Abstract
High mobility group box 1 (HMGB1), a non-histone nuclear protein, was associated with a variety of biological important processes, such as transcription, differentiation, extracellular signaling. As a cytokine or inflammatory mediator, more and more data showed that HMGB1 was involved in inflammatory diseases, cancers or autoimmune disease. However, few data focused on nucleic or cytoplasmic function of HMGB1. Therefore, the present study focused on cancer cells biological characteristics following HMGB1 silence. HMGB1 siRNAs were designed and chemically synthesized, and then transfected into the breast cancer cell line MCF-7 with lipofectamine 2000. The transcription and translation level of HMGB1 expression, proliferation, apoptosis, migration of MCF-7 were determined. The results demonstrated that HMGB1 silence inhibit invasion and migration and promote apoptosis of human breast cells; which indicated that HMGB1 silence might be a potential therapy targets.
Keywords: HMGB1, migration, apoptosis, proliferation
Introduction
High mobility group box protein 1 (HMGB1), a highly conserved nuclear protein, is very abundant expression and plays an important structural function in chromatin organization [1]. As a chromatin-binding factor, HMGB1 exerts its key functions within the nucleus by binding the minor groove of DNA and facilitating the assembly of site-specific DNA-binding proteins, which regulate the transcription of a number of genes [2-5]. In addition to its nuclear roles, HMGB1 can be actively secreted by inflammatory cells and passively released from necrotic cells into the local microenvironment, acting as an extracellular signalling molecule that binds individual surface receptors, including the receptor for advanced glycation end products (RAGE) and Toll-like receptors (TLRs) -2, -4 and -9 during inflammation, cell migration, cell differentiation, and cancer metastasis [6-8]. The up-regulation of HMGB1 has been confirmed in a variety of cancers, such as prostate cancer [9], bladder cancer [10], hepatocellular carcinoma [11], gastric cancer [12], and lung cancer [13]. Furthermore, up-regulation of HMGB1 is associated with all the hallmarks of cancer, including limitless replicative potentiality, evasion of apoptosis and tissue invasion and metastasis; which indicated that HMGB1 might be a new potential therapeutic target for the treatment of human malignancies [14].
As a cytokine or inflammatory mediator, more and more data showed that HMGB1 was involved in inflammatory diseases, cancers or autoimmune disease. However, few data focused on nucleic or cytoplasmic function of HMGB1, especially for the cancer cells. Therefore, the present study focused on cancer cells biological characteristics following HMGB1 silence. The results demonstrated that HMGB1 silence inhibit invasion and migration and promote apoptosis of human breast cells; which indicated that HMGB1 silence might be a potential therapy targets.
Materials and methods
Cell culture and transfection
Human breast cancer cell line MCF-7 was cultured in DMEM medium (KeyGEN BioTECH) supplemented with 10% fetal bovine serum (FBS; Hyclone, USA) at 37°C, in the presence of 5% CO2. Based on the sequence of HMGB1, siRNAs and the negative control were designed and chemically synthesized in GenePhama. The cells were seeded at a density of 1×105/well in a 24-well plate before transfection to achieve more than 30-50% confluence. For transfection, 1 μl of Lipofectamine 2000 (Invitrogen, USA) were added to 50 μl Opti-MEMI Serum free Medium (Invitrogen, USA) and then mixed gently at room temperature for 5 min; 2 μl FAM-siRNA were added to 50 μl Opti-MEMI Serum free Medium and then mixed gently. After 5 min incubation, diluted Lipofectamine2000 mixed with diluted FAM-siRNA gently at room temperature for 20 min. The cells were transfected with the different mixtures. The 24-well plate was incubated at 37°C, in the presence of 5% CO2 for 6 h and then changed medium into complete medium.
Western blotting
Total protein extracts were prepared with the Total Protein Extraction Kit (KeyGEN BioTECH). The proteins were electrophoresis by 12% SDS-PAGE before being transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% non-fat dried milk in TBST, and incubated with specific primary antibodies at 37°C for 24 h, followed by horseradish peroxidase (HRP)-conjugated anti-rabbit (1:5000 dilution) antibodies for 2 h at room temperature. Detection was performed with electrochemiluminesce (ECL) and relevant blots quantified by densitometry using the accompanying computerized image analysis program (Amercontrol Biosciences, USA).
