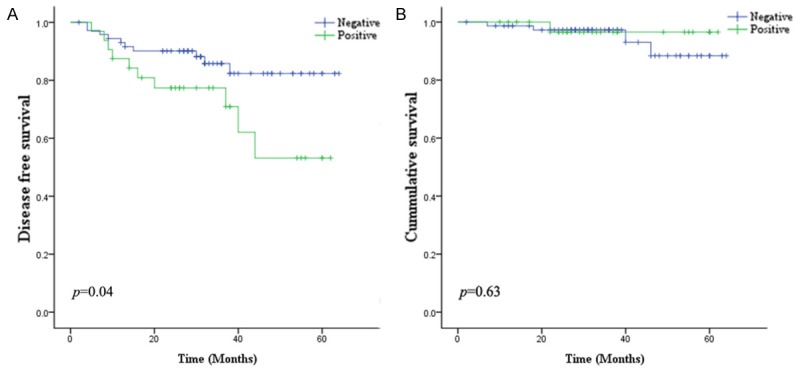
Kaplan-Meier survival curves. A. YAP nuclear expression in well differentiated adenocarcinoma is related with shorter disease free survival. B. Its expression was not related with overall survival.
Discussion
Hippo pathway is known to be involved in regulation of organ development and maturation. YAP which is a transcription coactivator of Hippo pathway has been shown to act as an oncogene in various human cancers [3]. On dephosphorylation, YAP translocates into nuclei to activate Hippo pathway transcriptional factors [3,4]. The role of Hippo pathway in lung is also being discovered. A recent study showed that Yap generate airway epithelium and regulates proximal-distal patterning of the developing lung in mouse [12]. Several studies investigated the role of Hippo pathway molecules in lung cancer, especially focusing on YAP [6,7,11,13]. According to one study, the expression of YAP was associated with expression of cell cycle markers, cyclin A and MAPK and with EGFR amplification in adenocarcinoma [13]. Although survival data was not shown, it shows that YAP may play as an oncogene in lung adenocarcinoma. LATS is an upstream molecule of YAP which phosphoryates YAP, inhibiting it translocating into nucleus and generally, LATS is regarded as a tumor suppressor gene as its name implies. LATS has not been studied as extensively while YAP is investigated in various cancers including lung cancer. One study observed that LATS expression is down-regulated in mRNA level in lung cancer [14] but very hard to find an article examining the expression of LATS protein. Hence, we investigated the expression of YAP and LATS in lung adenocarcinoma together. To our best knowledge, this is the first study to evaluate both markers simultaneously in protein level using immunohistochemistry in lung adenocarcinoma.
We could observe that YAP was expressed in both of nucleus and cytoplasm of the tumor cells because cytoplasmic YAP is dephosphorylated to translocate to nucleus. We expected, cytoplasmic and nuclear YAP expressions are related inversely to each other so that the cases which express nuclear YAP didn’t show cytoplasmic YAP. Although not perfectly related, 74 cases showed inverse relationship which is statistically significant. Nuclear expression was more frequently observed in well differentiated adenocarcinoma which exhibits lepidic, acinar and papillary growth pattern. In poorly differentiated adenocarcinomas including solid, micropapillary, and cribriform pattern, nuclear expression of YAP was not well observed. Cytoplasmic expression of YAP was seen in both of well differentiated and poorly differentiated adenocarcinomas. In well differentiated histology group, nuclear and cytoplasmic YAP showed inverse relationship better (P<0.01). Subsequently, nuclear YAP expression was more frequently observed in adenocarcinomas with EGFR mutation as shown in the other study [6]. While histologic grading was correlated with better prognosis, nuclear YAP expression was associated with more frequent cancer relapses in well differentiated histology. This finding is well consistent with that YAP plays as an oncogene. The overall survival was not significant, but this may be due to the fact that only 5 patients were dead among well differentiated adenocarcinoma group. Lung adenocarcinoma is notorious for its early recurrence even if diagnosed at an early stage and surgically removed [15,16]. In one study, 12 of 33 who had stage I bronchioloalvolear carcinoma developed recurrences [16]. Hence, the finding that YAP is associated with recurrence in well differentiated adenocarcinoma may be applied to risk stratification for recurrence and guide patient management after surgery.
The link between EGFR and Hippo pathway has been suggested in a few recent studies that EGFR regulate Hippo pathway molecules [13,17]. One study showed that inhibition of YAP reverses the resistance of tyrosine kinase inhibitor in lung adenocarcinoma [13]. The more frequent nuclear localization of YAP expression in well differentiated histology group may imply that YAP is downstream molecule activated by EGFR signal in that EGFR is more frequently mutated in well differentiated adenocarcinoma. These data can support that YAP take part in adenocarcinogenesis. Future studies can be conducted to investigate its functional role in lung adenocarcinoma and may help to develop molecules to target YAP in therapeutic field.
