Abstract
WRKY proteins are one of the largest transcription factor families in higher plants and play diverse roles in various biological processes. Previous studies have shown that some WRKY members act as negative regulators of secondary cell wall formation in pith parenchyma cells. However, the regulatory mechanism of pith secondary wall formation in tree species remains largely unknown. In this study, PtrWRKY19 encoding a homolog of Arabidopsis WRKY12 was isolated from Populus trichocarpa. PtrWRKY19 was expressed in all tissues tested, with highest expression in stems, especially in pith. PtrWRKY19 was located in the nucleus and functioned as a transcriptional repressor. Ectopic expression of PtrWRKY19 in an atwrky12 mutant successfully rescued the phenotype in pith cell walls caused by the defect of AtWRKY12, suggesting that PtrWRKY19 had conserved functions for homologous AtWRKY12. Overexpression of PtrWRKY19 in poplar plants led to a significant increase in the number of pith parenchyma cells. qRT-PCR analysis showed that lignin biosynthesis-related genes were repressed in transgenic plants. In transcient reporter assays, PtrWRKY19 was identified to repress transcription from the PtoC4H2 promoter containing the conserved W-box elements. These results indicated that PtrWRKY19 may function as a negative regulator of pith secondary wall formation in poplar.
Plant cells have a rigid cell wall that surrounds the cell membrane. In general, plant cell walls are composed of biopolymers, such as polysaccharides, phenolic compounds, and various proteins. All plant cells consist of a primary cell wall (PCW), but some specific type cells such as sclerenchyma cells also have a secondary cell wall (SCW), based on their biosynthetic composition and cellular location1,2. In stem tissues, PCW, formed at the cell plate during division, functions as a critical regulator of cell elongation and expansion3,4. In contrast, SCW, produced during the later phase of development of vascular tissues, provide mechanical strength to support physical weight of plants, and facilitate the transport of water and nutrients5,6. On the other hand, plant secondary cell walls are important for products such as paper, wood, fibers, renewable biofuel in human life7. Therefore, understanding the molecular mechanisms controlling SCW biosynthesis will have important implications in tree genetic improvement.
Plant secondary cell walls are mainly composed of cellulose, xylan, and lignin and their biosynthesis is regulated by a complex transcriptional network2,6. In this hierarchical network of transcription factors, SECONDARY WALL-ASSOCIATED NAC DOMAIN 1 protein (SND1/NST3) and its functional homologues (NST1 and NST2, vessel-specific VND6 and VND7) are master switches that turn on a subset of transcription factors i.e. SND3, MYB46, MYB83, MYB1032,8, which also directly activate the expression of SCW biosynthetic genes9. In Arabidopsis, AtMYB46 and AtMYB83 act as second layer-master switches of SCW biosynthesis and overexpression of AtMYB46 and AtMYB83 was activated the entire SCW biosynthetic pathway9,10. Other R2R3 MYB genes PtMYB4 from Pinus taeda11, EgMYB2 from Eucalyptus gunnii12 and PtrMYB3 and PtrMYB20 from Populus trichocarpa13 were also demonstrated to be functional orthologs of AtMYB46 and AtMYB83, suggesting the evolutionary conservation of the transcriptional network regulating SCW biosynthesis in vascular plants14.
