Abstract:
Racial and ethnic disparities in cardiovascular disease are well established; however, there is limited information about survival differences following veno-venous extracorporeal membrane oxygenation (VV-ECMO) in contemporary adult populations. The purpose of this study was to assess survival at discharge, 30 days, and at 1 year following institution of VV-ECMO in an ethnically diverse population, and to examine potential risk factors for mortality. This was a single-center study of 41 patients (49% female, 27% minorities, 7% > 65 years) who received VV-ECMO between the years 2004 and 2013 at an academic medical center. Kaplan–Meier estimates were calculated to assess survival up to 1 year, and cox proportional hazard models were used to evaluate the association between risk factors, mortality, and confounders. Overall, 76% (n = 31) of VV-ECMO patients survived to discharge and 30 days and 71% (n = 29) survived to 1 year. Whites (n = 30) had a higher survival at 1 year compared to minorities (n = 11) (83% vs. 36%, respectively, p = .01). Minorities had a significantly increased risk of mortality at 30 days (hazard ratio [HR] = 5.07, 95% confidence interval [CI] = 1.42–18.09) and at 1 year (HR = 5.19, 95% CI = 1.63–16.55). Race/ethnicity remained a significant independent predictor of survival at 30 days except when history of shock or lung transplantation was included in adjusted regression models. VV-ECMO was associated with an excellent overall survival up to 1 year. Racial/ethnic minorities had a 5-fold increased risk for 30-day mortality, which was largely explained by a lower likelihood of lung transplantation and increased risk of shock.
Keywords: survival, veno-venous extracorporeal membrane oxygenation, mortality, race/ethnicity
Veno-venous extracorporeal membrane oxygenation (VV-ECMO) is a form of extracorporeal life support (ECLS) indicated for patients with severe respiratory disease unresponsive to medical therapy and mechanical ventilation. According to the 2014 Extracorporeal Life Support Organization (ELSO) international report, the amount of recorded adult respiratory ECLS cases has increased over the last decade. Clinical outcomes analyzed according to racial/ethnic groups post VV-ECMO are limited. Such information could help to identify and to rectify disparities that may be due to modifiable causes.
Observational studies examining VV-ECMO patients have reported survival to discharge between 51% and 68% (1–4). A recent review documented adult respiratory ECLS survival between 51% and 79% (5); however, these results did not report survival for racial/ethnic sub-populations. One limitation of the literature is that many studies report survival to discharge, however length of stay among institutions may vary. Moreover, this method may over-estimate survival if the sickest patients are transferred, suggesting that longer-term survival estimates may provide more robust information about potential disparate outcomes. Previous work on respiratory ECLS patients has identified a number of factors associated with mortality (1–3,5,6), but potential confounders have not been systematically examined to explain variations in outcomes among diverse patient populations.
The purpose of this study was to evaluate survival at standardized time points (discharge, 30 days, and 1 year), and to determine if survival varied by demographics (racial/ethnic status) after adjustment for potential confounders in a diverse contemporary patient population at a large academic medical center.
MATERIALS AND METHODS
This was a single-center study that included 41 consecutive patients (49% female, 27% racial/ethnic minorities, 7% ≥ 65 years) who received VV-ECMO between the years 2004 and 2013 at an academic medical center. A subset of patients underwent conversion from VV-ECMO to veno-arterial (VA)-ECMO (n = 10), and another 10 were converted from VA-ECMO to VV-ECMO. Participants in this retrospective analysis were identified through an institution-based database of all patients who received ECMO during the above time frame. Corresponding electronic medical records were accessed via a secure comprehensive clinical information system at the medical center by a Health Insurance Portability and Accountability Act (HIPAA)-trained research assistant and were validated by a physician. The institutional review board at Columbia University Medical Center approved this study.
Mortality Ascertainment
Mortality information was obtained from electronic medical records updated monthly via the Social Security Death Index (SSDI). Mortality status for all patients was ascertained through August 2014. The minimum mortality ascertainment for a given patient was 1 year and the maximum was 10 years follow-up.