LATS was frequently expressed in low level in cytoplasm of tumor cells. In 18 cases, LATS was strongly expressed and its subcellular location was perinuclear. This perinuclear expression of LATS was also more frequently observed in well differentiated histology. We expected that LATS expression will exhibit inverse relationship with nuclear YAP expression but they didn’t. LATS perinuclear expression was more frequently detected in early T stage adenocarcinomas but didn’t demonstrate any relationship with other clinicopathologic factors except histologic grading. The reason why LATS expression shows little significances with YAP expression or other clinicopathologic factors may be explained by following reasons. First, we used LATS1 antibody, but actually LATS acts by two molecules, LATS1 and LATS2. Although LATS1 is known as major molecule, LATS2 may attribute to its action [12,14]. Second, actions of LATS seem to be more complicated than YAP involving many downstream molecules such as BCL2, cyclinE, cyclinB, and p53 besides YAP/TAZ [18]. Because there has been no study of LATS expression by immunohistochemistry, the perinuclear expression of LATS is intriguing new finding. It was postulated that LATS can shuffle to nucleus [18]. This finding may explain our observation of perinuclear localization of LATS but the meaning of perinuclear location of LATS still remains to be discovered.
In conclusion, Hippo pathway molecule YAP is overexpressed in well differentiated adenocarcinoma in lung and may predict relapse of well differentiated adenocarcinoma. Furthermore, association of EGFR mutation and nuclear YAP overexpression suggest the link of EGFR and Hippo pathway and the active role of Hippo pathway in lung adenocarcinogenesis.
Acknowledgements
This work was supported by grant 30-2014-004 from the Research Institute for Convergence of Biomedical Science and Technology, Pusan National University, Yang San, Republic of Korea.
Disclosure of conflict of interest
None.
References
- 1.Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2000;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 3.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 4.Saucedo LJ, Edgar BA. Filling out the Hippo pathway. Nat Rev Mol Cell Biol. 2007;8:613–621. doi: 10.1038/nrm2221. [DOI] [PubMed] [Google Scholar]
- 5.Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, Lauwers GY, Thasler W, Lee JT, Avruch J, Bardeesy N. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JM, Kang DW, Long LZ, Huang SM, Yeo MK, Yi ES, Kim KH. Differential expression of Yes-associated protein is correlated with expression of cell cycle markers and pathologic TNM staging in non-small-cell lung carcinoma. Hum Pathol. 2011;42:315–323. doi: 10.1016/j.humpath.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Dong Q, Zhang Q, Li Z, Wang E, Qiu X. Overexpression of yes-associated protein contribute to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci. 2010;101:1279–1285. doi: 10.1111/j.1349-7006.2010.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rusch VW, Appleman HD, Blackstone E, et al. Lung. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. American joint commision on cancer. 7th edition. Chicago: Springer; 2009. pp. 253–70. [Google Scholar]
- 9.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger K, Yatabe Y, Powell CA, Beer D, Riely G, Garg K, Austin JH, Rusch VW, Hirsch FR, Jett J, Yang PC, Gould M American Thoracic Society. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc. 2011;8:381–385. doi: 10.1513/pats.201107-042ST. [DOI] [PubMed] [Google Scholar]
- 10.Yoshizawa A, Motoi N, Riely GJ, Sima CS, Gerald WL, Kris MG, Park BJ, Rusch VW, Travis WD. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24:653–64. doi: 10.1038/modpathol.2010.232. [DOI] [PubMed] [Google Scholar]
- 11.Steinhardt AA, Gayyed MF, Klein AP, Dong J, Maitra A, Pan D, Montgomery EA, Anders RA. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahoney JE, Mori M, Szymaniak AD, Varelas X, Cardoso WV. The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev Cell. 2014;30:137–150. doi: 10.1016/j.devcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang JM, Nagatomo I, Suzuki E, Mizuno T, Kumagai T, Berezov A, Zhang H, Karlan B, Greene MI, Wang Q. YAP modifies cancer cell sensitivity to EGFR and survivin inhibitors and is negatively regulated by the non-receptor type protein tyrosine phosphatase 14. Oncogene. 2013;32:2220–2229. doi: 10.1038/onc.2012.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strazisar M, Mlakar V, Glavac D. LATS2 tumour specific mutations and down-regulation of the gene in non-small cell carcinoma. Lung Cancer. 2009;64:257–262. doi: 10.1016/j.lungcan.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Wu J, Tan Q, Zhu L, Gao W. Why do pathological stage IA lung adenocarcinomas vary from prognosis?: a clinicopathologic study of 176 patients with pathological stage IA lung adenocarcinoma based on the IASLC/ATS/ERS classification. J Thorac Oncol. 2013;8:1196–1202. doi: 10.1097/JTO.0b013e31829f09a7. [DOI] [PubMed] [Google Scholar]
- 16.Breathnach OS, Kwiatkowski DJ, Finkelstein DM, Godleski J, Sugarbaker DJ, Johnson BE, Mentzer S. Bronchioloalveolar carcinoma of the lung: recurrences and survival in patients with stage I disease. J Thorac Cardiovasc Surg. 2001;121:42–47. doi: 10.1067/mtc.2001.110190. [DOI] [PubMed] [Google Scholar]
- 17.Reddy BV, Irvine KD. Regulation of Hippo signaling by EGFR-MAPK signaling through Ajuba family proteins. Dev Cell. 2013;24:459–471. doi: 10.1016/j.devcel.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visser S, Yang X. LATS tumor suppressor: a new governor of cellular homeostasis. Cell Cycle. 2010;9:3892–3903. doi: 10.4161/cc.9.19.13386. [DOI] [PubMed] [Google Scholar]


