Abstract
Maternal stress exerts long-lasting postnatal growth on offspring, which persist into adulthood. However, the effect of maternal stress on appetizing system has not been widely reported. In this study, we found that maternal immobilization stress (IS) during lactation resulted in low body weight and food intake. Immunohistochemistry showed an increase in stomach ghrelin protein expression. The central regulation of body weight and food intake occurs in the hypothalamus, which contains multiple neuronal systems that play important roles in the regulation of energy homeostasis. These systems including multiple neuropeptides involve in the ghrelin pathway of appetite regulation. Therefore, real time reverse transcription polymerase chain reaction (RT-PCR) was used to measure the change of mRNA expression of ghrelin pathway related hormones in order to explore the mechanisms involved in the appetite regulation. Expression levels of the hypothalamic 5-hydroxytryptamine 2c receptor (5-HT2cR) and 5-HT2bR, which are essential for the development and function of ghrelin and leptin, were decreased, as well as those of corticotrophin releasing factor (CRF) and pro-opiomelanocortin (POMC). While the expression of growth hormone secretagogue receptor (GHSR), neuropeptide-Y (NPY) and agouti-related protein (AgRP) showed an increase with significant difference. These results suggest that stress in a postpartum mother has persistent effects on the body weight of their offspring. Increased ghrelin and decreased leptin expression in the stomach may play a role in these effects.
Keywords: Maternal stress, appetizing system, stomach, ghrelin
Introduction
Postpartum depression is one of the maternity affective disorder diseases. 50-75% of puerperas present unstable mood such as inexplicable crying depressed mood with the broth of child, among which 10-15% suffer more serious symptoms such as loss of interest in life and social ability, even as a tendency to commit suicide or infants [1]. The adverse effects of this emotional disorder on the offspring have caused concern world widely in recent years [2]. Studies found that postpartum stress can cause maternal connection obstacle including body contact between mother and baby, behavior of baby and emotional reactivity of mother. Children with attention deficit hyperactivity disorder due to maternal connection obstacle might result in depression, diabetes and heart disease [3,4]. Mother might refuse to take care of baby when maternal connection obstacle occurs, leading to the damage of infants or disordered development and growth. Because puerpera suffering postpartum depression are unable to intake essential nutrients, they have an earlier stopping lactation time, which resulting in a decrease in the body weight of infants compared to normal babies [5-8].
Ghrelin is a kind of endogenous brain-gut peptide composed by 28 amino acids [9]. Ghrelin mainly secreted by X/A-like cells in the astrosubmucosa possesses physiological functions such as regulating blood pressure, improving energy metabolism and the function of ventrisulus sinister [10]. The appetite regulation function of ghrelin has attracted more and more attention of researchers. In this study, the low maternal food intake caused by postpartum restraint stress will be interpreted through brain-gut ghrelin pathway.
In the present study, we examined the effect of immobilization stress (IS) during lactation on body weight gain and food intake of the maternal mice. The mother and pups were separated during immobilization and we found that IS resulted in low body weight of the mothers, while no significant difference was observed in the food intake of maternal mice. Furthermore, the mechanism involves in the regulation of appetite was explored using western blot and real time reverse transcription polymerase chain reaction (RT-PCR). The expression levels of ghrelin pathway related protein and gene including ghrelin, leptin, 5-hydroxytryptamine 2c receptor (5-HT2cR), 5-HT2bR, corticotrophin releasing factor (CRF), pro-opiomelanocortin (POMC), growth hormone secretagogue receptor (GHSR), neuropeptide-Y (NPY) and agouti-related protein (AgRP) announced the general appearance of regulation pathway of ghrelin in brain and gut.