Risk Factor Ascertainment
Risk factors and potential confounders for mortality at discharge, 30 days, and 1 year were abstracted from the New York Department of Health Database and the hospital’s electronic record system (EMR). These factors included age, sex, and medical history including congestive heart failure (CHF), myocardial infarction (MI), chronic renal failure, diabetes, coronary artery bypass grafting (CABG), cerebrovascular disease, ventricular arrhythmia, chronic obstructive pulmonary disease (COPD), lung transplantation, surgical priority, unplanned intervention, and cardiogenic shock.
Statistical Analysis
Descriptive data are presented as frequencies and percentages, overall and stratified by race/ethnicity (minority vs. white). Minorities included African-Americans, Asians, Hispanics, and those classified as “other” in the EMR. Univariate associations between baseline characteristics and racial/ethnic group were evaluated using Fisher’s exact statistics. Main outcomes were survival to discharge (yes vs. no), 30 days (yes vs. no), and 1 year (yes vs. no). Hazard ratios (HRs) were calculated to determine univariate associations between patient characteristics and mortality. Kaplan–Meier estimates were plotted to show probability of survival to discharge, 30 days, and to 1 year. Only the latter time points are presented because of the similarity between results for survival to discharge and survival to 30 days, owing to an average time to discharge of 46 days. Survival was calculated between the time of ECMO initiation and the death date as reported by SSDI.
Multivariate cox proportional hazard analyses with 95% confidence intervals (CIs) were used to evaluate univariate associations between race/ethnicity (minority vs. white) and mortality at discharge, 30 days, and 1 year. The multivariate models included adjustment for a priori established con-founders or those identified as significant in our univariate analyses including age (years), sex, and medical history (each condition was added separately as the fourth variable) to maintain parsimonious models because of the limited sample size. A maximum of four variables were included in the multivariate models because of the small sample size in each variable.
The data were double checked for errors and stored in a Microsoft Access database (Microsoft, Redmond, WA). Analyses were conducted using SAS software (version 9.3; SAS Institute, Cary, NC). Statistical significance was set a p < .05.
RESULTS
Table 1 shows the characteristics of the VV-ECMO population (n = 41) overall and by race/ethnicity. The mean age was 45.6 ± 13.7 years and did not differ between whites and racial/ethnic minorities (44.8 ± 14.9 years vs. 48 ± 10 years, respectively). The majority of VV-ECMO patients were white (73%) and male (51%). The minority sub-group (n = 11) included four Asians, two African-Americans, one Hispanic, and four unspecified non-whites. A small percentage of patients were 65 years or older (7%, n = 3) (Table 1). Patients had a variety of cardiac and pulmonary conditions and over half received lung transplants (54%, n = 22). Among patients who received lung transplants, three were minorities. Racial/ethnic minorities were significantly less likely to have COPD when compared with whites (18%, n = 2 vs. 70%, n = 21, p = .01) and significantly more likely to have shock when compared with whites (78%, n = 7 vs. 10%, n = 3, p < .001). All other demographic and medical history was not significantly different between whites and racial/ethnic minorities.
Table 1.
Patient characteristics overall and by race/ethnicity (n = 41).
| Overall (n = 41) n (%) | Whites (n = 30) n (%) | Minorities (n = 11) n (%) | |
|---|---|---|---|
| Demographics | |||
| Age (≥65 vs. <65) | 3 (7) | 2 (7) | 1 (9) |
| Sex (male vs. female) | 21 (51) | 13 (43) | 8 (72) |
| Medicaid vs. others (yes vs. no) | 2 (5) | 1 (3) | 1 (9) |
| Cardiovascular history | |||
| Congestive heart failure (yes vs. no) | 12 (29) | 8 (27) | 4 (36) |
| Myocardial infarction (yes vs. no) | 7 (17) | 4 (13) | 3 (27) |
| Coronary artery bypass graft surgery (yes vs. no) | 2 (5) | — | 2 (18) |
| Peripheral vascular disease (yes vs. no) | 1 (2) | 1 (3) | — |
| Cerebrovascular disease (yes vs. no) | 4 (10) | 2 (7) | 2 (18) |
| Malignant ventricular arrhythmia (Pre-operative) (yes vs. no)* | 4 (10) | 3 (10) | 1 (9) |
| Other medical history | |||
| Chronic renal failure (yes vs. no) | 13 (32) | 10 (30) | 3 (27) |
| Diabetes (yes vs. no) | 14 (34) | 10 (30) | 4 (36) |
| Hepatic failure (yes vs. no)† | 1 (3) | 1 (3) | — |
| Dialysis (yes vs. no) | 3 (7) | 2 (7) | 1 (9) |
| COPD (yes vs. no) | 23 (56) | 21 (70) | 2 (18) |
| Lung transplant (yes vs. no) | 22 (54) | 19 (63) | 3 (27) |
| Admission characteristics | |||
| Surgical priority level (emergency or emergent salvage vs. no) | 33 (81) | 23 (77) | 10 (91) |
| Unplanned cardiac reoperation or interventional procedure (yes vs. no)‡ | 8 (30) | 6 (27) | 2 (40) |
| Shock (yes vs. no)¶ | 10 (28) | 3 (11) | 7 (78) |
| Cohort year | |||
| Year 2010–2013 vs. year 2004–2009 | 29 (71) | 22 (73) | 7 (64) |
Missing n = 1.