Quantitative real-time PCR (RT-qPCR)
Total RNA was isolated from MCF-7 cell lines using Trizol reagent. RNA was reverse transcribed to cDNA using the PrimeScriptTM RT reagent Kit (TaKaRa, Dalian, China) according to the manufacturer’s protocols. The RT-qPCR assay was carried out using CFX96-Real-Time System. The PCR reaction conditions included an initial denaturation for 5 sec at 95°C followed by 40 cycles each of denaturation for 10 sec at 95°C, annealing for 20 sec at 60°C, extension for 15 sec at 72°C. All experiments were repeated in triplicate. The relative expression levels of mRNA were calculated with the 2-ΔΔCt method and expressed as the normalized to GAPDH. The primers used in the study were designed and chemically synthesized in Genecopoeia.
Cell proliferation assay (CCK-8)
After transcription, the cells were seeded in 96-well plates at a density of 1×104/well and incubated at 37°C, in the presence of 5% CO2. At 1 d, 2 d, 3 d, 4 d and 5 d post-transfection, 10 μl of CCK-8 solution was added to each well and incubated for 4 h at 37°C, respectively. Then, the OD (450 nm) was measured using a microplate reader (Bio-Rad, USA). All experiments were performed in triplicate.
Cell apoptosis assay
After 48 h post-transfection, the cells were washed twice with cold PBS and then resuspended in 1× Binding Buffer at a concentration of 1×106 cells/ml. The 100 μl solution was transfered to a 5 ml culture tube. 5 μl FITC Annexin and 5 μl PI were added in the tube and incubated for 15 min at 37°C. Then, 400 μl 1× Binding Buffer was added to each tube. The cells were evaluated by flow cytometry (BD Biosciences, USA).
Cell migration assay
After transfection, the cells were collected and resuspended in a serum-free medium at a concentration of 1×105 cells/ml. Then, the lower chamber was filled with 800 μl DMEM with 10% FBS and 400 μl cell suspension was added to the upper chamber. After incubation at 37°C, in the presence of 5% CO2 for 48 h, cells on the lower surface were fixed with 75% ethanol, stained with crystal violet and counted.
Wound healing assay
A linear scratch wound was performed using a pipette tip in a confluent monolayer of cells in 6-well plates. Medium without FBS was used in order to inhibit cell proliferation [15].
Statistical analysis
All statistical analysis was performed using GraphPad Prism. Data were expressed as the mean ± standard deviation (SD). Comparisons between groups were performed using the paired t-test or one-way ANOVA with Bonferroni correction. A P value of <0.05 was considered statistically significant.
Results
HMGB1 expression in MCF-7 cells was down-regulated by HMGB1-siRNA
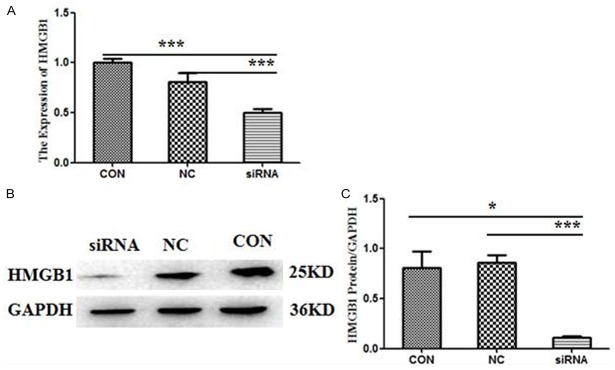
As Figure 1 shown, HMGB1 expression (both mRNA and protein) in MCF-7 cell line was obviously down-regulated following the HMGB1-siRNA transfection compared with the negative control (NC) group and the blank control (CON) group (P<0.05). However, there were no significant differences between the NC group and the CON group.
Figure 1.
HMGB1 expression in MCF-7 cells was down-regulated by HMGB1-siRNA. For the following experiments, the cells were divided into three groups: the siRNA group, the negative control (NC) group and the blank control (CON) group. The level of HMGB1 expression was measured by RT-qPCR and Western blotting after 48 h transfection. (A) The mRNA expression of HMGB1. Values were expressed compared with GADPH. B/C HMGB1 protein levels in MCF-7 cell line. GAPDH was also examined as a loading control. Representative blots were shown above (B) and densitometric analyses below (C). Data were means ± SD from three independent experiments. P values were calculated using one-way ANOVA. P<0.05 was considered significant.