Material and methods
Animal experiments
ICR mice (CLEA Japan, Inc., Tokyo, Japan) were housed in the animal facility of Jinshan Hospital of Fudan University, Shanghai, China, with the maintenance of environmental conditions at 21 ± 1°C and a 12/12 h light-dark cycle. Every five mice were kept in one cage (28 cm high × 20 cm wide × 13 cm long) with free access to standard food and water for 7 days, after which one female was housed with two males in each cage for 4 days until mated. On postpartum day 22 (PND22), the body weight and femur length of maternal mice were measured (Control: n = 8; IS: n = 7), followed by the sacrifice and remove of stomach and the entire pituitary gland from each mouse surgically for gene expression analysis (Control: n = 8; IS: n = 7).
Immunohistochemical analysis
A total of 6 maternal mice (Control: n = 3; IS: n = 3) were sacrificed on PND22. The stomachs of the mice were immediately collected and fixed with 4% paraformaldehyde phosphate-buffered saline (PBS) for 24 h, followed by the embedment into paraffin. Sections (4 μm thick) were prepared using a microtome and deparaffinized in xylene/ethanol for immunofluorescence and immunohistochemical staining. Alexa Fluor 488-labeled anti-rabbit IgG and 4’,6-diamino-2-phenylindole (DAPI) were used for immunofluorescence staining, in which a rabbit polyclonal anti-mouse ghrelin antibody (MAB10404, Millipore, Temecula, CA, USA) and a mouse monoclonal Leptin antibody (Ab 3583, Abeam, Canada) were diluted 1:5 with PBS and used as the primary antibodies. After the primary antibodies were applied to ghrelin and leptin, then the tissue sections were incubated with horseradish peroxidase-conjugated anti-rabbit IgG (sc-2004, Cosmo Bio Co, LTD) and anti-mouse IgG (sc-2005, Cosmo Bio Co, LTD), respectively, followed by rabbit and mouse peroxidase anti-peroxidase complexes for immunohistochemical staining. Results were visualized using ChemMate EnVision™ Kit/HRP (DAB) (DAKO Japan). According to the manufacturer’s protocol, the sections were mounted and photographed under FV1000 (Olympus, Tokyo) and OPTIP HOT-2 (Nikon, Tokyo) microscopes. Ghrelin- and Leptin-positive cells were identified and counted as follows. Five photographs (scale bar is 50 μm) were taken for each section and examined by a histopathologic researcher who was blinded to the experimental groups. The number of positive cells per square millimeter was calculated in each of the five photographs and then averaged. The results of three mice per group were compared.
Western blot
The protein expression level of ghrelin in stomach was measured using western blot. Samples collected from stomach of maternal mice (Control: n = 3; IS: n = 3) were fully lysed in a buffer containing 20 mM Tris-HCl (pH 7.4), 1 mM EDTA, 140 mM NaCl, 1% (w/v) Nonidet P-40, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 50 mM NaF, and 10 μg/ml aprotinin. Total protein was quantified and loaded onto a 12% sodium dodecyl sulfate-polyacrylamide electrophoresis gels (SDS-PAGE) and electrotransferred to a polyvinyllidene difluoride membrane (Bio-Rad, Hercules, CA, USA). The membranes were soaked in blocking buffer (1 × Tris-buffered saline, 1% BSA, 1% nonfat dry milk) for 1 h and incubated overnight at 4°C with rabbit anti-ghrelin (MAB10404, Millipore, Temecula, CA, USA) and a rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies. Blots were developed using a peroxidase-conjugated anti-rabbit IgG and a chemiluminescent detection system (Santa Cruz Biotechnology). The bands were visualized using a Chemic Doc XRS system (Bio-Rad) and quantified using Quantity One imaging software (Bio-Rad).