Missing n = 1.
Missing n = 14.
Missing n = 5.
Figure 1 shows overall survival at 30 days (76%, n = 31) and at 1 year (71%, n = 29). Whites had a significantly higher 30-day survival when compared to racial/ethnic minorities (87%, n = 26 vs. 46%, n = 5, p = .01). Whites also had a significantly higher 1-year survival when compared to racial/ethnic minorities (83%, n = 25 vs. 36%, n = 4, p = .01). Kaplan–Meier survival estimates for overall survival showed that survival decreased precipitously to 30 days and more gradually to 1 year. Kaplan–Meier survival estimates comparing whites to minorities showed a similar trend (Figure 2). Among the patients who died, 10 did so prior to discharge. Since results for survival to discharge were not different from survival to 30 days only the later curves are presented below.
Figure 1.
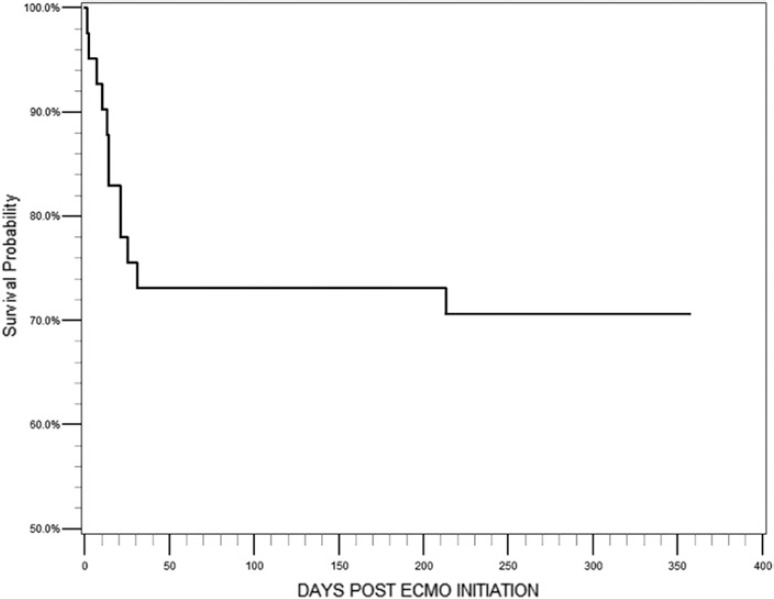
Kaplan–Meier survival curve for all patients who received VV-ECMO (n = 41) over the course of 1 year. Survival at 30 days and 1 year was 76% (n = 31) and 71% (n = 29), respectively.
Figure 2.
