Abstract
Background: To study the prognosis-predicting value of a risk score based on phosphorylated At (p-Akt), vascular endothelial growth factor (VEGF), and Nin one binding (NOB1) expression in patients with resected non-small-cell lung cancer (NSCLC). Methods: A prospective cohort among 98 consecutive patients with resected NSCLC was conducted in 2009 to 2010. Immunohistochemistry was used in the detection of p-Akt, VEGF, and NOB1 expression. Any of three genes with positive expression was allocated a score of 1, otherwise scored 0. The risk score ranged from 0-3. Prognosis outcomes included overall survival (OS) and progression-free survival (PFS). Log-rank test and Cox hazard model were used to investigate the prognosis predicting value for the risk score. Results: In the 98 NSCLC tissue specimens, p-Akt, VEGF and NOB1 positive Expression rates were 42.9%, 66.3%, and 60.2%, respectively. The median for OS was 44 month, with 95% CI 35-51 months, and the median for PFS was 36 months, with 95% CI 25-49 months. Log-rank test showed OS and PFS related with TMN stage, lymph node metastasis, p-Akt expression, VEGF expression, NOB1 expression, and gene-based risk score (P<0.05). Multivariate COX analysis showed pTMN stage, lymph node metastasis, p-Akt expression, VEGF expression, and gene-based risk score were independent prognosis factors for OS and PFS. The adjusted HR for gene-based risk score with every one score increase was 1.21 [1.04-1.56] for OS and 1.19 [1.02-1.79] for PFS. Conclusions: Our results suggest the risk scores based on p-Akt, VEGF, NOB1 expression can predict postoperative survival in patients with resected NSCLC.
Keywords: Non-small-cell lung cancer, prognosis, p-Akt, VEGF, NOB1
Introduction
In the recent years, much progress has been made for treatment of early stage non-small-cell lung cancer (NSCLC), such as better and more accurate staging by ultrasound-guided biopsy and aspiration of mediastinal nodes, intensive multimodality therapy for adjuvant treatment postoperatively [1]. However, the prognosis is still poor for early stage NSCLC after surgical resection. The 5-year disease specific mortality for resected NSCLC in stage I-IIIA subgroups had approximately a 30, 60, and 75%, respectively [2].
Accurate prediction of prognosis outcome in resected NSCLC also remains challenging. Previous studies have shown that early stage of IA, tumor size (<10 mm), histologic grade (well differentiated), and histologic subtype (bronchoalveolar cell) predicted favorable prognosis outcome [2-4]. A systematic review performed an electronic literature search on the MEDLINE database and found poor pulmonary function, cardiovascular disease, male gender, advanced age, TNM stage, non-squamous cell histology, pneumonectomy, low hospital volume and little experience of the surgeon were risk factors for postoperative prognosis outcome for resected NSCLC [5].
Recent studies have found many molecular markers were related to the prognosis of resecting NSCLC. Our previous study indicated that enhanced expression of Nin one binding 1 (NOB1) is related to poor prognosis outcomes in patients with resected NSCLC, the hazard ratio (HR) for high NOB1 expression was 1.7 (95% CI, 1.1-3.0, P = 0.027) for overall survival (OS), and 1.8 (95% CI, 1.3-3.7, P = 0.031) for progression-free survival (PFS) [6]. Other molecular markers related to the prognosis of resected NSCLC included high expression of phosphorylated Akt (pAkt) [7], vascular endothelial growth factor (VEGF) overexpression [8], fibroblast growth factor overexpression [9], overexpression and Xeroderma pigmentosum group G low expression [10], positive expression of CD98 [11], carbonic anhydrase XII overexpression [12], and others. These molecular markers related with tumorigenesis, tumor angiogenesis, and progression.
In this study, we constructed a three gene-based risk score by three typical molecular markers of p-Akt, VEGF, and NOB1. We hypothesis these three gene-based risk score can predict prognosis outcomes in patients with resected NSCLC.