HMGB1 silence did not inhibit MCF-7 cell proliferation but promote apoptosis
As a nuclear molecule, HMGB1 modulate transcription, repair and recombination through exerting effects on chromosomal architecture [16]. And then whether HMGB1 silence would affect biological characteristics of MCF-7 cell line. Therefore, the proliferation and apoptosis of MCF-7 cell were detected following the HMGB1 silence. As Figure 2 shown, there were no significant differences in cell proliferation among HMGB1 siRNA, NC and CON groups (P>0.05, Figure 2).
Figure 2.
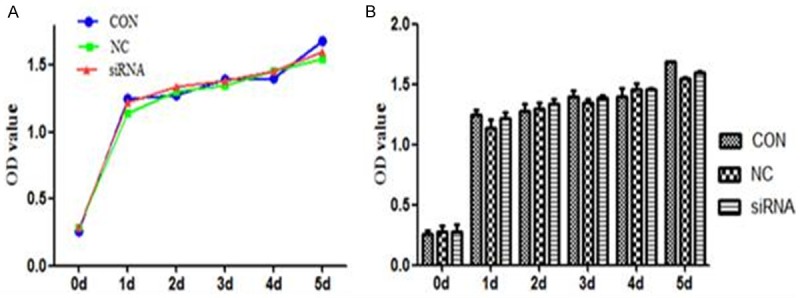
HMGB1 silence did not inhibit MCF-7 cell proliferation. The proliferation of transfected MCF-7 cells was measured by CCK-8 assay on 1 d, 2 d, 3 d, 4 d, 5 d post-transfected. No significant differences in the cell proliferation were found between the siRNA, the CON and NC groups (P>0.05). Data were means ± SD from three independent experiments. P values were calculated using one-way ANOVA. P<0.05 was considered significant.
Since HMGB1 silence didn’t inhibit MCF-7 cell proliferation; and then whether the apoptosis was affected. As Figure 3 shown, the apoptosis frequency was higher in the siRNA group (15.2±2.5%) comparing with CON (8.2±1.3%) and NC (12.3±2.8%) groups after 48 h post-transfected (Figure 3). However, no significant differences in cell apoptosis between the CON and NC groups were observed (P>0.05).
Figure 3.
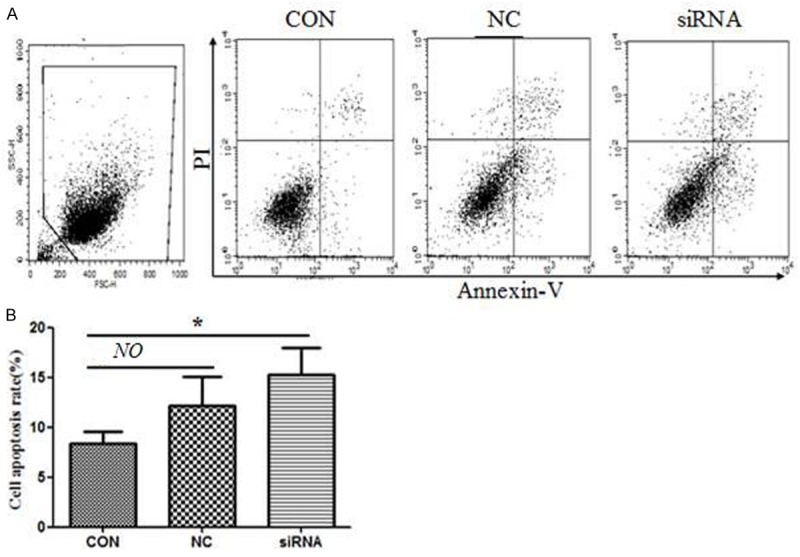
HMGB1 silence promoted MCF-7 cell apoptosis. Data are the mean ± SD from three independent experiments. Representative images are shown (above) and the statics analysis (below). P values were calculated using one-way ANOVA. P<0.05 was considered significant.
HMGB1 silence inhibited MCF-7 cell invasion and wound healing ability
Transwell assay was employed to evaluate the effect of HMGB1 silence on MCF-7 cell invasion. The numbers of invasive cells for HMGB1 siRNA, CON, NC group under the microscope were 20.1±3.5, 78.3±4.1 and 88.3±4.7. The cell number was significantly different in HMGB1 siRNA group comparing with CON and NC group Figure 4A (P<0.01).
