Abstract
Background: MicroRNAs (miRNAs) play key roles in cardiac development, and the expression of miRNAs is altered in the diseased heart. The aim of this study was to explore the value of circulating microRNA-122-5p (miR-122-5p) as a potential biomarker for acute myocardial infarction (AMI). Methods: Plasma samples from 50 patients with AMI and 39 healthy adults (non-AMI controls) were collected. The abundance of circulating miR-122-5p was measured using quantitative real-time PCR (qRT-PCR). The cTnI concentrations of these samples were analyzed by ELISA. Results: Our findings revealed that circulating miR-122-5p expression were increased in AMI patients at 4 h, 8 h, 12 h, and 24 h by contrast to those non-AMI controls and displayed similar trends to that of cTnI concentrations in AMI patients. Further study showed that there is a high correlation between circulating miR-122-5p and cTnI concentrations. At last, the receiver operating characteristic (ROC) curve was performed and showed that circulating miR-122-5p had considerable diagnostic accuracy for AMI with an area under curve (AUC) of 0.855. Conclusions: Our results implied that circulating miR-122-5p could be a potential biomarker for AMI.
Keywords: Acute myocardial infarction, biomarker, diagnosis, miR-122-5p
Introduction
Acute myocardial infarction (AMI) is one of the most serious cardiovascular diseases with high morbidity and mortality [1]. An early detection and correct diagnosis of AMI are required to prevent the progressive development of AMI, and reduce the mortality rate in patients [2]. Traditional methods, including an electrocardiogram, coronary angiography, and enzymatic indicators, are important in the diagnosis of AMI [3]. However, new biomarkers that can early detection of AMI are still lacking. Recent studies suggested that circulating myocardial derived miRNAs might be useful as potential biomarkers for AMI [4].
MicroRNAs (miRNAs), non-coding small RNA consisting of 21-23 nucleotides, are negative regulators of gene expression by binding to 3’-UTR of mRNA [5]. The miRNAs play versatile roles in cellular biology and pathophysiologic regulatory pathways [6]. Recent reports showed that miRNAs are abundantly present in body fluid and can be used as biomarkers for types of diseases [7]. For example, Dhayat et al. showed that circulating miR-200 family members were significantly deregulated in hepatocellular carcinoma patients, indicating their potential diagnostic value as non-invasive serum markers [8]. Peng et al. found that circulating let-7e was significantly increased in ischemic stroke patients at the acute stage, suggesting that the expression levels of let-7e in serum may serve as a useful non-invasive biomarker for the acute stage of ischemic stroke [9]. Lorenzen et al. indicated that circulating levels of miR-210 was downregulated in the plasma of acute kidney injury patients and could predict the survival in patients with acute kidney injury [10].
Previous studies demonstrated that plasma miRNAs could act as important predictors in the progression and diagnosis of AMI. For example, He at al showed that circulating miR-328 and miR-134 could be potential indicators for AMI, and the miRNA levels were associated with increased risk of mortality or development of heart failure [11]. Liu et al. showed circulating miR-133, miR-1291 and miR-663b were significantly increased in the AMI cases. Through ROC analyses, they showed that those miRNAs were potential predictors of AMI [12]. Wang et al. suggested that circulating miR-133a level was significantly increased and could be a new biomarker for AMI patients [13]. However, the expression levels of circulating miR-122-5p in AMI remains unknown. Therefore, the present study was designed to determine the diagnostic value of circulating miR-122-5p in AMI patients.
Materials and methods
Blood samples
50 patients with AMI and 39 healthy adults (non-AMI controls) from the Department of Cardiology, Huaihe Hospital of HeNan University were enrolled in our study between 2013 and 2014. AMI was diagnosed based on combination of several parameters: ischemic symptoms plus increased cardiac troponin I (cTnI) and creatine kinase MB (CK-MB), pathological Q wave. ST-segment elevation or depression defined by the European Society of Cardiology/American College of Cardiology [14,15]. Baseline ECG was recorded in all patients. This study was approved by the Medical Ethics Committee in Huaihe Hospital of Henan University, and written informed consents were obtained from all the participants.