Materials and methods
Study design
This is a secondary data analysis of our previous study. Inclusion criteria included: patients undergoing resection for NSCLC, NSCLC diagnosis was confirmed by histopathological examination. Exclusion criteria were patients received pre-operative chemotherapy or radiotherapy. The study was approved by the medical ethics committee of our hospital. Written informed consent for participation in the study was obtained from participants before enrollment.
Patients and clinicopathological data
Consecutive 98 patients with resected NSCLC in January 2009 to March 2010 were included. The patients consisted of 70 males (71.4%) and 28 females (28.6%) ranging from 34 to 82 years (median, 61 years) of age. Seventy-four patients (75.5%) had smoking history, and 24 patients (24.5%) never smoked. According to the current UICC criteria, 65 patients (66.3%) were at stages I-II; 33 were at stages III (33.7%). There were 63 patients (64.3%) with tumor size <3 cm, and 35 patients (35.7%) with tumor size ≥3 cm; 32 patients (71.1%) had lymph node metastasis, and 13 patients (28.9%) without. From tumor type, 35 patients (35.7%) were assigned to squamous cell carcinoma type, 51 patients (52.0%) adenocarcinoma type, and 12 patients (12.2%) other type. From histopathological grade, there were 2 well-differentiated cases (2.0%), 72 moderately differentiated cases (73.5%) and 24 poorly differentiated cases (24.5%).
Immunohistochemistry and construction of gene-based risk score
We collected tissue blocks of the resected lung cancer tissue of the 98 patients. Immunohistochemistry (IHC) for NOB1 expression as described previously [14]. IHC staining for p-Akt and VEGF were also conducted according to the operating instruction. All staining were analyzed by two observers unaware of clinicopathological data. Quantitative analysis included percentages of positive cells and the staining intensities. Percentages of positive cells were scored into four categories according to staining: 0 for 0%, 1 for 1-33%, 2 for 34-66%, and 3 for 67-100%. The staining intensities were also scored into four grades: 0 for no stain, 1 for light yellow, 2 for yellowish brown, and 3 for brown. The sum of the percentages and intensity scores for the final staining score ranged form o to 9. All cases scoring product 2 or less were defined as negative expression and all cases scoring product 3 or greater were defined as positive expression.
Any positive expression of p-Akt, VEGF, and NOB1 was allocated a score of 1, negative expression scored 0. The risk score ranged from 0-3.
Follow-up and prognosis outcomes
All patients received follow-up after surgery. The follow-up period was set as 60 months. Lost to follow-up, follow-up reach to 60 months, and died for other reason, were recognized as censored data. The prognosis outcomes included overall survival (OS) and progression-free survival (PFS). OS was calculated from the date of diagnosis to death, and the PFS was calculated from the date of diagnosis to the day with signs of progression.
Statistical analysis
Univariate analysis with log-rank test was used to detect possible risk factors for the prognosis outcomes (OS and PFS). The possible risk factors included gender, age, smoke, tumor diameter, pTNM stage, lymph node metastasis, histopathological grade, p-Akt, VEGF, NOB1 expression, and gene-based risk score. We also used Cox proportional hazard model to adjust the effects by other factors related to OS and PFS for multivariate analyses. Hazard ratios (HR), their 95% confidence intervals (CI) and P values were calculated. All statistical analyses were performed using IBM SPSS software (version 19.0).
Results
p-Akt, VEGF and NOB1 expression in NSCLC tissues
IHC staining showed p-Akt protein was primarily localized in the cytoplasm, and less frequently in the nuclear. In the 98 NSCLC tissue specimens, 42.9% (42 specimens) were detected p-Akt positive expression. VEGF protein was localized in the cytoplasm. 66.3% (65 specimens) were detected VEGF positive expression. NOB1 protein was also localized both in the cytoplasm and the nucleus, but intensely localized in the cytoplasm. 60.2% (59 specimens) were detected NOB1 positive expression. The p-Akt, VEGF and NOB1 expression in NSCLC tissue by IHC was shown in Figure 1.
Figure 1.
