Abstract
Objective: This study aimed to investigate the effect of bone marrow mesenchymal stem cells (BMSCs) transplantation on the expression of high mobility group box 1 protein (HMGB1) in the serum and liver of rats with acute liver failure (ALF). Methods: Healthy male SD rats were randomly divided into control group, ALF group and BMSCs group. ALF was induced by intraperitoneal injection of 900 mg/kg D-GalN and 10 μg/kg LPS. In BMSCs group, rats received BMSCs (1.0×107) transplantation via the tail vein at 2 h after ALF induction. Results: Intraperitoneal injection of 900 mg/kg D-GalN and 10 μg/kg LPS was able to induce ALF in rats. In ALF group, serum ALT and AST increased gradually over time. At 72 h, the serum ALT and AST in BMSCs group were significantly different from those in ALF group. HMGB1 expression in the serum and liver remained at a low level at any time point in control group, but increased significantly in ALF group and BMSCs group. The serum and liver HMGB1 expression increased progressively in ALF group, but reduced gradually in BMSCs group. Significant difference in serum and liver HMGB1 expression was observed between ALF group and BMSCs group at 24 h and 72 h. In addition, there was marked difference in the survival rate among three groups at 24 h (χ2=21.098, P<0.01). Conclusion: BMSCs transplantation is able to improve the liver function and liver pathology in ALF rats and decrease the serum and liver HMGB1.
Keywords: Bone marrow mesenchymal stem cells, acute liver failure, high mobility group box 1 protein, rat, transplantation
Introduction
Acute liver failure (ALF) is a syndrome caused by multiple factors and characterized by massive hepatocyte degeneration and necrosis as well as infiltration of inflammatory cells shortly after injury. ALF has a rapid progression and numerous complications; its treatment is very difficult, which renders it a disease with high mortality and poor prognosis. To date, ALF has been a refractory disease in clinical practice [1]. In recent years, stem cells bring a promise to the therapy of liver failure due to their transdifferentiation. Increasing studies confirm that bone marrow mesenchymal stem cells (BMSCs) may promote the reconstruction of endogenous hepatocytes via cell replacement and paracrine and attenuate the oxidative stress after liver injury [2]. Thus, stem cells transplantation becomes another promising strategy for the therapy of liver failure following orthotopic liver transplantation. The pathogenesis of ALF has involvement of a lot of inflammatory cytokines. After infection, hepatocyte necrosis and apoptosis mediated by inflammatory cytokines are important factors causing the progression of ALF [3]. High mobility group box 1 protein (HMGB1) is a group of non-histone nucleoproteins. In the presence of exogenous microorganisms or the endogenous injury, HMGB1 may be released out of the cells and act as a signal of stress and an inflammatory mediator. There is evidence showing that excellular HMGB1 may induce the production of several cytokines in monocytes to participate in innate immunity. In addition, it may also induce the activation and proliferation of naïve T cells and promote their differentiation into T help (Th1) cells to induce the adaptive immunity [4]. Thus, we speculate that HMGB-1 plays an important role in the pathophysiology of liver failure. In the present study, ALF was induced in rats and the therapeutic effect of BMSCs transplantation on ALF was investigated. At the same time, we explored the influence of BMSCs transplantation on the serum and liver HMGB-1 in ALF rats, which may provide experimental evidence for the clinical BMSCs transplantation in the therapy of ALF.
Materials and methods
Materials
Healthy male Sprague-Dawley rats weighing 200-250 g were purchased from Kunming Experimental Animal Institute of Chinese Academy of Sciences. D-galactosamine and lipopolysaccharide (Sigma, USA), TRIzol (Beijing TransGen Biotech, Co., Ltd), kits for reverse transcription and polymerase chain reaction (PCR) (Beijing Tiangen Biotech, Co., Ltd), rabbit anti-rat HMGB1 polyclonal antibody (Anbo Biotech, Co., Ltd), horse anti-rabbit HRP conjugated secondary antibody (Wuhan Boster Biotech, Co., Ltd), HMGB1 ELISA kit (Shanghai Xitang Biotech, Co., Ltd) and two-step immunohistochemistry kit (Wuhan Boster Biotech, Co., Ltd) were used in the present study.
BMSCs culture and establishment of ALF model
Separation, culture and identification of BMSCs: BMSCs were separated and cultured according to previously reported [5]. BMSCs of the third generation were subjected to identification by flow cytometry. BMSCs of the forth generation were digested with trypsin, washed with PBS and re-suspended in normal saline at a density of 1.0×107/ml for further use.