Real-time RT-PCR
Total mRNA was extracted from the hypothalamus and stomachs of maternal mice (Control: n = 8; IS: n = 7) using an RNeasy Mini kit (Cat. No. 74104, Qiagen, Tokyo, Japan). Complementary DNA was synthesized through reverse transcription and amplified using a reverse transcriptase kit (Improm-IITM Reverse Transcription system Cat. No. A3800, Promega, Madison, WI, USA) under the reaction cycle of 25°C for 5 min, 42°C for 60 min, and 70°C for 10 min. Primer sets (ghrelin, leptin, GHSR, 5-HT 2bR, 5-HT 2cR, AgRP, NPY, POMC, CRF and GAPDH) used as PCR primers in TaqMan® gene expression assays (Applied Biosystems, Foster City, CA, USA) were designed and shown in Table 1. The primer sets were validated by DNA sequencing of the amplified products. Quantitative real-time RT-PCR was performed in triplicate using the QuantiFast® SYBR Green RT-PCR kit® (Qiagen K.K.). The cycle parameters involved an initial activation step at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 10 s, then annealing and extension at 60°C for 30 s. After amplification, the samples were incubated at 55°C for 1 min, and the temperature was gradually increased by 0.5°C every 10 s to perform the melting curve analysis. All RT-PCR procedures were performed on an iCycler iQTM Real-Time PCR Detection System (Bio-Rad Laboratories K.K., Tokyo, Japan). The threshold cycles (Ct) were used to quantify the mRNA levels of each gene after normalization for GAPDH. The relative mRNA expression data were analyzed using the 2-∆∆Ct method which allows relative quantification of the template and increases sample throughput by eliminating the need for standard curves by determining expression levels relative to a control [11].
Table 1.
Primers used in RT-PCR analysis
| Gene | Primers sequence | |
|---|---|---|
| Ghrelin | Forward | 5’-AAGAAGCCACCAGCTAAAC-3’ |
| Reverse | 5’-ATCGAAGGGAGCATTGAAC-3’ | |
| Leptin | Forward | 5’-TCTGTCTGGTGCTGTGAG-3’ |
| Reverse | 5’-GCCCTGAAATGCGGTATG-3’ | |
| 5-HT 2bR | Forward | 5’-GATGCCGATTGCCCTCTTGAC-3’ |
| Reverse | 5’-CTGGGATGGCGATGCCTATTG-3’ | |
| 5-HT 2cR | Forward | 5’-CATTCTTCATCCCGTTGAC-3’ |
| Reverse | 5’-TTCCTCATCACCCTTCTTG-3’ | |
| CRF | Forward | 5’-TTCTGCGGGAAGTCTTGG-3’ |
| Reverse | 5’-ATCGGAGCTGCGATATGG-3’ | |
| POMC | Forward | 5’-TTGGAAAGATAGCGGGAGAG-3’ |
| Reverse | 5’-GCAGAGGCAAACAAGATTGG-3’ | |
| NPY | Forward | 5’-GGTGATGGGAAATGAAAC-3’ |
| Reverse | 5’-CAACAACAAGGGAAATGG-3’ | |
| AgRP | Forward | 5’-CCACCTTTGCAGCATTCC-3’ |
| Reverse | 5’-GTGCCAACAGCAGAACAC-3’ | |
| GHSR | Forward | 5’-ATTTCCAATGCCCTGGTC-3’ |
| Reverse | 5’-CCTTGAACTCCTGGTAATCC-3’ | |
| GAPDH | Forward | 5’-ATCACTGCCACCCAGAAG-3’ |
| Reverse | 5’-TCCACGACGGACACATTG-3’ | |
Statistical analysis
Data are represented as means ± standard deviation. All data were analyzed with SPSS 10.0 using Fischer’s analysis or unpaired t tests. Differences were considered significant at P values < 0.05.
Results
Maternal IS reduces maternal weight
Body weight and food intake of the maternal mice were measured from PND1 to PND16. As shown in Figure 1A, the body weight of the mothers in the IS group decreased significantly compared with the control group (P < 0.05). Although food intake of stressed maternal mice shown in Figure 1B was not significantly lower on any individual day, there was a tendency to decrease from PND3 to PND13. Besides, the total food intake in the stressed maternal mice (215.33 g) was lower than that in the control group (256.37 g).
Figure 1.