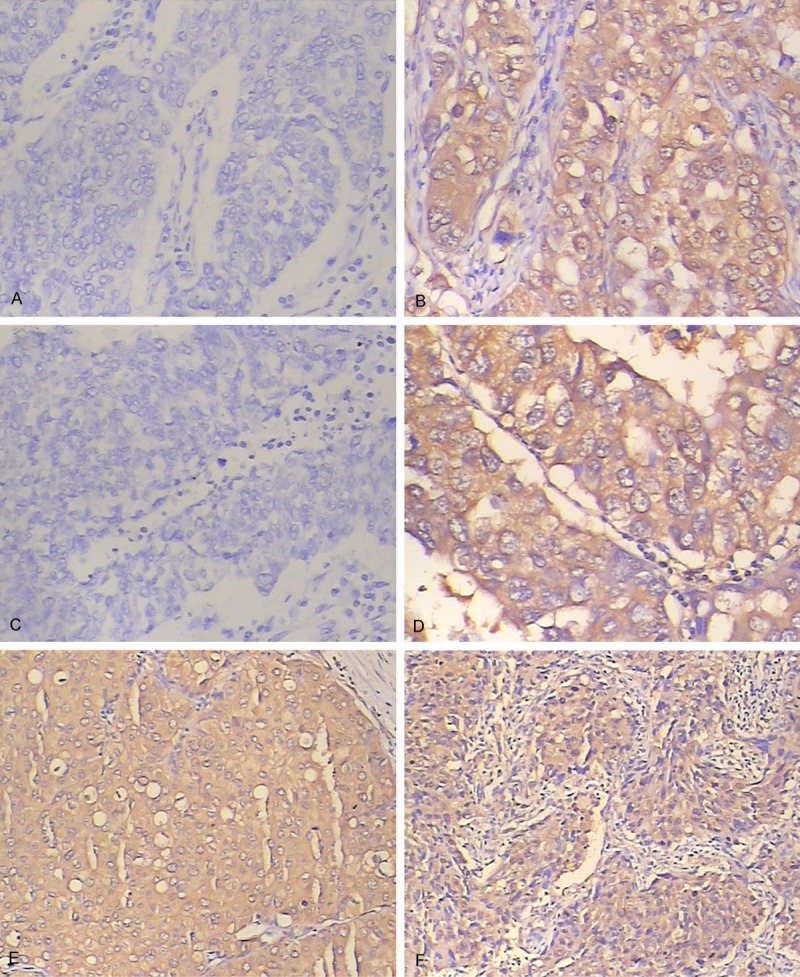
p-Akt, VEGF and NOB1 Expression in NSCLC tissue by IHC. A. p-Akt positive expression; B. p-Akt negative expression; C. VEGF positive expression; D. VEGF negative expression; E. NOB1 positive expression; F. NOB1 negative expression.
Univariate analysis for OS and PFS
Follow-up data were available for all patients. The median for OS was 44 months, with 95% CI 35-51 months, and the median for PFS was 36 months, with 95% CI 25-49 month.
Log-rank test showed OS and PFS has no relationship with patients’ gender, age, smoke status, tumor diameter, and histopathological grade (P>0.05), but related with TMN stage, lymph node metastasis, p-Akt expression, VEGF expression, NOB1 expression, as well as gene-based risk score (P<0.05). Univariate Log-rank test analysis for OS and PFS of clinical, histopathological and immunohistochemical parameters were listed in the Table 1.
Table 1.