Establishment of ALF model: A total of 90 SD rats (specific pathogen free; SPF) were randomly assigned into 3 groups according to random number (n=30 per group): blank control group, ALF group and BMSCs group. The diagnostic criteria for ALF were previously reported [6]. According to the method described in previous study [7], 900 mg/kg D-GalN and 10 μg/kg LPS were intraperitoneally injected to induce ALF. Two hours later, rats in BMSCs group received BMSCs (1.0×107) transplantation via the tail vein. In blank control group, 1 ml of normal saline was intraperitoneally injected. At 12, 24 and 72 h after transplantation, the serum and liver were collected from each rat (n=6 at each time point). The serum was stored at -20°C. The liver was immediately frozen in liquid nitrogen and then stored at -70°C. The survival rate was calculated in each group within 24 h.
Detections
Setection of serum ALT and AST: Serum ALT and AST were measured by using the Hitachi 7020 Biochemical analyzer.
Detection of serum HMGB1 by ELISA: Standards and samples were independently added to plates (100 μl/well), followed by incubation at 37°C for 120 min. After washing, the primary antibody solution was added, followed by incubation at 37°C for 60 min. Following addition of secondary antibody, the plates were incubated at 37°C for 120 min. After addition of subtrate, the plates were incubated at 37°C in dark for 5-10 min. After addition of stop solution, absorbance (A) was measured at 492 nm and standard curve was delineated. The serum content of HMGB1 was calculated according to the standard curve.
Detection of HMGB1 mRNA expression by RT-PCR: In brief, total RNA was extracted from 250 mg of liver tissues with Trizol reagent, and reverse transcription and PCR were conducted according to manufacturer’s instructions [8]. β-actin served as an internal control. Primers used in this study were as follows: HMGB1: 5’-GATGACAAGCAGCCCTAT-3’ (forward) and 5’-TCCATGCCAATTTACAAC-3’ (reverse), and anticipated length was 481 bp; β-actin: 5’-CCAACCGTGAAAAGATGACC-3’ (forward) and 5’-CAGGAGGAGCAATGATCTTG-3’ (reverse), and anticipated length was 660 bp. HMGB1 mRNA expression was determined with 2-ΔΔCt method.
HE staining of liver tissues: Liver tissues were fixed in 10% neutral formalin, embedded in paraffin and cut into 4-μm sections. After deparaffinization, staining, dehydration, and transparentization, sections were mounted, and pathological examination was performed under a light microscope.
Detection of HMGB1 protein expression by immunohistochemistry: Paraffin-embedded sections were subjected to deparaffinization and hydration. After incubation at room temperature and antigen retrieval, sections were subjected to blocking of non-specific binding. The sections were treated with HMGB1 primary antibody (1:100) at 37°C for 1 h and then at 4°C over night. After incubation with HRP conjugated horse anti-rabbit secondary antibody at 37°C for 1 h, visualization, counterstaining with hematoxylin and mounting were conducted. In negative control group, the primary antibody was replaced with PBS. Evaluation of HMGB1 expression was performed according to previously reported [9].
Detection of HMGB1 protein expression by Western blot assay: Total protein was extracted from about 100 mg of liver tissues, and ground and lysed in lysis buffer for 3-5 min. After addition of loading buffer (1:3), the samples were heated at 96°C for 5 min for denaturation. After separation of proteins by 10% SDS-PAGE, proteins were transferred onto nitrocellulose membrane which was then blocked for 1 h at room temperature. The membrane was then incubated with primary antibody (1:200 for HMGB1; 1:1000 for GAPDH) at 4°C over night. After washing in TBST, the membrane was incubated in alkaline phosphatase conjugated secondary antibody. Following washing, the membrane was visualized in the presence of subtrate. Protein bands were analyzed with Quantity One image analysis system. Protein expression of HMGB1 was normalized to that of GAPDH.
Statistical analysis
Statistical analysis was conducted with SPSS version 18.0. Quantitative data are expressed as mean ± standard deviation and comparisons were performed with analysis of variance. Qualitative data were compared with analysis of variance after rank conversion. A value of P<0.05 was considered statistically significant.