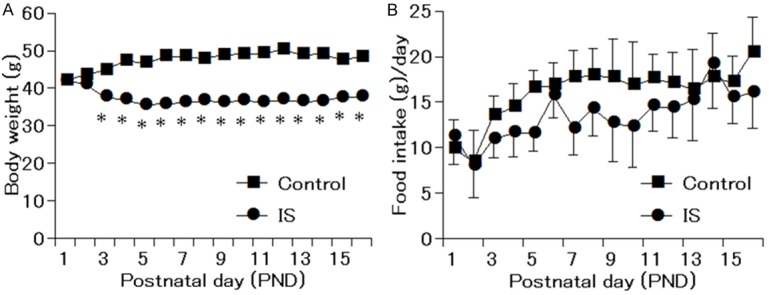
Body weight and food intake of maternal mice from postnatal day 1 to postnatal day 16. A. Body weight of maternal mice in IS group decreased significantly from postnatal 3 to postnatal 16. B. Food intake of maternal in the IS group showed a tendency to decrease without significant difference. *P < 0.05 compared with the control group.
Maternal IS induces expression level of ghrelin in the stomachs of maternal mice
The expression level of ghrelin in the stomachs of maternal mice was visualized using immunohistochemical analysis on PND22. As shown in Figure 2A, the positive cells presenting as fluorescently-labeled in the IS group were counted more intensive than that in the control group. Figure 2B shows the relative protein expression level of ghrelin measured by Western blot, while the result of SDS-PAGE was presented in Figure 2C. Correspond to the result obtained in immunohistochemical assay, IS group shows an increase in the expression level of ghrelin in the stomachs of maternal mice with significant difference (P < 0.05).
Figure 2.
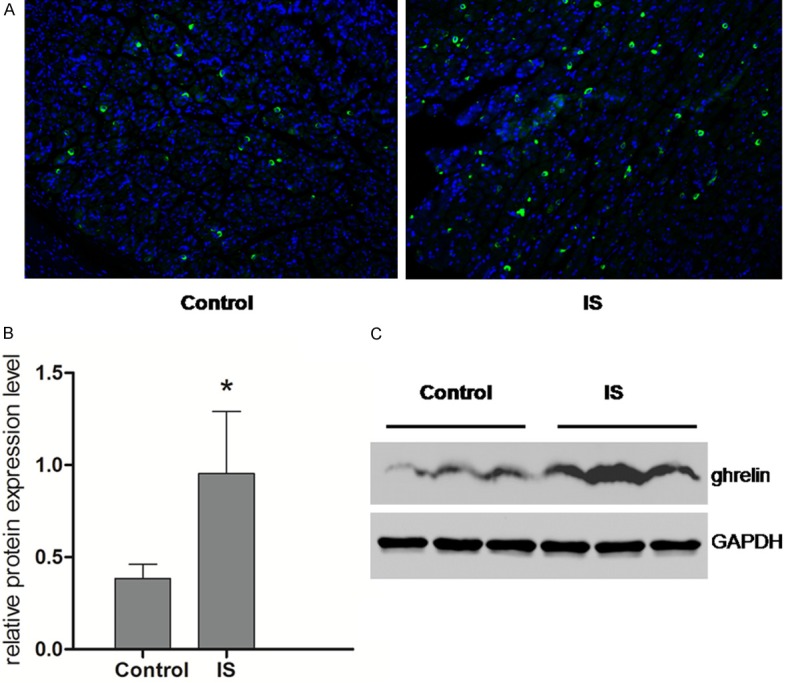
Expression of ghrelin was observed using immunflurescence staining and western blot in the stomach of maternal mice. A. Immunofluorescence images for the transmitted differential (TD) interference image, with ghrelin fluorescence and DAPI nuclear staining. The number of ghrelin-positive cells (shown as green) per square millimeter (mm2) in the stomach was lower in the IS group compared with the control. B. The expression level of ghrelin relative to GAPDH in the stomach of maternal mice showed a tendency to increase in the IS group with significant difference (P < 0.05). C. The expression level of ghrelin measured using SDS-PAGE. *P < 0.05 compared with the control group (Control: n = 3; IS: n = 3).