Univariate analysis for overall survival and progression-free survival of clinical, histopathological and immunohistochemical parameters
| Characteristics | Overall survival | Progression free survival | |||
|---|---|---|---|---|---|
|
| |||||
| HR [95% CI] | P-values | HR [95% CI] | P-values | ||
| Gender | Male | 1 | 1 | ||
| Female | 0.91 [0.65-1.58] | 0.663 | 0.89 [0.61-1.83] | 0.679 | |
| Age (years) | <60 | 1 | 1 | ||
| ≥60 | 1.62 [0.68-2.74] | 0.336 | 1.25 [0.76-3.22] | 0.216 | |
| Smoke | No | 1 | 1 | ||
| Yes | 1.35 [0.89-2.46] | 0.215 | 1.39 [0.81-2.99] | 0.201 | |
| Tumor diameter (cm) | <3 | 1 | 1 | ||
| ≥3 | 1.91 [0.91-4.12] | 0.115 | 1.81 [0.82-3.64] | 0.125 | |
| pTMN stage | I, II | 1 | 1 | ||
| III | 1.85 [1.12-3.51] | 0.032* | 1.98 [1.15-4.32] | 0.022* | |
| Lymph node metastasis | No | 1 | 1 | ||
| Yes | 1.42 [1.09-2.95] | 0.034* | 1.49 [1.15-2.88] | 0.035* | |
| Histopathological grade | Well | 1 | 1 | ||
| Medium | 1.14 [0.72-2.23] | 0.199 | 1.12 [0.76-2.46] | 0.225 | |
| Low | 1.46 [0.85-2.89] | 0.111 | 1.36 [0.84-2.97] | 0.105 | |
| p-Akt expression | Negative | 1 | 1 | ||
| Positive | 1.75 [1.26-2.95] | 0.014* | 1.94 [1.21-2.96] | 0.031* | |
| VEGF expression | Negative | 1 | 1 | ||
| Positive | 1.92 [1.24-2.52] | 0.031* | 1.99 [1.14-3.06] | 0.015* | |
| NOB1 expression | Negative | 1 | 1 | ||
| Positive | 1.62 [1.15-2.31] | 0.039* | 1.65 [1.12-2.24] | 0.021* | |
| Gene-based risk score | 1.25 [1.04-1.56] | 0.044* | 1.21 [1.05-1.51] | 0.022* | |
P<0.05;
HR: hazard ratio; CI: confidence interval.
Multivariate analysis for OS and PFS
Multivariate COX analysis showed pTMN stage, lymph node metastasis, p-Akt expression and gene-based risk score were independent prognosis factors for OS in patients with NSCLC. After adjusted by TMN stage and lymph node metastasis, p-Akt expression, the HR for gene-based risk score was 1.21 [1.04-1.56] for every one score increase.
Multivariate COX analysis also showed pTMN stage, lymph node metastasis, p-Akt expression, VEGF expression, and gene-based risk score were independent prognosis factors for PFS. The adjusted HR for gene-based risk score was 1.19 [1.02-1.79] for every one score increase.
Multivariate COX analysis for OS and PFS of clinical, histopathological and immunohistochemical parameters were listed in the Table 2. The Kaplan-Meier curves for OS and PFS stratified by gene-based risk score were listed in the Figure 2.
Table 2.
Multivariate for overall survival and progression-free survival of clinical, histopathological and immunohistochemical parameters
| Characteristics | Overall survival | Progression free survival | |||
|---|---|---|---|---|---|
|
| |||||
| HR [95% CI] | P-values | HR [95% CI] | P-values | ||
| pTMN stage | I, II | 1 | 1 | ||
| III | 1.82 [1.25-2.79] | 0.012* | 1.75 [1.21-3.16] | 0.017* | |
| Lymph node metastasis | No | 1 | 1 | ||
| Yes | 1.41 [1.02-3.54] | 0.045* | 1.69 [1.15-4.26] | 0.039* | |
| p-Akt expression | Negative | 1 | 1 | ||
| Positive | 1.52 [1.06-3.76] | 0.041* | 1.63 [1.04-3.44] | 0.034* | |
| VEGF expression | Negative | 1 | 1 | ||
| Positive | 1.45 [0.91-4.64] | 0.146 | 1.47 [1.02-3.75] | 0.028* | |
| NOB1 expression | Low | 1 | 1 | ||
| High | 1.55 [0.96-3.22] | 0.091 | 1.71 [0.95-3.79] | 0.111 | |
| Gene-based risk score | 1.21 [1.04-1.56] | 0.037* | 1.19 [1.02-1.79] | 0.041* | |
P<0.05;
HR: hazard ratio; CI: confidence interval.
Figure 2.