Results
Observation and identification of BMSCs
BMSCs of the third generation showed single cell growth, several cells were adherent to the wall, and most cells were short spindle-shaped. After culture for 7-10 days, cell monolayer formed, and cells were largely long spindle-shaped or polygonal. BMSCs of the third and forth generations had a good morphology and a favorable transmittance, became fibroblast-like and showed whirlpool growth. Flow cytometry showed more than 95% of cells of the third generation were negative for CD34 and CD45 but positive for CD29, CD44 and CD90. This suggests that these cells were BMSCs.
Survival rate at 24 h
At 24 h, 8 and 4 rats died in ALF group and BMSCs group, respectively, but none died in blank control group. Significant difference was observed in the survival rate among three groups (χ2=21.098, P<0.01).
Serum ALT and AST
After D-GalN/LPS treatment, the serum ALT and AST increased over time in ALF group. In BMSCs group, the serum ALT and AST reduced gradually over time. At 12 h, the serum ALT and AST were comparable between ALF group and BMSCs group (P>0.05). At 24 h and 72 h, significant differences were observed in the serum ALT and AST between ALF group and BMSCs group (P<0.01) (Table 1).
Table 1.
Serum ALT and AST in different groups
| Group | 12 h | 24 h | 72 h | |||
|---|---|---|---|---|---|---|
|
| ||||||
| ALT (U/L) | AST (U/L) | ALT (U/L) | AST (U/L) | ALT (U/L) | AST (U/L) | |
| Blank control | 36.0±3.2 | 38.6±3.5 | 35.8±4.6 | 39.2±6.3 | 38.8±3.7 | 37.7±5.1 |
| ALF | 423.6±32.7 | 482.3±61.5 | 533.4±62.0 | 592.5±64.1 | 639.0±69.8 | 724.3±48.8 |
| BMSCs | 390.0±54.8 | 444.8±47.6 | 312.6±28.7a | 381.5±48.6a | 163.5±31.6a | 172.8±55.7a |
| F | 16.172 | 19.795 | 10.961 | 11.198 | 19.652 | 17.767 |
| P | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
P<0.01 vs. ALF.
Liver pathology
In blank control group, HE staining of the liver showed the hepatic lobules had normal and complete structure. In ALF group at 12 h, the hepatic lobules showed disarranged structure and hepatic cord dissociation, hepatocytes displayed patchy necrosis, cells with eosinophilic change increased, karyolysis and nuclear fragmentation were observed, cell debris increased, a variety of inflammatory cells infiltrated, and congestion and hemorrhage were also found in the hepatic sinus. At 72 h, normal hepatic lobules were almost not observed and massive patchy necrosis was also noted. At 12 h after BMSCs transplantation, the pathological findings were similar to those in ALF group, but the liver pathology improved gradually over time. At 72 h, the hepatic lobules showed nearly normal structure and only focal infiltration of inflammatory cells was noted in BMSCs group (Figure 1).
Figure 1.
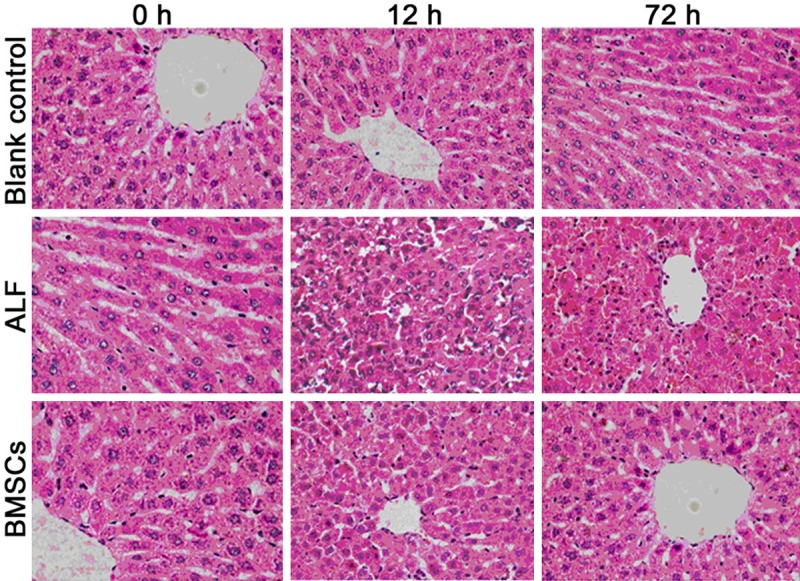
Liver pathology of rats in different groups (HE staining; ×400).