Figure 4.
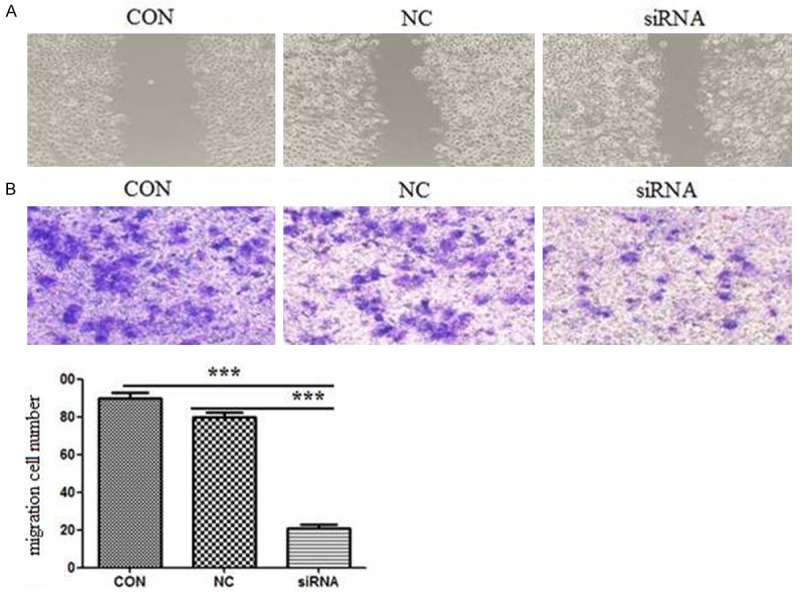
HMGB1 silence inhibited cell invasion and wound healing ability. A. Cell invasion in HMGB1 silence MCF-7 cell lines. The invasion cell number is presented by bar diagram. Each experiment was repeated for three times. ***P<0.01. B. Wound healing results. Each experiment was repeated for three times. P values were calculated using one-way ANOVA. P<0.05 was considered significant.
The wound healing assay was also employed to analysis cell migration ability. As Figure 4B shown, the migration of HMGB1 silence MCF-7 cells was apparently suppressed. These results demonstrate that HMGB1 silence suppresses MCF-7 cell invasion and migration.
Discussion
HMGB1 is a highly conserved nuclear protein, acting as achromatin-binding factor that bends DNA and promotes transcriptional protein assemblies on specific DNA targets [17,18]. In addition to its nuclear role, HMGB1 also functions as an extracellular signaling molecule during inflammation, cell differentiation, cell migration, and tumor metastasis [6,18,19]. Many tumor cells express RAGE [20], and the binding of HMGB1 to RAGE or TLR4 inhibits tumor cell apoptosis by promoting tumor cell autophagy [21] and increases tumor cell invasiveness [22,23]. Furthermore, previous studies have confirmed that HMGB1 is over-expressed in a variety of cancers [9-13]. Additionally, HMGB1 has been implicated in many hallmarks of cancer, including apoptosis, angiogenesis, invasion, metastasis and inflammatory microenvironment [14]. However, few data focused on nucleic or cytoplasmic function of HMGB1, especially for the cancer cells. Therefore, the present study focused on cancer cells biological characteristics following HMGB1 silence. Firstly, we constructed specific HMGB1-siRNA and transfected them into MCF-7 cell. The HMGB1 expression was efficiently suppressed (Figure 1). And then the biological characteristic of MCF-7 cell line was assessed following HMGB1 silence. Our results demonstrated that HMGB1 silence did not inhibit MCF-7 cell proliferation but promote apoptosis. Although some data also indicated that HMGB1 could promote the cell proliferation by modulating cyclin D1, a critical regulator of G1-phase progression [24-26]; our data only focus on the nuclear function not as a cytokine. Additionally, HMGB1 over-expression in cancer cell increased NF-κB activity and led to c-IAP2 up-regulation in colon carcinoma, which could inhibit apoptosis via suppressing caspase-3 and caspase-9 activity [27].
Cell migration invasion and wound healing ability assay also indicated that HMGB1 silence suppresses MCF-7 cell invasion and migration; which was consistent with other report that HMGB1 is also associated with tumor progression, including invasion and metastasis [28]; however the mechanism need to be investigated.