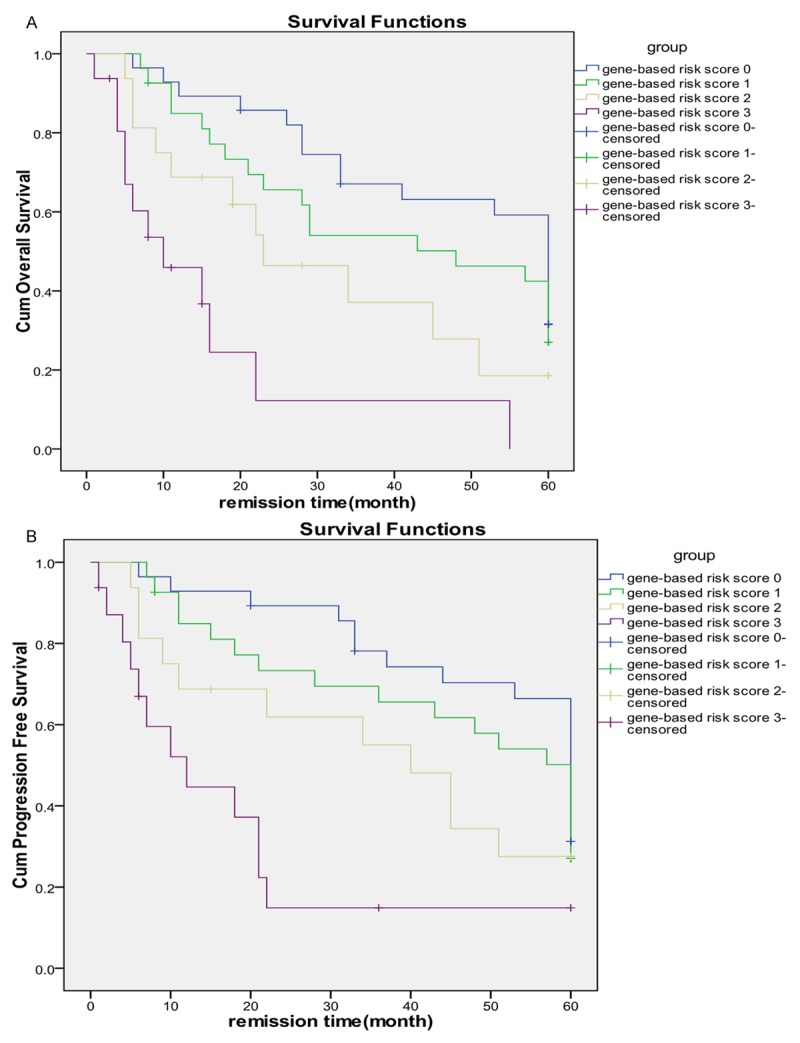
The Kaplan-Meier curves for OS and PFS, stratified by gene-based risk score. A. OS; B. PFS.
Discussion
In Log-rank test univariate analysis, we found prognosis outcomes in patients with resected NSCLC were related with TMN stage, lymph node metastasis, p-Akt expression, VEGF expression, NOB1 expression, and gene-based risk score. However, in multivariate COX analysis, we found only pTMN stage; lymph node metastasis, p-Akt expression and gene-based risk score were independent prognosis factors for OS. For PFS prognosis, only pTMN stage, lymph node metastasis, p-Akt expression, VEGF expression, gene-based risk score were independent prognosis factors. The gene-based risk score is constructed by p-Akt, VEGF, and NOB1 expression. This maybe the reason why VEGF and NOB1 expression didn’t became the independent prognosis.