Maternal IS alters gene expression level in stomachs and hypothalamus of maternal mice
The mRNA expression levels of ghrelin pathway related genes including ghrelin, leptin, GHSR and 5-HT2bR in stomachs were measured using RT-PCR, as well as the expression levels of ghrelin, NPY, AgRP, GHSR, CRF, POMC and 5-HT2cR in hypothalamus of maternal mice on PND22. As shown in Figure 3A, the mRNA expression of ghrelin in the stomach, which is the main source of serum ghrelin, increased significantly in the IS group compared with the control group (P < 0.001). Besides, the expression level of GHSR also showed an increase with significant difference compared with that in the control group (P < 0.01), while the mRNA expression levels of leptin and 5-HT2bR decreased significantly (P < 0.001). The gene expression levels of major ghrelin pathway in the hypothalamus of maternal mouse were also analyzed, which was shown in Figure 3B. The expression levels of ghrelin, GHSR, AgRP and NPY were significantly higher than that in the control group (P < 0.001), while the expression levels of POMC, CRF and 5-HT2cR decreased significantly (P < 0.001). These data suggest that the major targets in hypothalamus of IS may be ghrelin-producing cells and Leptin-producing cells.
Figure 3.
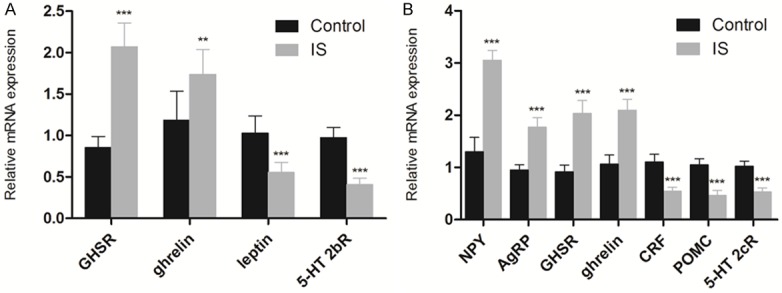
Effects of IS on the expression levels of ghrelin pathway related genes in maternal mice using RT-PCR relative to GAPDH. A. Gene expression profiles of ghrelin, leptin, GHSR and 5-HT 2bR in the stomach. B. Gene expression profiles of ghrelin, 5-HT 2cR, AgRP, NPY, POMC and CRF in the hypothalamus. Gene expression of GHSR, (Control: n = 8; IS: n = 7). **P < 0.01, ***P < 0.001 compared with the control group.
Discussion
A series of studies were conducted in order to explore the mechanism involves in the influence of postpartum depression on the development and growth of the offspring [12-14]. Low body weight in faints due to the separation of mother and baby was found using maternal deprivation stress model. In the previous work, postpartum restraint stress model was developed using ICR mice based on the maternal deprivation stress model [15]. A 3 h per day extra restraint stress was given to maternal mice for 3 weeks continually, resulting in the low body weight of offspring. Different to the growth disorder due to the limitation of maternal food intake, the offspring exhibits significant obesity or emaciation after weaning [16]. Previous studies in the growth disorder caused by postpartum restraint stress demonstrated the significant drop of expression levels of insulin-like growth factor (IGF-1) and growth hormone (GH) in serum and hypophyse, respectively, in the restraint stress group after weaning. Further study indicated the mechanism involves in the inhibition of prolactin (PRL) was related to the low expression levels of transcription factors Pit-1 and PitX2 which regulate GH and PRL. However, the expression levels of IGF-1, GH, PRL, Pit-1 and PitX2 showed no significant difference to the control group after free diet for 5 weeks, suggesting the direct relation between the growth disorder and the changed metabolism due to the decrease in the IGF-1. In view of the ‘matabolic programming’ caused by the maternal restraint stress during lactation period, the low nutrition condition including low body weight and food intake might be related to the metabolism correlative factors ghrelin, leptin, IL-6 and TNFα [17,18]. Therefore, the mechanism involves in the growth disorder of offspring was explored through the low nutrition condition due to the restraint stress during the lactation period.