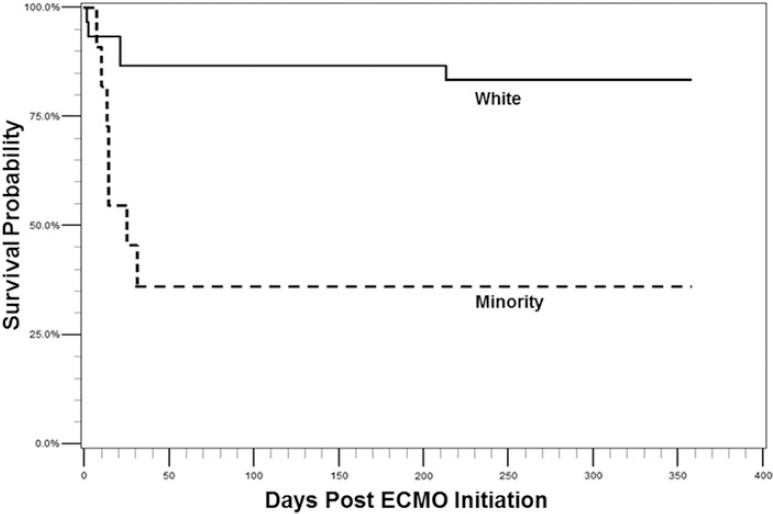
Kaplan–Meier survival curves generated for white (n = 30) and minority (n = 11) VV-ECMO patients over the course of a year. Survival at 30 days was 87% (n = 26) and 46% (n = 5), respectively. Survival at 1 year was 83% (n = 25) and 36% (n = 4) among whites and minorities respectively. A log-rank test was performed at discharge (χ2 = 7.36), 30 days (χ2 = 7.77), and 1 year (χ2 = 9.65).
Table 2 shows significant and non-significant univariate predictors of mortality at 30 days and at 1 year. Minorities had an increased likelihood of mortality compared with whites at 30 days (HR = 5.07, 95% CI = 1.42–18.09) and at 1 year (HR = 5.19, 95% CI = 1.63–16.55). Other significant positive predictors of mortality at 30 days and at 1 year included history of CABG, cerebrovascular disease, dialysis, shock, and MI. Having received a lung transplant was associated with a significantly reduced risk of mortality at 30 days (HR = .07, 95% CI = .01–.58) and at 1 year (HR = .12, 95% CI = .03–.56). There was no significant difference in survival at 30 days or at 1 year if a patient received VV-ECMO or a combination of VV and VA-ECMO. There was no difference in mortality at 30 days and at 1 year for patients who received VV-ECMO between the years 2010 and 2013 (n = 29) when compared to those who received VV-ECMO between 2004 and 2009 (n = 12).
Table 2.
Univariate predictors of mortality.
| Risk factors | Death prior to 30 days (n = 10, 24%) |
Death prior to 1 year (n = 12, 29%) |
||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age (≥65 vs. <65) | 1.41 (.18–11.13) | .75 | 2.59 (.57–11.82) | .22 |
| Sex (male vs. female) | .96 (.28–3.30) | .94 | .68 (.21–2.13) | .50 |
| Minority vs. white | 5.07 (1.42–18.09) | .01* | 5.19 (1.63–16.55) | .01* |
| Medicaid vs. other (yes vs. no) | 2.32 (.29–18.44) | .43 | 2.00 (.26–15.56) | .51 |
| 2010–2013 vs. 2004–2009 | 1.03 (.27–3.97) | .97 | .87 (.26–2.90) | .82 |
| VV vs. VV and VA | .90 (.26–3.11) | .87 | .90 (.29–2.79) | .86 |
| Emergent vs. non-emergent | .88 (.19–4.13) | .87 | 1.12 (.25–5.10) | .89 |
| Prior CABG | 22.39 (3.09–162.18) | .002* | 22.39 (3.09–162.18) | .002* |
| Prior MI | 4.43 (1.24–15.79) | .02* | 3.49 (1.04–11.66) | .04* |
| Shock | 5.42 (1.52–19.35) | .01* | 5.57 (1.74–17.77) | .004* |
| Pre-operative ventricular arrhythmia | 3.26 (.69–15.46) | .14 | 2.63 (.57–12.05) | .21 |
| CHF | 1.69 (.48–6.00) | .42 | 1.29 (.39–4.29) | .68 |
| Cerebrovascular disease | 8.23 (2.05–33.08) | .003* | 6.77 (1.77–25.83) | .01* |
| Diabetes | .42 (.09–1.97) | .27 | .33 (.07–1.50) | .15 |
| Chronic renal failure | 2.29 (.66–7.94) | .19 | 2.37 (.76–7.37) | .14 |
| Unplanned intervention | 1.97 (.44–8.81) | .38 | 2.21 (.59–8.28) | .24 |
| Dialysis | 6.88 (1.71–27.61) | .01* | 6.88 (1.71–27.61) | .01* |
| Lung transplant | .07 (.01–.58) | .01* | .12 (.03–.56) | .01* |
| COPD | .31 (.08–1.20) | .09 | .34 (.10–1.14) | .08 |
p < .05
