Abstract
The present study demonstrates the effect of rubriflordilactone on lipopolysaccharide (LPS)-induced acute kidney injury in rats and MLE-15 cells. LPS administration in rats resulted in formation of edema which was inhibited by pretreatment with rubriflordilactone. The pulmonary tissues of LPS administered rats and MLE-15 cells showed a significant increase in the expression of matrix metalloproteinase-9, interleukin-6 and inducible nitric oxide synthase. However, rubriflordilactone treatment prior to LPS administration caused a significant reduction in the expression of these factors at a concentration of 10 nm/kg. Analysis of the Sirtuin 1 (Sirt1) expression revealed significant (P=0.002) reduction on exposure to LPS in MLE-15 cells. However, rubriflordilactone treatment at 10 nm/ml concentration before LPS exposure caused inhibition of LPS induced reduction in Sirt1 expression. Silencing of Sirt1 by siRNA in MLE-15 cells led to inhibition of increased Sirt1 expression by rubriflordilactone in LPS administered rats. These findings suggest that rubriflordilactone inhibits LPS induced acute lung injury in rats and MLE-15 cells through promotion of Sirt1 expression.
Keywords: Edema, inflammation, interleukin, lung injury, silencer RNA
Introduction
Lung cancer is a frequently detected disease and is considered to be the challenging threat to human health by clinicians globally. Administration of various chemotherapeutic agents has been found to induce toxicity in lung tissues and exposure to radiation therapy causes pneumonitis [1,2]. In the beginning of lung injury, pulmonary tissues show inflammation which then subsequently result in collagen deposition on the pulmonary walls [1,2]. The mortality rate of the patients with acute lung injury is estimated to be 50% [3]. The acute lung injury animal model for various studies is prepared by the administration of lipopolysaccharide (LPS) to the animals [4]. Acute lung injury characterized by inflammation of alveoli because of enhanced expression of tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1) and transforming growth factor-β (TGF-β) in animals is caused by various cytokines [5]. It is also reported that TNF-α which is a signaling protein is associated with the initiation, amplification and regulation of inflammatory processes [6].
Studies have shown that Sirtuin 1 (Sirt1), a histone deacetylase plays a vital role in the inhibition of inflammatory processes through regulation of various mediators involved in inflammation [7]. Increased expression of Sirt1 inhibits tumor necrosis factor-α and interleukin (IL)-8 release [8]. Thus for the inhibition of inflammatory processes Sirt1 is considered to be the potential target [9].
Rubriflordilactones A was isolated in the year 2006 by Sun et al. from the plant Schisandrarubriflora. The plant has a long traditional Chinese Medicinal importance particularly as antitumor and anti-HIV agent [10]. The present study was designed and performed to investigate the effect of rubriflordilactone on the acute lung injury in rats induced by administration of LPS. The results revealed that rubriflordilactone inhibited the formation of lung edema, reduced neutrophil count and inhibited the expression of inflammatory factors.
Materials and methods
Cell culture and treatment strategy
The Mice Lung Epithelial cell line, MLE-15 was purchased from Shanghai Institute of Biochemistry and Cellular Biology Chinese Academy of Sciences (Shanghai, China). The cells were cultured in Dulbecco’s Modified Eagle’s medium containing penicillin (100 U/ml) and streptomycin (100 mg/ml) along with 10% fetal bovine serum in an incubator with humidified atmosphere of 5% CO2. The cells were treated with 10 nm/ml concentration of rubriflordilactone 2 h before exposure to LPS. Rubriflordilactone at a concentration of 10 nm/kg was injected to the rats 2 h prior to the LPS administration.
RNA interference
Silencer RNA (siRNA) for Sirt1 was prepared and transfected into MLE-15 cells according to the reported protocol [11].