It was reported that two systems, appetite promotion and suppression systems, exist in human body [19,20]. Ghrelin is the only hormone with the appetite regulation function produced in peripheral nerve, especially in stomach. Under stressed condition, the production of 5-HT increases and activates 5-HT2b receptors, leading to the inhibition of acylated ghrelin secreted by X/A-like cells in the gastric mucosa. The decreased acylated ghrelin in blood inhibits the combination of ghrelin with GHSR, resulting in the inhibition of the activation of NPY/AgRP in hypothalamus, finally leading to the down-regulation of appetite. While in the central nervous system, especially in hypothalamus, CRF was activated by increased 5-HT, resulting in the combination of CRF and 5-HT2R, and then leading to the secretion of ghrelin in blood. At the same time, POMC and 5-HT2c receptor activated by 5-HT also inhibit the secretion of ghrelin. Besides, the acylated ghrelin secreted by ghrelin nerve could be inhibited by the activated 5-HT2cR in the hypothalamus, resulting in the loss of appetite [19,20]. In a word, the appetite promotion system including NPY/AgRP pathway in hypothalamus and ghrelin pathway in stomach are inhibited, while the appetite suppression system containing CRF and 5-HT2cR in hypothalamus and 5-HT2bR in stomach are enhanced under stressed condition, resulting in the down-regulation of appetite. According to the results obtained from real time RT-PCR assay, the increased gene expression level of ghrelin suggested the up regulation of ghrelin. In the study, there was no significant difference in the food intake of maternal mice in the IS group, suggesting the increased ghrelin level in stomach which resulting in the activation of ghrelin pathway so as to up regulate the appetite, which correspond to the gene expression level measured by real time RT-PCR assay. However, in view of the decreased IGF-1 level in the blood of maternal mice based on the previous study, indicating the malnutrition condition in maternal mice, and leading to the low body weight of faints.
The limitation existing in the study is the lack of ghrelin data in the blood. Further study will be conducted to analyze the levels of acylated and deacylated ghrelin in the blood in order to supply reliable theory base for the study of postpartum stress clinically.
Conclusion
In conclusion, the low body weight of maternal mice due to the IS was related to the expression of ghrelin in the stomach which involves in the brain-gut ghrelin pathway. On the other hand, the ghrelin pathway stimulated by the increased ghrelin in stomach up regulated the appetite in order to feed the offspring, resulting in the decreased food intake without significant difference. Our study revealed the potential relation and mechanism involve in the effect of IS on appetite and ghrelin pathway in maternal mice.
Acknowledgements
The National Natural Science Foundation of China (Grant No. 81473610).
Disclosure of conflict of interest
None.