Cardiac troponin I determination
Plasma cTnI concentrations were detected by ELISA assay according to manufacturer’s protocol (Abnova).
RNA isolation and quantitative real-time PCR
Small RNA was isolated from 2 mL of plasma using the miRcute miRNA Isolation Kit (Tiangen) according to the manufacturer’s protocol. The quality and concentration of miRNA were detected in a multivolume spectrophotometer system (Epoch). cDNA synthesis was performed with the miRcute miRNA First-Strand cDNA Synthesis Kit (Tiangen). Subsequently, the miRcute miRNA qPCR Detection kit (SYBR Green) was used in real-time PCR for relative quantification of miRNAs with U6 as an internal control. The PCR primers were synthesized by Sangon Biotechnology (Shanghai). The amplification reactions were performed using the 7500 FAST Real-Time PCR System (Applied Biosystems). Relative miRNA expression level was calculated by 2-ΔΔCt method.
Statistical analysis
All computations were carried out using the software of SPSS version 18.0 for Windows. Data were expressed as means ± standard deviation (SD). Comparison among groups was assessed by the chi-square test or ANOVA. Spearman’s correlation test was used to analyze correlations between miR-122-5p and cTnI. Receiver operating characteristic (ROC) curves were used for evaluation of diagnostic accuracy of miR-122-5p and cTnI. P<0.05 was considered statistically significant.
Results
Characteristics of patients
A total of 50 patients with AMI and 39 healthy adults (non-AMI controls) were enrolled in this study. The details of clinical characteristics were shown in Table 1. Age, gender, history of diabetes, smoking status, systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol (CHOL), triglyceride (TG), glucose (Glu), blood urine nitrogen (BUN), serum creatinine (Scr), high density lipo-protein (HDL), low density lipo-protein (LDL) were compared to analyze the differences separately. There are no significant differences in these clinical characteristics between AMI patients and healthy adults (Table 1, P>0.05).
Table 1.
Clinical characteristics of AMI patients
| Characteristics | AMI group (n=50) | non-AMI group (n=39) | P value |
|---|---|---|---|
| Age | 63.2±11.4 | 62.7±10.5 | 0.376 |
| Gender (male/female) | 33/17 | 27/12 | 0.447 |
| Smoking (yes/no) | 29/21 | 23/14 | 0.426 |
| Diabetes (yes/no) | 21/29 | 18/21 | 0.295 |
| SBP | 128±21 | 120±23 | 0.576 |
| DBP | 81±14 | 76±18 | 0.193 |
| CHOL | 4.62±0.16 | 4.17±0.19 | 0.226 |
| TG | 1.76±0.09 | 1.53±0.07 | 0.187 |
| Glu | 6.36±1.93 | 6.63±1.74 | 0.703 |
| BUN | 7.83 ±1.92 | 5.63±0.96 | 0.097 |
| Scr | 80.19±17.83 | 77.61±26.1 | 0.285 |
| HDL | 1.37±0.43 | 1.22±0.39 | 0.491 |
| LDL | 2.53±0.69 | 2.31±0.53 | 0.183 |
Comparison of circulating miR-122-5p and cTnI levels in AMI patients
Using qRT-PCR, we analyzed the expression levels of circulating miR-122-5p in AMI patients and non-AMI controls. Our data showed that circulating miR-122-5p levels exhibited 4.63±0.82 fold, 16.91±3.73 fold, 7.08±1.63 fold, and 3.13±0.64 fold increase at 4 h, 8 h, 12 h, and 24 h respectively, compared with non-AMI controls (Figure 1A; P<0.05). Meanwhile, the circulating cTnI concentrations were also assayed by ELISA assay. miR-122-5p and cTnI were measured from the same samples at same time points. cTnI levels in AMI patients increased significantly at 4 h, 8 h, 12 h, and 24 h, respectively, compared with non-AMI controls (Figure 1B; P<0.05).
Figure 1.