Preparation of animal model treatment protocol
The present study was approved by the Ethics Committee of the Shanghai Institute of Biochemistry and Cellular Biology Chinese Academy of Sciences. Male Wistar rats 10-12 week old were obtained from Laboratory Animal Center, Academy of Military Medical Sciences, Beijing, China. The animals were housed under specific pathogen-free conditions having free access to food and water. LPS was administered to the mice intra-peritoneally at a concentration of 10 mg/kg body weight whereas the rubriflordilactone was injected at the same concentration prior to 2 h of LPS administration. The animals were sacrificed following 24 of LPS administration to extract the lungs from each animal.
Ratio of wet and dry lung weight
For each of the animal ratio of wet to dry lung weight was calculated using the reported protocol. Immediately after extraction of lung, it was weighed followed by drying in microwave oven to get wet weight.
Immunohistochemistry for Sirt1
The lungs from each of the animal were extracted under liquid nitrogen atmosphere and subjected to formalin fixing in 3% buffered formaldehyde. The lungs were then paraffin embedded and cut into thin 2 µm sections followed by hematoxylin and eosin (H&E) staining. The sections were de-paraffined by heating in xylene, rehydrated in gradient ethyl alcohol and treated with hydrogen peroxide for quenching the activity of peroxidase activity. Treatment of the sections with BSA for 45 min was followed by incubation for overnight withanti-Sirt1 monoclonal antibody. The sections were then washed again with PBS before treatment with a secondary antibody for 40 min.
Analysis of the matrix metalloproteinase-9 (MMP-9), iNOS, IL-6 and Sirt1 mRNA expression
For the analysis of MMP-9, iNOS, IL-6 and Sirt1 gene expression quantitative real-time RT-PCR was used. The PCR primers needed to target genes were obtained from Invitrogen Life Technologies (Carlsbad, CA, USA).
Western blot analysis
MLE-15 cells were incubated with 10 nm/ml concentration of rubriflordilactone and then harvested. Electrophoresis on 10% sodium dodecyl sulfate polyacrylamide gel was used for the isolation of proteins which were transferred onto a nitrocellulose membrane. The membranes were incubated with primary antibodies in blocking buffer, washed with PBS and incubated for 1 h with secondary antibody. Following primary antibodies and dilutions were used: MMP-9 (Wuhan Boster Biological Technology, Ltd., Wuhan, China; 1:1,000) and iNOS, IL-6 and Sirt1 (Wuhan Boster Biological Technology, Ltd., Wuhan, China; 1:1,000). The horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody was obtained from Wuhan (Boster Biological Technology, Ltd.; 1:10,000). β-actin was used as the loading control and relative levels of the target protein were represented as the optical density (OD).
Statistical analysis
Analysis of the data obtained was performed using unpaired Student’s t-test (Graph Pad Prism V.4). The differences were considered significant statistically at P-values of <0.05.
Results
Rubriflordilactone inhibits lung edema formation by LPS in rats
Administration of LPS to rats caused increase in the lung weight and volume because of the edema formation. However, rubriflordilactone injection at 10 nm/kg concentration prior to the LPS administration inhibited the formation of lung edema significantly (Figure 1, P<0.01).
Figure 1.
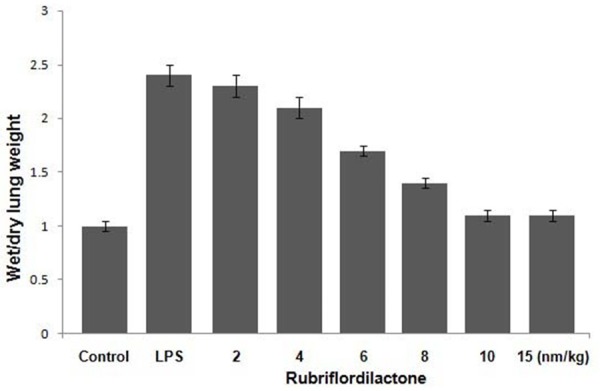
Inhibition of edema formation by rubriflordilactone treatment in LPS administered rats. The effect of various doses of rubriflordilactone on ratio of wet to dry weight of lungs in rats in the LPS+rub, LPS and untreated rats was determined. LPS and Rub stand for lipopolysaccharide and rubriflordilactone, respectively.