Serum HMGB1 in different groups
In blank control group, the serum HMGB1 remained unchanged at different time points. At different time points, significant difference was found in the serum HMGB1 among blank control group, ALF group and BMSCs group (P<0.01). In ALF group, serum HMGB increased gradually over time, but it reduced progressively after BMSCs transplantation. At 24 and 72 h after BMSCs transplantation, the serum HMGB1 was significantly different from that in ALF group (P<0.01) (Table 2).
Table 2.
Serum HMGB1 content (ng/ml) in different groups (x ± s)
| Group | 12 h | 24 h | 72 h |
|---|---|---|---|
| Blank control | 40.2±5.8 | 41.5±6.7 | 40.5±6.5 |
| ALF | 180.9±21.6 | 290.4±30.9 | 486.5±57.2 |
| BMSCs | 188.7±18.1 | 146.8±35.2a | 90.6±17.4a |
| F | 4.637 | 15.347 | 11.824 |
| P | 0.006 | 0.000 | 0.000 |
P<0.01 vs. ALF.
HMGB1 mRNA expression in the liver
At different time points, there was marked different in the HMGB1 mRNA expression in the liver among three groups (P<0.01). When compared with ALF group, the HMGB1 mRNA expression reduced gradually over time and significant difference was observed at 24 h and 72 h after BMSCs transplantation (P<0.01) (Table 3).
Table 3.
HMGB1 mRNA expression in the liver of different groups (x ± s)
| Group | 12 h | 24 h | 72 h |
|---|---|---|---|
| Blank control | 0.5±0.1 | 0.5±0.2 | 0.5±0.1 |
| ALF | 0.9±0.2 | 1.2±0.3 | 1.4±0.1 |
| BMSCs | 0.7±0.1 | 0.8±0.2a | 0.6±0.2a |
| F | 6.372 | 13.467 | 7.167 |
| P | 0.004 | 0.000 | 0.003 |
P<0.01 vs. ALF group.
Immunohistochemistry for HMGB1 in the liver
At different time points, a small amount of light brown granules were found in the nucleus and cytoplasm of cells in blank control group, and HMGB1 expression remained unchanged. In ALF group, the brown granules in the cytoplasm increased over time and finally merged to form patchy brown staining regions. In BMSCs group, the HMGB1 expression at 12 h was comparable to that in ALF group, but it reduced gradually over time. At 24 h and 72 h, significant difference was noted in the HMGB1 expression between ALF group and BMSCs group (P<0.01) (Table 4 and Figure 2).
Table 4.
HMGB1 protein expression in the liver of different groups (x ± s)
| Group | 12 h | 24 h | 72 h |
|---|---|---|---|
| Blank control | 1.5±0.3 | 1.5±0.2 | 1.6±0.3 |
| ALF | 4.4±1.2 | 6.2±1.6 | 8.5±3.0 |
| BMSCs | 4.2±1.1 | 3.8±2.2a | 2.6±0.8a |
| F | 3.552 | 12.195 | 18.423 |
| P | 0.008 | 0.000 | 0.000 |
P<0.01 vs. ALF group.
Figure 2.
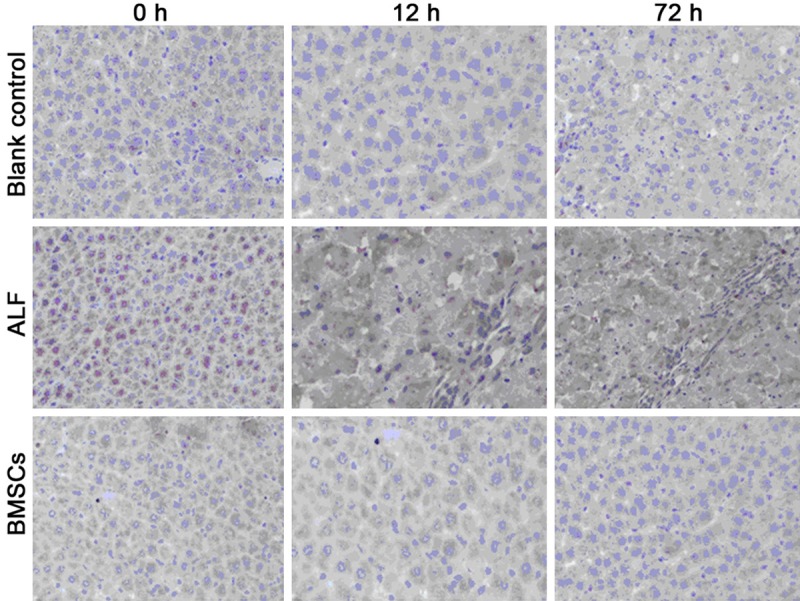
HMGB1 protein expression in the liver of different groups (super vision two step immunohistochemistry; ×400).