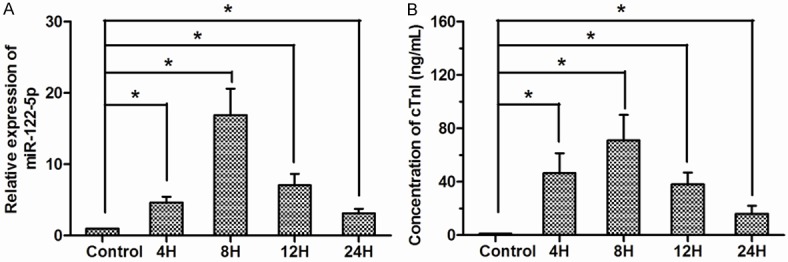
Expression of circulating miR-122-5p and concentrations of cTnI in AMI patients. A. The relative expression of circulating miR-122-5p in AMI patients was explored at 4 h, 8 h, 12 h, and 24 h, compared with that in non-AMI controls. B. Concentrations of cTnI were also investigated at the same time point. *P<0.05.
Correlations between circulating miR-122-5p and cTnI in AMI patients
To evaluate the significance of increased levels of circulating miR-122-5p following myocardial infarction, we analyzed whether the levels of miR-122-5p correlated with clinical parameters cTnI. Our data revealed that the level of miR-122-5p was significantly correlated with cTnI (Figure 2; P<0.05). These findings indicated that circulating miR-122-5p could be considered as a novel biomarker of AMI.
Figure 2.

Correlation of the miR-122-5p with cTnI in AMI patients.
Circulating miR-122-5p expression as a potential predictor of AMI
Receiver-operator characteristic (ROC) curve analysis was performed to evaluate the predictive power of circulating miR-122-5p levels for AMI. The ROC curves of miR-122-5p reflected separation between AMI and non-AMI control groups, with an area under curve (AUC) of 0.855 (95% CI, 0.746-0.964), compared with cTnI with an AUC of 0.902 (95% CI, 0.811-0.993) respectively (Figure 3). These data indicated that miR-122-5p was a novel biomarker for AMI.
Figure 3.
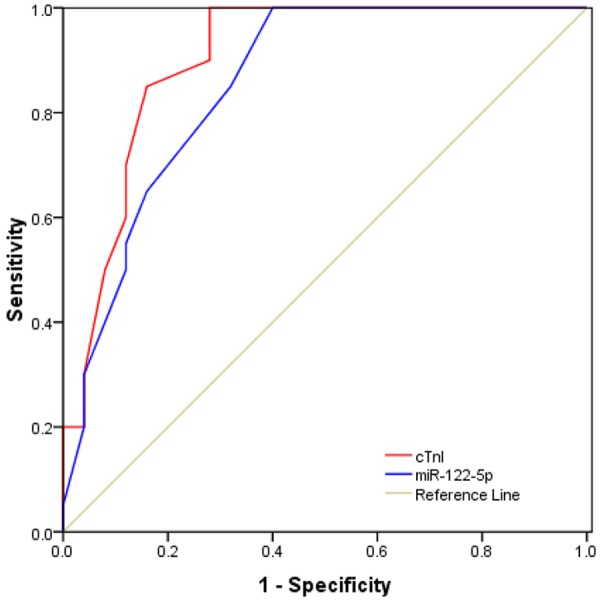
Receiver operator characteristic (ROC) curve analyses of miR-122-5p and cTnI in AMI patients.
Discussion
Acute myocardial infarction (AMI) is the leading cause of human mortality and morbidity in the world [16]. The existing biomarkers for AMI, including CK, CK-MB, MYO, cTnI, and BNP were considered as the biochemical criteria of AMI diagnosis [3]. However, the sensitivity and specificity of these biomarkers remain to be further enhanced, and it is difficult to identify new proteomic markers because of a technical bottleneck [2]. Recent studies showed that miRNAs were abundantly present in a remarkably stable form and they could be detected in peripheral circulation [17]. In addition, more and more circulating miRNAs were reported as new biomarkers in cardiovascular diseases, For example, Fichtlscherer et al. suggested that plasma levels of miR-145 were associated with severity of coronary artery disease [18]. Fabiola et al. indicated that circulating miR-499-5p could emerge in a geriatric population as a promising complementary biomarker of acute non ST-elevation myocardial infarction [19].