Rubriflordilactone suppresses LPS induced alterations in the lung morphology of rats
LPS administration led to a significant increase in the accumulation of neutrophils in the lung tissues compared to control group of rats (Figure 2). When the rats were treated with rubriflordilactone before the administration of LPS, the LPS induced increase in the level of neutrophils was suppressed. LPS treated rats showed significantly higher level of neutrophils in the pulmonary tissues compared to the control and LPS plus rubriflordilactone treated rats. The rats in the LPS administered group showed inflammatory vesicles which were not observed in the rubriflordilactone treated rats.
Figure 2.
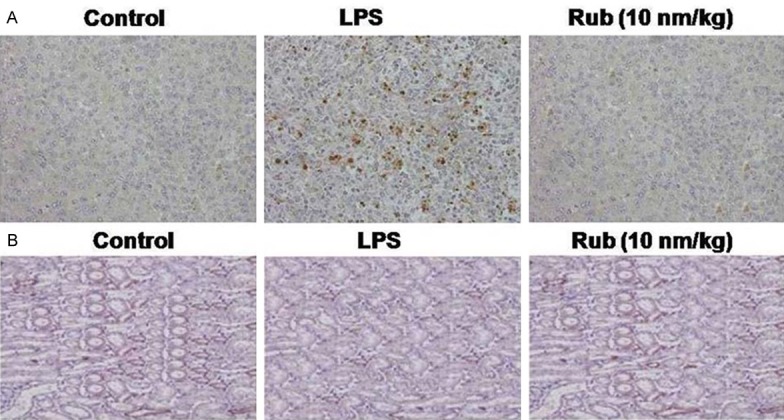
Effect of rubriflordilactone on the acute lung injury induced in rats by LPS administration. A. The pulmonary tissues of the rats in LPS, LPS+Rub and control groups were stained with HE-stain (magnification, ×200). B. Rubriflordilactone treatment increased expression of Sirt1 significantly compared to LPS group was analyzed by immunohistochemistry. Rub, LPS and Sirt1 stand for rubriflordilactone, lipopolysaccharide and sirtuin 1, respectively.
Administration of LPS to the rats resulted in a marked reduction in the level of Sirt1 expression than the animals in untreated group (Figure 2). However, when the animals were injected with 10 nm/kg concentration of rubriflordilactone prior to the administration of LPS, the expression of Sirt1 was increased significantly (P=0.005). The level of Sirt1 in the rubriflordilactone treated and control animals were found to be similar.
Inhibition of MMP-9 and inflammatory factors by rubriflordilactone in LPS administered rats
Administration of LPS markedly increased the expression level of MMP-9 in the pulmonary tissues of rats (Figure 3). In addition, the level of mRNA corresponding to iNOS, MMP-9 and IL-6 was enhanced whereas the level of Sirt1 was decreased in MLE-15 cells administered with LPS (Figure 3).
Figure 3.
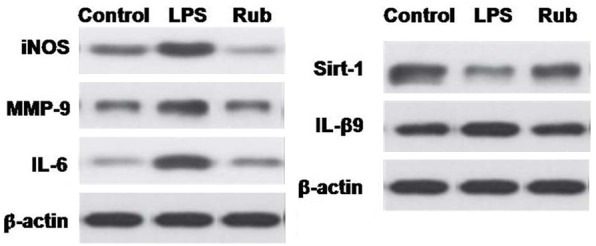
Inhibition of factors involved in inflammatory processes by rubriflordilactone treatment in LPS administered MLE-15 cells. In MLE-15 cells LPS exposure increased expression of MMP-9, however, rubriflordilactone treated suppressed the effect of LPS onMMP-9 expression. Rubriflordilactone inhibited the LPS induced increase in the expression of mRNA corresponding to iNOS, MMP-9 and IL-6. Rubriflordilactone inhibited the LPS induced reduction in the expression of Sirt1 in MLE-15 cells. Rub, LPS, Sirt1, MMP-9, IL and iNOS stands for rubriflordilactone, lipopolysaccharide, sirtuin 1, matrix metalloproteinase-9, interleukin and inducible nitric oxide synthase.