Detection of HMGB1 expression in the liver by Western blot assay
In BMSCs group, the HMGB1 expression reduced gradually with the improvement of liver function. At 12 h, 24 h and 72 h, the HMGB1 protein expression was 0.46±0.24, 0.38±0.25 and 0.20±0.11, respectively, in BMSCs group, which were significantly different from that in blank control group and ALF group at corresponding time point (P<0.05) (Figure 3).
Figure 3.
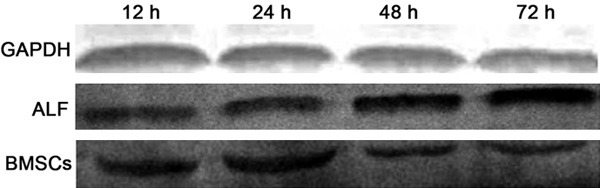
HMGB1 protein expression in the liver (Western blot assay).
Discussion
Liver failure is a severe liver diease and may be caused by different factors. Ultimately, liver failure is manifestated as excess necrosis of hepatocytes and other cells in the liver. Currently, the masive hepatocyte necrosis is found to be related to the apoptosis of heptocytes and microcirculation disorder, and hepatocyte apoptosis is an important mechanism underlying the second attack to the liver [10]. In recent years, great progress has been achieved in the intracellular signal transduction related to cell death. In ALF, numerous necrotic hepatocytes and activated monocyte-macrophages may release a large amount of HMGB1 out of cells, which is found to be ascribed to NF-κB activation. The binding of NF-κB to TLR2 or TLR4 may stimulate the release of inflammatory cytokines such as IL-18 and IL-1 which form a positive feedback in the immune response and further amplify the inflammation [11]. In the liver, focal inflammation may cause microcirculation disorder and ischemia/hypoxia injury. In the presence of liver ischemia, HMGB1 translocates from the nucleus to the cytoplasm and then interact with pro-inflammatory reaction induced by ischemia; in the presence of ischemia/reperfusion, HMGB1 may interact with TLR4 dependent, free radicals/calcium mediated signaling pathway [12]. Ischemia/hypoxia may not only cause hepatocyte death, but induce the endotoxemia. LPS is a major endotoxin in the intestine and may bind to TLR to induce NF-κB activation and stimulate the release of a large amount of pro-inflammatory cytokines, resulting in the progression of secondary inflammation. Taken together, HMGB1 may promote the release of pro-inflammatory cytokines and their cascade activation to amplify the cascade of inflammation and deteriorate the pre-existing disease. In 2013, Majumdar et al [13] found high circulating HMGB1 level was a biomarker of ALF secondary to E hepatitis and played an important role of fulminant hepatitis. In the present study, intraperitoneal injection of D-GalN+LPS was employed to induce ALF in rats. HE staining and detection of serum ALT/AST showed characteristics of ALF. In ALF rats, not only serum HMGB1 increased significantly, the HMGB1 mRNA and protein expressions in the liver also elevated markedly. In addition, ELISA was conducted to detect serum HMGB1. Results showed serum HMGB1 remained at a low level in blank control group, but gradually increased over time in ALF group. However, BMSCs transplantation progressively increased the serum HMGB1. RT-PCR indicated that the HMGB1 mRNA expression in the liver of ALF group was significantly higher than in blank control group at different time points. In addition, HMGB1 mRNA expression in ALF group increased gradually over time. After BMSCs transplantation, the HMGB1 mRNA expression reduced progressively over time (P<0.01). Immunohistochemistry showed the change in HMGB1 protein expression in the liver was similar to the HMGB1 mRNA expression. On the basis of above findings, the serum HMGB1 and liver HMGB1 (mRNA and protein) increased gradually over time in ALF, which was consistent with the deterioration of liver injury over time. This also indicates that the serum and liver HMGB1 is positively related to the serverity of liver injury and may become an important predictor of liver injury in ALF.