miR-122 was initially discovered as a liver specific miRNA, and its precursors were found on human chromosome 18, at position 18q21 [20]. Recent evidences showed that miR-122-5p play important roles in various human diseases. For example, Tan et al. showed that miR-122-5p targets ADAM10 to regulate breast cancer cell progression [21]. Panach et al. found that circulating miR-122-5p were increased and could act as biomarkers of osteoporotic fracture patients [22]. Florczyk et al. revealed that miR-122-5p could act as a plasma biomarker of liver injury in fish exposed to microcystin-LR [23]. However, there is no information available regarding miR-122-5p in AMI.
In the present study, we explored the expression level of miR-122-5p by qRT-PCR, we found that miR-122-5p was overexpressed at 4 h, 8 h, 12 h, and 24 h in AMI patients contrasted by that in non-AMI controls. Furthermore, we detected the plasma concentrations of cTnI, a classic marker of myocardial injury, and assessed the correlation between plasma cTnI and miR-122-5p. Our data showed that cTnI was increased in AMI patients and highly positively correlated with circulating miR-122-5p. At last, ROC curve of miR-122-5p was plotted to investigate the informativeness for AMI diagnosis. Our results showed that AUC of circulating miR-122-5p for the diagnosis of AMI was 0.855. Thus, our results suggested that circulating miR-122-5p could act as useful indicators for AMI. However, we should noted that the consideration of circulating miR-122-5p as a biomarker for AMI was at present based on our results from a relatively small sample size and larger clinical studies are definitely required to establish the case.
In conclusion, circulating miR-122-5p is associated with AMI and might be potentially novel biomarkers for AMI identification.
Acknowledgements
This study was supported by Henan Province foundation and advanced technology research project, “the transcriptional control of isl1, a maker of cardiacprogenitor” (No. 152300410065).
Disclosure of conflict of interest
None.
References
- 1.White HD, Chew DP. Acute myocardial infarction. Lancet. 2008;372:570–584. doi: 10.1016/S0140-6736(08)61237-4. [DOI] [PubMed] [Google Scholar]
- 2.Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, Fox K, Huber K, Kastrati A, Rosengren A, Steg PG, Tubaro M, Verheugt F, Weidinger F, Weis M. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909–2945. doi: 10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- 3.Keller T, Zeller T, Peetz D, Tzikas S, Roth A, Czyz E, Bickel C, Baldus S, Warnholtz A, Frohlich M, Sinning CR, Eleftheriadis MS, Wild PS, Schnabel RB, Lubos E, Jachmann N, Genth-Zotz S, Post F, Nicaud V, Tiret L, Lackner KJ, Munzel TF, Blankenberg S. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868–877. doi: 10.1056/NEJMoa0903515. [DOI] [PubMed] [Google Scholar]
- 4.Meder B, Keller A, Vogel B, Haas J, Sedaghat-Hamedani F, Kayvanpour E, Just S, Borries A, Rudloff J, Leidinger P. MicroRNA signatures in total peripheral blood as novel biomarkers for acute myocardial infarction. Basic Res Cardiol. 2011;106:13–23. doi: 10.1007/s00395-010-0123-2. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shukla GC, Singh J, Barik S. MicroRNAs: Processing, Maturation, Target Recognition and Regulatory Functions. Mol Cell Pharmacol. 2011;3:83–92. [PMC free article] [PubMed] [Google Scholar]
- 7.Cortez MA, Calin GA. MicroRNA identification in plasma and serum: a new tool to diagnose and monitor diseases. Expert Opin Biol Ther. 2009;9:703–711. doi: 10.1517/14712590902932889. [DOI] [PubMed] [Google Scholar]