However, rubriflordilactone treatment before the administration of LPS in rats inhibited the LPS induced increase in the level of iNOS, MMP-9 and IL-6 (Figure 3). The level of Sirt1 was increased by rubriflordilactone treatment.
Effect of Sirt1 siRNA on rubriflordilactone treated MLE-15 cells
In order to confirm whether rubriflordilactone exhibits its effect through Sirt1, MLE-15 cells were transfected with silencer RNA for Sirt1. It was observed that transfection of siRNA for Sirt1 inhibited the rubriflor-dilactone induced reduction in MMP-9, iNOS and IL-6 expression (Figure 4).
Figure 4.
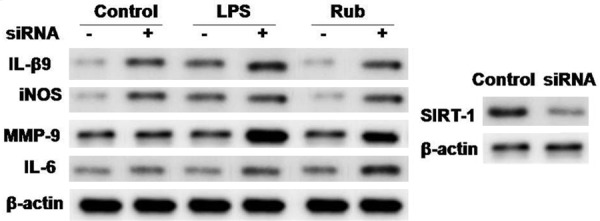
Effect of silencer RNA for Sirt1 on inhibition of acute lung injury by rubriflordilactone inMLE-15 cells. The cells transfected with siRNA for Sirt1 were exposed to LPS after rubriflordilactone treatment and then analyzed by western blot analysis. Rub, LPS, Sirt1, MMP-9, IL and iNOS stand for rubriflordilactone, lipopolysaccharide, sirtuin 1, matrix metalloproteinase-9, interleukin and inducible nitric oxide synthase.
Discussion
Administration of LPS to the animals in order to induce pulmonary injury and prepare sepsis model has been used in several studies [12,13]. Acute pulmonary injury is a serious concern at present for the clinicians throughout the globe. Studies have revealed that Sirt1 activation by chemotherapeutic agents can be a promising treatment strategy for inhibition of acute lung injury [14].
It is reported that LPS administration causes formation of edema leading to the increase of both lung weight and volume of the rats [15]. Results from the present study revealed that rubriflordilactone exhibited inhibitory effect on the formation of edema in rats which was consistent with the earlier reports. However, pretreatment of the rats with rubriflordilactone before LPS administration prevented the formation of lung edema. Rubriflordilactone treatment also resulted reduction in the accumulation of neutrophil concentration in the pulmonary tissues of the rats which was markedly enhanced by LPS administration. It is well established that administration of LPS to rats causes significant increase in the expression of factors involved in the inflammatory processes [15]. In consistence with these reports, our results showed a marked increase in the expression level of MMP-9 in LPS administered rats. However, rubriflordilactone treatment prior to LPS administration led to anincrease in the expression level of MMP-9. The results from the present study showed that LPS administration in the rats enhanced the expression levels of Inos and IL-6 and reduced the level of Sirt1. On the other hand, when rats were treated with rubriflordilactone prior to LPS the expression of iNOS and IL-6 was reduced whereas the expression of Sirt1 was increased. Therefore, it appeared that rubriflordilactone exhibited its effect through increase in the expression of Sirt1. The confirmation of the involvement of Sirt1 was performed by inhibiting its expression through silencer RNA transfection. In the cells transfected with siRNA, the effect of rubriflordilactone on inflammatory factors was inhibited. Therefore, it was evident that rubriflordilactone exhibited its effect through increase in the expression of Sirt1.
Thus, rubriflordilactone treatment led to reduction in pulmonary edema formation, inhibited the expression of inflammatory factors in LPS administered rats and MLE-15 cells through increase in Sirt1 expression.
Disclosure of conflict of interest
None.