Racial/ethnic minority status was examined in multi-variate analyses to determine if it remained an independent predictor of mortality at 30 days and at 1 year after adjustment for demographics and potential confounders (Tables 3 and 4). Racial/ethnic minority status remained an independent predictor of mortality at 30 days except when models were adjusted for shock or lung transplantation. When lung transplantation was added to the 30-day model, the HR decreased by almost 50% and was no longer statistically significant (HR = 5.61, 95% CI = 1.51–20.82 to HR = 2.95, 95% CI = 0.79–10.99), but a trend for higher risk of mortality persisted for minorities. A similar trend was observed at 1 year; however, a residual effect of racial/ethnic status on mortality was observed. When shock was added to the basic models at 30 days and 1 year the impact of race/ethnicity was reduced and no longer statistically significant.
Table 3.
Multivariate models: association between race/ethnicity and mortality at 30 days.
| Death at 30 days |
||||||
|---|---|---|---|---|---|---|
| Demographic model |
Demographic and lung transplant/shock models |
|||||
| Predictors | β | HR(95% CI) | p | β | HR(95% CI) | p |
| Race/ethnicity (minority vs. white) | 1.72 | 5.61 (1.51–20.82) | .01* | 1.08 | 2.95 (.79–10.99) | .11 |
| Age (≥65 vs. <65) | .44 | 1.55 (.19–12.76) | .38 | .04 | 1.05 (.12–9.32) | .97 |
| Sex (male vs. female) | −.44 | .65 (.18–2.36) | .43 | −.24 | .79 (.21–2.94) | .72 |
| Lung transplant (yes vs. no) | — | — | — | −2.33 | .10 (.01–.82) | .03* |
| Race/ethnicity (minority vs. white) | 1.72 | 5.61 (1.51–20.82) | .01* | 1.12 | 3.07 (.48–19.54) | .23 |
| Age (≥65 vs. <65) | .44 | 1.55 (.19–12.76) | .38 | −.14 | .87 (.10–7.95) | .90 |
| Sex (male vs. female) | −.44 | .65 (.18–2.36) | .43 | −.26 | .77 (.21–2.81) | .70 |
| Shock (yes vs. no) | — | — | — | .99 | 2.69 (.41–17.54) | .30 |
p < .05
Table 4.
Multivariate models: association between race/ethnicity and mortality at 1 year.
| Death at 1 year |
||||||
|---|---|---|---|---|---|---|
| Demographic model |
Demographic and lung transplant/shock models |
|||||
| Predictors | β | HR(95% CI) | p | β | HR(95% CI) | p |
| Race/ethnicity (minority vs. white) | 1.98 | 7.24 (2.07–25.31) | .002* | 1.30 | 3.68 (1.04–13.01) | .04* |
| Age (≥65 vs. <65) | 1.25 | 3.51 (.72–17.09) | .12 | 1.07 | 2.93 (.55–15.72) | .21 |
| Sex (male vs. female) | −.98 | .38 (.11–1.30) | .12 | −.90 | .41 (.11–1.45) | .17 |
| Lung transplant (yes vs. no) | — | — | — | –1.80 | .17 (.03–.84) | .03* |
| Race/ethnicity (minority vs. white) | 1.98 | 7.24 (2.07–25.31) | .002* | 1.40 | 4.04 (.71–22.95) | .12 |
| Age (≥65 vs. <65) | 1.25 | 3.51 (.72–17.09) | .12 | .77 | 2.16 (.40–11.56) | .37 |
| Sex (male vs. female) | −.98 | .38 (.11–1.30) | .12 | −.80 | .45 (.13–1.53) | .20 |
| Shock (yes vs. no) | — | — | — | .86 | 2.35 (.42–13.32) | .33 |
p < .05
DISCUSSION
Overall survival for the VV-ECMO population was high at discharge and 30 days (76%) and at 1 year (71%). We documented that survival among minorities was substantially lower than whites, likely owing to differential rates of lung transplantation. Overall survival declined rapidly to 30 days and the vast majority of patients who survived to 30 days survived to 1 year suggesting that 30-day survival may serve as a prognostic indicator for longer-term survival to 1 year. Our results are consistent with a recent review that reported survival to discharge for respiratory ECLS between 51% and 79% (5). In addition, the 2014 ELSO international report presented a 60% survival to discharge for adult respiratory ECLS. Our data report a similar but higher overall survival to discharge (76%). The high survival achieved in this study may be related to the institution’s expertise in ECMO and/or the fact that most patients received lung transplantation.