We found the gene-based risk score was related with prognosis outcomes, with HR for 1.21 [1.04-1.56] for OS and 1.19 [1.02-1.79] for PFS. p-Akt is a serine/threonine-specific protein kinase that plays a key role in multiple cellular processes such as glucose metabolism, apoptosis, cell proliferation, transcription and cell migration. Activation of the intracellular prosurvival signal transduction protein p-Akt has been proposed as a central signaling event in carcinogenesis [13]. It has also been found confer resistance to chemotherapy and radiation for NSCLC [14]. New blood vessel formation is a fundamental event in the process of tumor growth and metastatic dissemination, the VEGF pathway is well established as one of the key regulators of this process [15]. Chen SC also reported VEGF was also an independent risk factor in predicting early recurrence of resected stage I NSCLC (HR = 3.98, P = 0.02); patients with strong VEGF staining also had poor 3-year DFS (P = 0.008) and OS (P = 0.007) [8]. The function of NOB1 is necessary for ubiquitin proteasome pathway-mediated proteolysis for the cell cycle progression [16]. NOB1 has a critical function of ubiquitin-dependent proteolysis. Silencing the NOB1 gene the functions of the NOB1protein in cell cycle progression was inhibited and thereby cancer cells proliferation was suppressed [17]. NOB1 also has been identified as one of six genes discriminated chronic phase from blast crisis for chronic myeloid leukemia [18]. These three molecular markers are important molecular markers for tumorigenesis, tumor angiogenesis, and progression. This maybe the reason the gene-based risk score was related with prognosis outcomes in resected NSCLC.
Previous studies also in identified gene-expression profiles were related with lung cancer prognosis. Potti reported a lung metagene model predicted prognosis for individual patients significantly better than did clinical prognostic factors across all early stages of NSCLC [19]. Wan also identified a 12-gene signature which can predict poor prognosis for NSCLC patients [20]. In our next studies, we will compare our three gene base score with these gene-expression profiles in predicting prognosis in patients with NSCLC.
In conclusions, our results suggest the risk score based on p-Akt, VEGF, NOB1 expression can predict postoperative survival in patients with resected NSCLC. Our study provides another useful tool for predicting prognosis in patients with resected NSCLC.
Acknowledgements
This research was supported by Nantong city social development project (HS2012025), Jiangsu province postdoctoral research foundation (1301072C), Six talent peaks project in Jiangsu Province (2014-YY-006) and China postdoctoral science foundation (2103M541705).
Disclosure of conflict of interest
None.
References
- 1.Spiro SG, Tanner NT, Silvestri GA, Janes SM, Lim E, Vansteenkiste JF, Pirker R. Lung cancer: progress in diagnosis, staging and therapy. Respirology. 2010;15:44–50. doi: 10.1111/j.1440-1843.2009.01674.x. [DOI] [PubMed] [Google Scholar]
- 2.Ravdin PM, Davis G. Prognosis of patients with resected non-small cell lung cancer: impact of clinical and pathologic variables. Lung Cancer. 2006;52:207–12. doi: 10.1016/j.lungcan.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Asamura H, Suzuki K, Watanabe S, Matsuno Y, Maeshima A, Tsuchiya R. A clinicopathological study of resected subcentimeter lung cancers: a favorable prognosis for ground glass opacity lesions. Ann Thorac Surg. 2003;76:1016–1022. doi: 10.1016/s0003-4975(03)00835-x. [DOI] [PubMed] [Google Scholar]
- 4.Goya T, Asamura H, Yoshimura H, Kato H, Shimokata K, Tsuchiya R, Sohara Y, Miya T, Miyaoka E Japanese Joint Committee of Lung Cancer Registry. Prognosis of 6644 resected non-small cell lung cancers in Japan: a Japanese lung cancer registry study. Lung Cancer. 2005;50:227–34. doi: 10.1016/j.lungcan.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Birim O, Kappetein AP, van Klaveren R, Bogers AJ. Prognostic factors in non-small cell lung cancer surgery. Eur J Surg Oncol. 2006;32:12–23. doi: 10.1016/j.ejso.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Liu K, Chen HL, Gu MM, You QS. Relationship between NOB1 expression and prognosis of resected non-small cell lung cancer. Int J Biol Markers. 2015;30:e43–8. doi: 10.5301/jbm.5000120. [DOI] [PubMed] [Google Scholar]