Piscaglia et al [14] found cytokines secreted by BMSCs were able to inhibit the necrosis of hepatocytes and increased the survival rate after fulminant hepatic failure. In addition, studies also reveal that BMSCs may inhibit hepatocyte apoptosis and promote their proliferation. BMSCs may secret a lot of cytokines and growth factors in a paracrine dependent manner, to induce the hepatocyte regeneration and inhibit the inflammation and hepatocyte apoptosis [15]. To elucidate the pathogenesis of ALF and to inhibit the hepatocyte apoptosis and promote hepatocyte regeneration are crucial for the elevation of survival rate following ALF. In the presence of severe liver injury and inhibition of hepatocyte proliferation, BMSCs have the capability to differentiate into hepatocytes and cholangiocytes which may participate in the liver repair after liver injury [16]. A variety of studies have confirmed that there is implantation of BMSCs in the liver after BMSCs transplantation, and the number of BMSCs implanted in the liver after liver injury is significantly higher than in normal liver [17]. Takami et al [18] used specific cytokines to induce the differentiation of BMSCs into hepatocytes, and they found BMSCs showed characteristics (morphology and functions) of hepatocytes. These BMSCs were then transplanted into the liver of immunodeficient mice, and results showed BMSCs implantation in the liver, and they could store glycogen, secret the albumin and express hepatocyte specific antigens. These suggest that the transplanted cells have functions of hepatocytes. Thus, BMSCs may become promising cells used for the therapy of liver disease.
In the present study, intraperitoneal injection of D-GalN/LPS was employed to induce ALF. In ALF rats, serum ALT and AST increased markedly, and liver pathology deteriorated significantly. At 24 h and 72 h after BMSCs transplantation, the liver function improved and hepatocyte necrosis and apoptosis attenuated gradually, which were consistent with previously reported [19-23]. This suggests that BMSCs transplantation is able to inhibit the inflammation and programmed cell death to repair the injured liver. In addition, after BMSCs transplantation, the HMGB1 mRNA and protein expressions in the liver showed a decreased tendency, indicating that BMSCs transplantation improves the immunity of ALF rats and exerts protective effect on ALF. To reduce serum HMGB1 and regulate the production of pro-inflammatory cytokines may be one of mechanism underlying the therapeutic effect of BMSCs transplantation on ALF.
In rats with ALF caused by D-GalN and LPS, serum ALT, AST and HMGB1 increased significantly, and mRNA and protein expressions of HMGB1 in the liver elevated dramatically, and liver pathology showed deterioration, suggesting that HMGB1 plays a crucial role in the pathophysiology of ALF and may become an important predictor of severity of liver injury in ALF. BMSCs may significantly attenuate ALF, delay its progression and may become an alternative strategy for the therapy of ALF after pharmacotherapy, orthotopic liver transplantation and bioartificial liver.
Acknowledgements
This study was supported by Natural Science Foundation of China (81360072); Yunnan Natural Science Fund (2012FD095); Key Program of Yunnan Provincial Research Fund of Department of Education (2014Z125, 2015Z146); Yunnan Key Clinical Specialist Construction Project (YWYF[2015]18#).
Disclosure of conflict of interest
None.