Patients who received a lung transplant had a high survival at 1 year (91%), likely attributed to replacement of the diseased lung(s). Minorities were less likely to receive a lung transplant when compared to whites (27%, n = 3 vs. 63%, n = 19, respectively); however, we did not evaluate differences in indication or eligibility for lung transplantation, which may explain disparity in lung allocation. It is also possible that racial/ethnic differences in comorbidities, adherence to medical regimens, or socioeconomic and lifestyle habits could account for differences in rates of lung transplantation. These factors are important parts of transplantation candidacy and may affect a patient’s eligibility or likelihood of receiving one (7). There have been a number of studies that have evaluated the differences in lung transplant allocation and survival between whites and non-whites, but information in patients who have received VV-ECMO is limited. A study looking at survival following lung transplantation found that there was no significant difference in 5-year survival between white and non-whites during the years 2001–2009 (8). However, this study did not perform a sub-group analysis of those patients who received VV-ECMO. One study found that African-Americans were less likely to undergo lung transplantation after being listed and were more likely to die or be removed from the list (9). The relation between minorities and access to care needs to be further examined, especially in the realm of critical care.
The majority of patients included in this study were less than 65 years of age with a mean age of approximately 45 years. Advanced age may be considered a relative contraindication for ECMO as it has been associated with increased mortality (10). However, mortality associated with age may be related to the presence of multiple comorbidities and not a direct effect of age itself. This study comprises half females and over a quarter of the population were minorities. Inclusion of females and minorities is important as they may be linked to different outcomes post ECLS (11,12). Univariate analyses revealed a number of significant and non-significant predictors of mortality. Factors associated with significantly increased mortality at 30 days and at 1 year included racial/ethnic status, having a medical history of CABG, cerebrovascular disease, dialysis, shock, and MI. It is possible that these comorbidities, especially those related to cardiac function, could have complicated the patient’s recovery on VV-ECMO requiring conversion to VA-ECMO. However, it is important to note that there was no significant difference in mortality found among those patients who were converted between the two ECMO modalities. Receiving a lung transplant was the only factor that was significantly associated with decreased mortality at 30 days and at 1 year.
Previous studies have identified a number of factors that increase the risk of mortality at discharge for patients undergoing respiratory ECLS. These include older age (3,6), more pre-ECMO ventilation days (3,6), multisystem organ failure (3), pre-ECMO renal failure (2,3), degree of respiratory impairment (3), PaCO2 ≥ 75 mmHg (6), PIP ≥ 42 cmH2O (6), pre-ECMO nitric oxide therapy (6), or bicarbonate infusion (6). Factors that have been shown to decrease mortality at discharge include shorter latency to ECMO (1), fewer pre-ECMO ventilation days (1), lower peak blood urea nitrogen (1), prone position (5), and pre-ECMO neuromuscular blockade (6). We recently showed that older age was an important predictor of mortality following VA-ECMO and that greater risk was largely a function of greater comorbidities, underscoring the importance of evaluating potential confounders for survival following ECMO among different demographic groups (13). There were a number of non-significant predictors of mortality at 30 days and at 1 year (Table 2). These factors warrant further examination, in studies with larger sample sizes.
Multivariate analysis revealed that the increased risk of 30-day mortality associated with minority status was largely mitigated by adjustment for lung transplantation and history of shock. In contrast, at 1 year racial and ethnic status remained a significant contributor to mortality after adjustment for lung transplantation suggesting unmeasured factors associated with minority status may be important for survival.