- 7.Yip PY, Cooper WA, Kohonen-Corish MR, Lin BP, McCaughan BC, Boyer MJ, Kench JG, Horvath LG. Phosphorylated Akt expression is a prognostic marker in early-stage non-small cell lung cancer. J Clin Pathol. 2014;67:333–40. doi: 10.1136/jclinpath-2013-201870. [DOI] [PubMed] [Google Scholar]
- 8.Chen SC, Shih CM, Tseng GC, Cheng WE, Chiou J, Hsiao M, Kuo ML, Su JL, Chen CY. Vascular endothelial growth factor C as a predictor of early recurrence and poor prognosis of resected stage I non-small cell lung cancer. Ann Acad Med Singapore. 2011;40:319–24. [PubMed] [Google Scholar]
- 9.Ohgino K, Soejima K, Yasuda H, Hayashi Y, Hamamoto J, Naoki K, Arai D, Ishioka K, Sato T, Terai H, Ikemura S, Yoda S, Tani T, Kuroda A, Betsuyaku T. Expression of fibroblast growth factor 9 is associated with poor prognosis in patients with resected non-small cell lung cancer. Lung Cancer. 2014;83:90–6. doi: 10.1016/j.lungcan.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Bartolucci R, Wei J, Sanchez JJ, Perez-Roca L, Chaib I, Puma F, Farabi R, Mendez P, Roila F, Okamoto T, Taron M, Rosell R. XPG mRNA expression levels modulate prognosis in resected non-small-cell lung cancer in conjunction with BRCA1 and ERCC1 expression. Clin Lung Cancer. 2009;10:47–52. doi: 10.3816/CLC.2009.n.007. [DOI] [PubMed] [Google Scholar]
- 11.Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Kawashima O, Kamide Y, Ishizuka T, Kanai Y, Nakajima T, Mori M. CD98 expression is associated with poor prognosis in resected non-small-cell lung cancer with lymph node metastases. Ann Surg Oncol. 2009;16:3473–81. doi: 10.1245/s10434-009-0685-0. [DOI] [PubMed] [Google Scholar]
- 12.Ilie MI, Hofman V, Ortholan C, Ammadi RE, Bonnetaud C, Havet K, Venissac N, Mouroux J, Mazure NM, Pouysségur J, Hofman P. Overexpression of carbonic anhydrase XII in tissues from resectable non-small cell lung cancers is a biomarker of good prognosis. Int J Cancer. 2011;128:1614–23. doi: 10.1002/ijc.25491. [DOI] [PubMed] [Google Scholar]
- 13.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 14.Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in nonsmall cell lung cancer and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–97. [PubMed] [Google Scholar]
- 15.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005;23:1011–27. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 16.Veith T, Martin R, Wurm JP, Weis BL, Duchardt-Ferner E, Safferthal C, Hennig R, Mirus O, Bohnsack MT, Wöhnert J, Schleiff E. Structural and functional analysis of the archaeal endonuclease Nob1. Nucleic Acids Res. 2012;40:3259–74. doi: 10.1093/nar/gkr1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen B, Liu J, Wu D, Qin Y, Peng C, Li C, Wang J. Gene silencing of NOB1 by lentivirus suppresses growth and migration of human osteosarcoma cells. Mol Med Rep. 2014;9:2173–9. doi: 10.3892/mmr.2014.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oehler VG, Yeung KY, Choi YE, Bumgarner RE, Raftery AE, Radich JP. The derivation of diagnostic markers of chronic myeloid leukemia progression from microarray data. Blood. 2009;114:3292–8. doi: 10.1182/blood-2009-03-212969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potti A, Mukherjee S, Petersen R, Dressman HK, Bild A, Koontz J, Kratzke R, Watson MA, Kelley M, Ginsburg GS, West M, Harpole DH Jr, Nevins JR. A genomic strategy to refine prognosis in early-stage non-small-cell lung cancer. N Engl J Med. 2006;355:570–80. doi: 10.1056/NEJMoa060467. [DOI] [PubMed] [Google Scholar]
- 20.Wan YW, Sabbagh E, Raese R, Qian Y, Luo D, Denvir J, Vallyathan V, Castranova V, Guo NL. Hybrid models identified a 12-gene signature for lung cancer prognosis and chemoresponse prediction. PLoS One. 2010;5:e12222. doi: 10.1371/journal.pone.0012222. [DOI] [PMC free article] [PubMed] [Google Scholar]