Conclusion
In conclusion, our research suggests that HMGB1, as a nuclear molecule, plays an essential role in the apoptotic, migratory and invasive activity of breast cancer cells. siRNA interference could effectively suppress the expression of HMGB1, and inhibit the migration, invasion and induce the apoptosis of human breast cells; which indicated that HMGB1 represents a potential target for breast cancer therapy.
Disclosure of conflict of interest
None.
References
- 1.Taniguchi N, Yoshida K, Ito T, Tsuda M, Mishima Y, Furumatsu T, Ronfani L, Abeyama K, Kawahara K, Komiya S, Maruyama I, Lotz M, Bianchi ME, Asahara H. Stage-specific secretion of HMGB1 in cartilage regulates endochondral ossification. Mol Cell Biol. 2007;27:5650–5663. doi: 10.1128/MCB.00130-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bianchi ME, Agresti A. HMG proteins: dynamic players in gene regulation and differentiation. Curr Opin Genet Dev. 2005;15:496–506. doi: 10.1016/j.gde.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Brickman JM, Adam M, Ptashne M. Interactions between an HMG-1 protein and members of the Rel family. Proc Natl Acad Sci U S A. 1999;96:10679–10683. doi: 10.1073/pnas.96.19.10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imamura T, Izumi H, Nagatani G, Ise T, Nomoto M, Iwamoto Y, Kohno K. Interaction with p53 enhances binding of cisplatin-modified DNA by high mobility group 1 protein. J Biol Chem. 2001;276:7534–7540. doi: 10.1074/jbc.M008143200. [DOI] [PubMed] [Google Scholar]
- 5.Onate SA, Prendergast P, Wagner JP, Nissen M, Reeves R, Pettijohn DE, Edwards DP. The DNA-bending protein HMG-1 enhances progesterone receptor binding to its target DNA sequences. Mol Cell Biol. 1994;14:3376–3391. doi: 10.1128/mcb.14.5.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellerman JE, Brown CK, de Vera M, Zeh HJ, Billiar T, Rubartelli A, Lotze MT. Masquerader: high mobility group box-1 and cancer. Clin Cancer Res. 2007;13:2836–2848. doi: 10.1158/1078-0432.CCR-06-1953. [DOI] [PubMed] [Google Scholar]
- 7.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 8.Su Z, Sun C, Zhou C, Liu Y, Zhu H, Sandoghchian S, Zheng D, Peng T, Zhang Y, Jiao Z, Wang S, Xu H. HMGB1 blockade attenuates experimental autoimmune myocarditis and suppresses Th17-cell expansion. Eur J Immunol. 2011;41:3586–3595. doi: 10.1002/eji.201141879. [DOI] [PubMed] [Google Scholar]
- 9.Ishiguro H, Nakaigawa N, Miyoshi Y, Fujinami K, Kubota Y, Uemura H. Receptor for advanced glycation end products (RAGE) and its ligand, amphoterin are overexpressed and associated with prostate cancer development. Prostate. 2005;64:92–100. doi: 10.1002/pros.20219. [DOI] [PubMed] [Google Scholar]
- 10.Yang GL, Zhang LH, Bo JJ, Huo XJ, Chen HG, Cao M, Liu DM, Huang YR. Increased expression of HMGB1 is associated with poor prognosis in human bladder cancer. J Surg Oncol. 2012;106:57–61. doi: 10.1002/jso.23040. [DOI] [PubMed] [Google Scholar]
- 11.Jiang W, Wang Z, Li X, Fan X, Duan Y. Highmobility group box 1 is associated with clinicopathologic features in patients with hepatocellular carcinoma. Pathol Oncol Res. 2012;18:293–298. doi: 10.1007/s12253-011-9442-3. [DOI] [PubMed] [Google Scholar]
- 12.Akaike H, Kono K, Sugai H, Takahashi A, Mimura K, Kawaguchi Y, Fujii H. Expression of high mobility group box chromosomal protein-1 (HMGB-1) in gastric cancer. Anticancer Res. 2007;27:449–457. [PubMed] [Google Scholar]
- 13.Liu PL, Tsai JR, Hwang JJ, Chou SH, Cheng YJ, Lin FY, Chen YL, Hung CY, Chen WC, Chen YH, Chong IW. High-mobility group box 1-mediated matrix metalloproteinase-9 expression in non-small cell lung cancer contributes to tumor cell invasiveness. Am J Respir Cell Mol Biol. 2010;43:530–538. doi: 10.1165/rcmb.2009-0269OC. [DOI] [PubMed] [Google Scholar]