References
- 1.Limper AH. Chemotherapy-induced lung disease. Clin Chest Med. 2004;25:53–64. doi: 10.1016/S0272-5231(03)00123-0. [DOI] [PubMed] [Google Scholar]
- 2.Graves PR, Siddiqui F, Anscher MS, Movsas B. Radiation pulmonary toxicity: from mechanisms to management. Semin Radiat Oncol. 2010;20:201–207. doi: 10.1016/j.semradonc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS Canadian Critical Care Trials Group. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 4.Chung YJ, Jarvis B, Pestka J. Modulation of lipopolysaccharide-induced proinflammatory cytokine production by satratoxins and other macrocyclictrichothecenes in the murine macrophage. J Toxicol Environ Health A. 2003;66:379–391. doi: 10.1080/15287390306363. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia M, Zemans RL, Jeyaseelan S. Role of chemokines in the pathogenesis of acute lung injury. Am J Respir Cell Mol Biol. 2012;46:566–572. doi: 10.1165/rcmb.2011-0392TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukhopadhyay S, Hoidal JR, Mukherjee TK. Role of TNF alpha in pulmonary pathophysiology. Respir Res. 2006;7:125. doi: 10.1186/1465-9921-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 8.Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke‑induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol. 2007;292:L567–L576. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- 9.Olholm J, Paulsen SK, Cullberg KB, Richelsen B, Pedersen SB. Antiinflammatory effect of resveratrol on adipokine expression and secretion in human adipose tissue explants. Int J Obes (Lond) 2010;34:1546–1553. doi: 10.1038/ijo.2010.98. [DOI] [PubMed] [Google Scholar]
- 10.Xiao WL, Yang LM, Gong NB, Wu L, Wang RR, Pu JX, Li XL, Huang SX, Zheng YT, Li RT, Lu Y, Zheng QT, Sun HD. Rubriflordilactones A and B, two novel bisnortriterpenoids from Schisandra rubriflora and their biological activities. Org Lett. 2006;8:991. doi: 10.1021/ol060062f. [DOI] [PubMed] [Google Scholar]
- 11.Marshall JC, Vincent JL, Guyatt G, Angus DC, Abraham E, Bernard G, Bombardier C, Calandra T, Jørgensen HS, Sylvester R, Boers M. Outcome measures for clinical research in sepsis: a report of the 2nd Cambridge Colloquium of the International Sepsis Forum. Crit Care Med. 2005;33:1708–1716. doi: 10.1097/01.ccm.0000174478.70338.03. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda N, Hattori Y, Takahashi Y, Nishihira J, Jesmin S, Kobayashi M, Gando S. Therapeutic effect of in vivo transfection of transcription factor decoy to NF-kappaB on septic lung in mice. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1248–L1255. doi: 10.1152/ajplung.00164.2004. [DOI] [PubMed] [Google Scholar]
- 13.Oshikawa K, Sugiyama Y. Gene expression of Toll-like receptors and associated molecules induced by inflammatory stimuli in the primary alveolar macrophage. Biochem Biophys Res Commun. 2003;305:649–655. doi: 10.1016/s0006-291x(03)00837-4. [DOI] [PubMed] [Google Scholar]
- 14.Bishayee A, Waghray A, Barnes KF, Mbimba T, Bhatia D, Chatterjee M, Darvesh AS. Suppression of the inflammatory cascade is implicated in resveratrol chemoprevention of experimental hepatocarcinogenesis. Pharm Res. 2010;27:1080–1091. doi: 10.1007/s11095-010-0144-4. [DOI] [PubMed] [Google Scholar]
- 15.Rojas M, Woods CR, Mora AL, Xu J, Brigham KL. Endotoxin-induced lung injury in mice: structural, functional and biochemical responses. Am J Physiol Lung Cell Mol Physiol. 2005;288:L333–L341. doi: 10.1152/ajplung.00334.2004. [DOI] [PubMed] [Google Scholar]