The strengths of this study include measurement of survival at standardized time points (including up to 1-year follow-up), the diverse population, and the inclusion of risk factors not previously examined as potential con-founders. This study is limited by its small size and the single-center experience. However, given that the annual incidence of VV-ECMO has not grown until recently, the sample is still a sizable contribution from a center with substantial experience in ECMO. This study did not record specific indications for ECMO nor did it include some risk factors that have previously been reported in the literature. A casual connection among race/ethnicity, the lower frequency of lung transplantation, and increased mortality for minorities cannot be determined because of the observational design of this study.
Overall survival for this study population was high relative to the previous reports for respiratory ECLS owing to the efficacy of this therapy to treat patients with severe respiratory illness (5). Kaplan-Meier survival curves show that the major risk for mortality occurs within the first 30 days. There was a striking difference in survival between whites and minorities that warrants further investigation and highlights the importance of performing sub-group analysis to determine if survival varies by demographic sub-populations. Racial/ethnic status was a significant independent predictor of mortality and although it was largely explained by an increased likelihood of shock and/or decreased likelihood of receiving a lung transplant, a potential significant residual risk likely remains due to causes we were not able to document. More research needs to be conducted on survival in ECMO patient sub-groups. A better understanding of modifiable causes of disparities in ECMO survival could potentially lead to improved clinical outcomes.
REFERENCES
- 1.Lee JJ, Hwang SM, Ko JH, et al. . Efficacy of veno-venous extracorporeal membrane oxygenation in severe acute respiratory failure. Yonsei Med J. 2015;56:212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camboni D, Philipp A, Lubnow M, et al. . Support time-dependent outcome analysis for veno-venous extracorporeal membrane oxygenation. Eur J Cardiothorac Surg. 2011;40:1341–6. [DOI] [PubMed] [Google Scholar]
- 3.Schmid C, Philipp A, Hilker M, et al. . Venovenous extracorporeal membrane oxygenation for acute lung failure in adults. J Heart Lung Transplant. 2012;31:9–15. [DOI] [PubMed] [Google Scholar]
- 4.Gray BW, Haft JW, Hirsch JC, Annich GM, Hirschl RB, Bartlett RH.. Extracorporeal life support: Experience with 2,000 patients. ASAIO J. 2015;61:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leligdowicz A, Fan E.. Extracorporeal life support for severe acute respiratory distress syndrome. Curr Opin Crit Care. 2015;21:13–9. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt M, Bailey M, Sheldrake J, et al. . Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014;189:1374–82. [DOI] [PubMed] [Google Scholar]
- 7.Orens JB, Estenne M, Arcasov S, et al. . International guidelines for the selection of lung transplantation candidates: 2006 update-a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25:745–55. [DOI] [PubMed] [Google Scholar]
- 8.Liu V, Weill D, Bhattacharya J.. Racial disparities in survival after lung transplantation. Arch Surg. 2011;156:286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lederer DJ, Benn EK, Barr RG, et al. . Racial differences in waiting list outcomes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayes D Jr, Whitson BA, Black SM, Preston TJ, Papdimos TJ, Tobias JD.. Influence of age on survival in adult patients on extracor–poreal membrane oxygenation before lung transplantation. J Heart Lung Transplant. 2015;34:832–8. [DOI] [PubMed] [Google Scholar]
- 11.Berlit S, Hornermann A, Schaible T, et al. . Influence of gender on mortality and need for extracorporeal membrane oxygenation in neonates with congenital diaphragmatic hernia. In Vivo. 2012;26:481–6. [PubMed] [Google Scholar]
- 12.Qureshi FG, Jackson HT, Brown J, et al. . The changing population of the United States and use of extracorporeal membrane oxygenation. J Surg Res. 2013;184:572–6. [DOI] [PubMed] [Google Scholar]
- 13.Narotsky DL, Mosca MS, Mochari-Greenberger H, et al. . Short-term and longer-term survival after veno-arterial extracorporeal membrane oxygenation in an adult patient population; does older age matter? Perfusion. 2015. 5 October [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