References
- 1.Ushiroyama T, Sakuma K, Ueki M, Ueki M. Efficacy of the kampo medicine xiong-gui-tiaoxue-yin (kyuki-chouketsu-in), a traditional herbal medicine, in the treatment of maternity blues syndrome in the postpartum period. Am J Chin Med. 2005;33:117–126. doi: 10.1142/S0192415X05002710. [DOI] [PubMed] [Google Scholar]
- 2.Fumagalli F, Molteni R, Racagni G, Riva MA. Stress during development: Impact on neuroplasticity and relevance to psychopathology. Prog Neurobiol. 2007;81:197–217. doi: 10.1016/j.pneurobio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Batten SV, Aslan M, Maciejewski PK, Mazure CM. Childhood maltreatment as a risk factor for adult cardiovascular disease and depression. Batten SV. 2004;65:249–254. doi: 10.4088/jcp.v65n0217. [DOI] [PubMed] [Google Scholar]
- 4.Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- 5.Downey G, Coyne JC. Children of depressed parents: an integrative review. Psychol Bull. 1990;108:50–76. doi: 10.1037/0033-2909.108.1.50. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien LM, Heycock EG, Hanna M, Jones PW, Cox JL. Postnatal depression and faltering growth: a community study. Pediatrics. 2004;113:1242–1247. doi: 10.1542/peds.113.5.1242. [DOI] [PubMed] [Google Scholar]
- 7.Paulson JF, Dauber S, Leiferman JA. Individual and combined effects of postpartum depression in mothers and fathers on parenting behavior. Pediatrics. 2006;118:659–668. doi: 10.1542/peds.2005-2948. [DOI] [PubMed] [Google Scholar]
- 8.Wright CM, Parkinson Kn, Drewett RF. The influence of maternal socioeconomic and emotional factors on infant weight gain and weight faltering (failure to thrive): data from a prospective birth cohort. Arch Dis Child. 2006;91:312–317. doi: 10.1136/adc.2005.077750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kojima M, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 10.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Hendrick V, Smith LM, Hwang S, Altshuler LL, Haynes D. Weight gain in breastfed infants of mothers taking antidepressant medications. J Clin Psychiatry. 2003;64:410–412. doi: 10.4088/jcp.v64n0409. [DOI] [PubMed] [Google Scholar]
- 13.Patel V, Rahman A, Jacob KS, Hughes M. Effect of maternal mental health on infant growth in low income countries: new evidence from South Asia. BMJ. 2004;328:820–823. doi: 10.1136/bmj.328.7443.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surkan PJ, Kawachi I, Ryan LM, Berkman LF, Carvalho Vieira LM, Peterson KE. Maternal depressive symptoms, parenting self-efficacy, and child growth. Am J Public Health. 2008;98:125–132. doi: 10.2105/AJPH.2006.108332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Moura EG, Passos MC. Neonatal programming of body weight regulation and energetic metabolism. Biosci Rep. 2005;25:251–269. doi: 10.1007/s10540-005-2888-3. [DOI] [PubMed] [Google Scholar]
- 16.Teixeira C, Passos M, Ramos C, Dutra S, Moura E. Leptin serum concentration, food intake and body weight in rats whose mothers were exposed to malnutrition during lactation. J Nutr Biochem. 2002;13:493. doi: 10.1016/s0955-2863(02)00197-3. [DOI] [PubMed] [Google Scholar]
- 17.Nakata H, Watanabe K, Murakami Y, Gao P, Tsuiji K, Nishimura K, Plotnikoff GA, Kurihara N, Irie Y, Ishige A. Stress on a postpartum mother inhibits the secretion of growth hormone in the offspring and causes persistent growth impairment. Methods Find Exp Clin Pharmacol. 2009;31:433–441. doi: 10.1358/mf.2009.31.7.1407221. [DOI] [PubMed] [Google Scholar]
- 18.Gao P, Ishige A, Murakami Y, Nakata H, Oka JI, Munakata K, Yamamoto M, Nishimura K, Watanabe K. Maternal stress affects postnatal growth and the pituitary expression of prolactin in mouse offspring. J Neurosci Res. 2011;89:329–340. doi: 10.1002/jnr.22550. [DOI] [PubMed] [Google Scholar]
- 19.Yakabi K, Sadakane C, Noguchi M, Ohno S, Ro S, Chinen K, Aoyama T, Sakurada T, Takabayashi H, Hattori T. Reduced ghrelin secretion in the hypothalamus of rats due to cisplatin-induced anorexia. Endocrinology. 2010;151:3773–3782. doi: 10.1210/en.2010-0061. [DOI] [PubMed] [Google Scholar]
- 20.Nahata M, Muto S, Nakagawa K, Ohnishi S, Sadakane C, Saegusa Y, Iizuka S, Hattori T, Asaka M, Takeda H. Serotonin 2C receptor antagonism ameliorates novelty-induced hypophagia in aged mice. Psychoneuroendocrinology. 2013;38:2051–2064. doi: 10.1016/j.psyneuen.2013.03.014. [DOI] [PubMed] [Google Scholar]