References
- 1.Kim TY, Kim DJ. Acute-on-chronic liver failure. Clin Mol Hepatol. 2013;19:349–359. doi: 10.3350/cmh.2013.19.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Zhang L, Xin J, Jiang L, Li J, Zhang T, Jin L, Li J, Zhou P, Hao S, Cao H, Li L. Immediate intraportal transplantation of human bone marrow mesenchymal stem cells prevents death from fulminant hepatic failure in pigs. Hepatology. 2012;56:1044–1052. doi: 10.1002/hep.25722. [DOI] [PubMed] [Google Scholar]
- 3.Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet. 2010;376:190–201. doi: 10.1016/S0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]
- 4.Li XP, Niu ZG, Han JX, Gao C, Liu W, Song XF, Gao ZT, Wang H. Influences of recombinant human HMGB1 on the expression of HMGB1-related receptors in HTLV-1 infected MT2 cells. Chin J Microbiol Immunol. 2014;34:47–50. [Google Scholar]
- 5.Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terblanche J, Hickman R. Animal models of fulminant hepatic failure. Dig Dis Sci. 1991;36:770–774. doi: 10.1007/BF01311235. [DOI] [PubMed] [Google Scholar]
- 7.Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, Hood LE, Galas DJ. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci U S A. 2009;106:4402–4407. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beggs KM, Fullerton AM, Miyakawa K, Ganey PE, Roth RA. Molecular mechanisms of hepatocellular apoptosis induced by trovafloxacin-tumor necrosis factor-alpha interaction. Toxicol Sci. 2014;137:91–101. doi: 10.1093/toxsci/kft226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng S, Xiao QY, Yin F, Guo ZP, Liu HQ, Wang JG, Zhu WM, Wang YB. Effects of bone marrow mesechymal stem cells on microRNA-155 and tumor necrosis factor alpha expression in liver tissue of rats with acute liver failure. Chin J Cell Stem Cell. 2014:79–83. [Google Scholar]
- 11.Zhu YC, Ling B, Sun J, Xiu GH, Zhu GX, Pan XH. Role of HMGB1 in inflammation regulation of multiple organ dysfunction syndrome. Chin J Crit Care Med. 2015:465–468. [Google Scholar]
- 12.Zheng S, Yin F, Xiao QY, Guo ZP. Therapeutic effects and approach optimizing of human umbilical cord mesenchymal stem cells transplantation on rat acute liver failure model. Chin Hepatol. 2014:918–923. [Google Scholar]
- 13.Majumdar M, Ratho R, Chawla Y, Singh MP. High levels of circulating HMGB1 as a biomarker of acute liver failure in patients with viral hepatitis E. Liver Int. 2013;33:1341–1348. doi: 10.1111/liv.12197. [DOI] [PubMed] [Google Scholar]
- 14.Piscaglia AC, Shupe TD, Oh SH, Gasbarrini A, Petersen BE. Granulocyte-colony stimulating factor promotes liver repair and induces oval cell migration and proliferation in rats. Gastroenterology. 2007;133:619–631. doi: 10.1053/j.gastro.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook D, Genever P. Regulation of mesenchymal stem cell differentiation. Adv Exp Med Biol. 2013;786:213–229. doi: 10.1007/978-94-007-6621-1_12. [DOI] [PubMed] [Google Scholar]
- 16.Zheng S, You LY. Research progress in the differentiation of mesenchymal stem cells into functional hepatocytes. Chin J Hepatobill Surg. 2013;19:396–400. [Google Scholar]
- 17.Li M, Ikehara S. Bone-marrow-derived mesenchymal stem cells for organ repair. Stem Cells Int. 2013;2013:132642. doi: 10.1155/2013/132642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takami T, Terai S, Sakaida I. Stem cell therapy in chronic liver disease. Curr Opin Gastroenterol. 2012;28:203–208. doi: 10.1097/MOG.0b013e3283521d6a. [DOI] [PubMed] [Google Scholar]
- 19.Margini C, Vukotic R, Brodosi L, Bernardi M, Andreone P. Bone marrow derived stem cells for the treatment of end-stage liver disease. World J Gastroenterol. 2014;20:9098–9105. doi: 10.3748/wjg.v20.i27.9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao P, Hu DR, Wang S, Wen W, Zhou YM, Gong LJ. Treatment of chronic hepatic failure with autologous bone marrow stem cells transplantation. Chin Hepatol. 2005;10:171–173. [PubMed] [Google Scholar]
- 21.Pai M, Zacharoulis D, Milicevic MN, Helmy S, Jiao LR, Levicar N, Tait P, Scott M, Marley SB, Jestice K, Glibetic M, Bansi D, Khan SA, Kyriakou D, Rountas C, Thillainayagam A, Nicholls JP, Jensen S, Apperley JF, Gordon MY, Habib NA. Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol. 2008;103:1952–1958. doi: 10.1111/j.1572-0241.2008.01993.x. [DOI] [PubMed] [Google Scholar]
- 22.Li Q, Zhou X, Shi Y, Li J, Zheng L, Cui L, Zhang J, Wang L, Han Z, Han Y, Fan D. In vivo tracking and comparison of the therapeutic effects of MSCs and HSCs for liver injury. PLoS One. 2013;8:e62363. doi: 10.1371/journal.pone.0062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui L, Shi Y, Han Y, Fan D. Immunological basis of stem cell therapy in liver diseases. Expert Rev Clin Immunol. 2014;10:1185–1196. doi: 10.1586/1744666X.2014.930665. [DOI] [PubMed] [Google Scholar]