- 8.Dhayat SA, Mardin WA, Kohler G, Bahde R, Vowinkel T, Wolters H, Senninger N, Haier J, Mees ST. The microRNA-200 family--a potential diagnostic marker in hepatocellular carcinoma? J Surg Oncol. 2014;110:430–438. doi: 10.1002/jso.23668. [DOI] [PubMed] [Google Scholar]
- 9.Peng G, Yuan Y, Wu S, He F, Hu Y, Luo B. MicroRNA let-7e Is a Potential Circulating Biomarker of Acute Stage Ischemic Stroke. Transl Stroke Res. 2015;6:437–445. doi: 10.1007/s12975-015-0422-x. [DOI] [PubMed] [Google Scholar]
- 10.Lorenzen JM, Kielstein JT, Hafer C, Gupta SK, Kumpers P, Faulhaber-Walter R, Haller H, Fliser D, Thum T. Circulating miR-210 predicts survival in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2011;6:1540–1546. doi: 10.2215/CJN.00430111. [DOI] [PubMed] [Google Scholar]
- 11.He F, Lv P, Zhao X, Wang X, Ma X, Meng W, Meng X, Dong S. Predictive value of circulating miR-328 and miR-134 for acute myocardial infarction. Mol Cell Biochem. 2014;394:137–144. doi: 10.1007/s11010-014-2089-0. [DOI] [PubMed] [Google Scholar]
- 12.Peng L, Chun-guang Q, Bei-fang L, Xue-zhi D, Zi-hao W, Yun-fu L, Yan-ping D, Yang-gui L, Weiguo L, Tian-yong H, Zhen-wen H. Clinical impact of circulating miR-133, miR-1291 and miR-663b in plasma of patients with acute myocardial infarction. Diagn Pathol. 2014;9:89. doi: 10.1186/1746-1596-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J, Qin YW, Jing Q. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J. 2010;31:659–666. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- 14.Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow AB, Wu AH, Christenson RH, Apple FS, Francis G, Tang W. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin Chem. 2007;53:552–574. doi: 10.1373/clinchem.2006.084194. [DOI] [PubMed] [Google Scholar]
- 15.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–969. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 16.Boersma E, Mercado N, Poldermans D, Gardien M, Vos J, Simoons ML. Acute myocardial infarction. Lancet. 2003;361:847–858. doi: 10.1016/S0140-6736(03)12712-2. [DOI] [PubMed] [Google Scholar]
- 17.Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012;110:483–495. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 18.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Roxe T, Muller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 19.Olivieri F, Antonicelli R, Lorenzi M, D’Alessandra Y, Lazzarini R, Santini G, Spazzafumo L, Lisa R, La Sala L, Galeazzi R, Recchioni R, Testa R, Pompilio G, Capogrossi MC, Procopio AD. Diagnostic potential of circulating miR-499-5p in elderly patients with acute non ST-elevation myocardial infarction. Int J Cardiol. 2013;167:531–536. doi: 10.1016/j.ijcard.2012.01.075. [DOI] [PubMed] [Google Scholar]
- 20.Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA, Xu C, Mason WS, Moloshok T, Bort R. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and maydownregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004;1:106–113. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- 21.Ergun S, Ulasli M, Igci YZ, Igci M, Kirkbes S, Borazan E, Balik A, Yumrutas O, Camci C, Cakmak EA, Arslan A, Oztuzcu S. The association of the expression of miR-122-5p and its target ADAM10 with human breast cancer. Mol Biol Rep. 2015;42:497–505. doi: 10.1007/s11033-014-3793-2. [DOI] [PubMed] [Google Scholar]
- 22.Panach L, Mifsut D, Tarín JJ, Cano A, García-Pérez MÁ. Serum Circulating MicroRNAs as Biomarkers of Osteoporotic Fracture. Calcif Tissue Int. 2015;97:495–505. doi: 10.1007/s00223-015-0036-z. [DOI] [PubMed] [Google Scholar]
- 23.Florczyk M, Brzuzan P, Krom J, Woźny M, Łakomiak A. miR1225p-- as a plasma biomarker of liver injury in fish exposed to microcystin-LR. J Fish Dis. 2015 doi: 10.1111/jfd.12406. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