- 14.Guo ZS, Liu Z, Bartlett DL, Tang D, Lotze MT. Life after death: targeting high mobility group box 1 in emergent cancer therapies. Am J Cancer Res. 2013;3:1–20. [PMC free article] [PubMed] [Google Scholar]
- 15.Jia Y, Cao B, Yang Y, Linghu E, Zhan Q, Lu Y, Yu Y, Herman JG, Guo M. Silencing NKD2 by promoter region hypermethylation promotes gastric cancer invasion and metastasis by upregulating SOX18 in human gastric cancer. Oncotarget. 2015;6:33470–85. doi: 10.18632/oncotarget.5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 17.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 18.Muller S, Scaffidi P, Degryse B, Bonaldi T, Ronfani L, Agresti A, Beltrame M, Bianchi ME. New EMBO members’ review: the double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J. 2001;20:4337–4340. doi: 10.1093/emboj/20.16.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong Xda E, Ito N, Lotze MT, Demarco RA, Popovic P, Shand SH, Watkins S, Winikoff S, Brown CK, Bartlett DL, Zeh HJ 3rd. High mobility group box I (HMGB1) release from tumor cells after treatment: implications for development of targeted chemoimmunotherapy. J Immunother. 2007;30:596–606. doi: 10.1097/CJI.0b013e31804efc76. [DOI] [PubMed] [Google Scholar]
- 20.Gebhardt C, Riehl A, Durchdewald M, Nemeth J, Furstenberger G, Muller-Decker K, Enk A, Arnold B, Bierhaus A, Nawroth PP, Hess J, Angel P. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med. 2008;205:275–285. doi: 10.1084/jem.20070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X, Zhu H, Wang T, Sun Y, Ni P, Liu Y, Tian S, Amoah Barnie P, Shen H, Xu W, Xu H, Su Z. Exogenous high-mobility group box 1 inhibits apoptosis and promotes the proliferation of lewis cells via RAGE/TLR4-dependent signal pathways. Scand J Immunol. 2014;79:386–394. doi: 10.1111/sji.12174. [DOI] [PubMed] [Google Scholar]
- 22.Livesey KM, Tang D, Zeh HJ, Lotze MT. Not just nuclear proteins: ‘novel’ autophagy cancer treatment targets - p53 and HMGB1. Curr Opin Investig Drugs. 2008;9:1259–1263. [PubMed] [Google Scholar]
- 23.Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM, Schmidt AM. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 24.Poser I, Golob M, Buettner R, Bosserhoff AK. Upregulation of HMG1 leads to melanoma inhibitory activity expression in malignant melanoma cells and contributes to their malignancy phenotype. Mol Cell Biol. 2003;23:2991–2998. doi: 10.1128/MCB.23.8.2991-2998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren M, Zhong X, Ma CY, Sun Y, Guan QB, Cui B, Guo J, Wang H, Gao L, Zhao JJ. Insulin-like growth factor-1 promotes cell cycle progression via upregulation of cyclin D1 expression through the phosphatidylinositol 3-kinase/nuclear factor-kappaB signaling pathway in FRTL thyroid cells. Acta Pharmacol Sin. 2009;30:113–119. doi: 10.1038/aps.2008.8. [DOI] [PubMed] [Google Scholar]
- 26.Palumbo R, Galvez BG, Pusterla T, De Marchis F, Cossu G, Marcu KB, Bianchi ME. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-kappaB activation. J Cell Biol. 2007;179:33–40. doi: 10.1083/jcb.200704015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volp K, Brezniceanu ML, Bosser S, Brabletz T, Kirchner T, Gottel D, Joos S, Zornig M. Increased expression of high mobility group box 1 (HMGB1) is associated with an elevated level of the antiapoptotic c-IAP2 protein in human colon carcinomas. Gut. 2006;55:234–242. doi: 10.1136/gut.2004.062729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang D, Kang R, Zeh HJ 3rd, Lotze MT. High-mobility group box 1 and cancer. Biochim Biophys Acta. 2010;1799:131–140. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]