Recent studies have shown that the NAC master switches (NST1/NST2, VND6/VND7) of SCW formation are also regulated by the upstream transcription factors. Arabidopsis myb26 mutant exhibited a failure of anther dehiscence and male sterility15. A similar phenotype was observed in double mutants of NST1 and NST216. Overexpression of AtMYB26 induced ectopic deposition of SCW in both Arabidopsis and tobacco17. These results indicate that MYB26 is an activator of the upstream of NAC transcription factors during SCW formation. More recently, a novel Arabidopsis WRKY transcription factor WRKY13 has also been shown to positively regulate lignin biosynthesis in stems by directly binding to the promoter of NST218. In addition, some negative regulators of NAC transcription factors have been identified in plant tissues. For example, in Arabidopsis wrky12 mutant, ectopic SCW formation appeared in pith parenchyma cells of inflorescence stems, and the expression of NST2 activated. Further studies showed that WRKY12 protein can bind to the NST2 promoter sequence in vitro, suggesting that WRKY12 negatively regulates SCW formation by directly inhibiting NST2 expression in pith cells19. An AtWRKY12 homologous gene MIWRKY12 was also isolated from monocotyledonous grass species Miscanthus lutarioriparius and heterologous expression of MlWRKY12 in an atwrky12 background mutant successfully rescued the phenotype of pith cell walls caused by the mutation of AtWRKY1220, implying their similar functions in SCW development. However, it remains unclear whether WRKY transcription factors play also similarly critical roles in SCW development in pith cell walls of tree species.
In this study, we isolated a group IIc WRKY subfamily member PtrWRKY19 from P. trichocarpa. Phylogenetic analysis revealed that PtrWRKY19 has a close relationship with MtSTP from Medicago truncatula19 and AtWRKY12. The phenotype of pith cell walls in atwrky12 mutant could be rescued by the heterologous expression of PtrWRKY19. Overexpression of PtrWRKY19 in transgenic poplar resulted in a signficant increase in pith diameter and a reduction in expression level of lignin biosynthetic genes. These results indicated that PtrWRKY19 as a function ortholog of AtWRKY12 negatively regulated SCW development in pith cells in poplar.
Materials and Methods
Plant materials and growth conditions
Populus trichocarpa plants were grown in the greenhouse at 25 °C under a 14-/10-h light/dark cycle with supplemental light (4500 lux). Seeds of the Arabidopsis thaliana ecotype Col-0 were incubated at 4 °C for 3 days before being surface-sterilized and germinated on 1/2 MS medium with the addition of 1.0% agar. Plants were grown in a growth chamber at 23–25 °C with the 8 h/16 h dark/light photoperiod, 70%–80% relative humidity and light intensity 150 μmol m−2s−1. The Arabidopsis atwrky12 mutant (SALK_080995) was obtained from Arabidopsis Biological Resource Center (ABRC).
Sequence analysis
The amino acid sequences of poplar WRKY transcription factors were obtained from website (http://www.phytozome.com). The deduced amino acid sequences were aligned with the program DNAMAN7.0 (Lynnon Corporation, USA). The phylogenetic relationships of WRKY proteins were analyzed with the neighbour-joining method using MAGE 5.021. The localization of the PtrWRKY19 protein was predicted by the WoLF PSORT program (http://wolfpsort.org/)22.
The GenBank accession numbers of the WRKY genes from different species were: MlWRKY12 (KC191597), MtSTP (HM622067), AtWRKY12 (At2g44745), OsWRKY36 (BK005039), AtWRKY13 (AF421153), VvWRKY20 (JQ782602), AtWRKY25 (AT2G30250), OsWRKY24 (AY676925), PtrWRKY13 (POPTR_0005s08860.1), PtrWRKY19 (POPTR_0014s04890.1), PtrWRKY25 (POPTR_0007s06930.1), OsWRKY78 (BK005212), VvWRKY2 (AY596466), PtrWRKY3 (POPTR_0008s09140.1), PtrWRKY4 (POPTR_0017s12430.1), PtrWRKY68 (POPTR_0004s12000.1), AtWRKY3 (At2g03340), AtWRKY4 (AF425835).
Gene expression analysis
Poplar tissues were collected from 6-month-old stems, flash frozen in liquid nitrogen and stored in a −80 °C freezer. Total RNA was isolated from different tissues of poplar plants according to Zhong et al.14. The 2nd and 6th leaves from the apex of plants were defined as young and mature leaves, respectively. Different tissues of stems were collected from the 2nd to 7th internodes. The pith/xylem tissues were separated using microknives under a stereoscopic microscope. First-stand cDNAs were treated with DNase I (Promega, USA) and then used for quantitative real-time PCR (qRT-PCR) analysis. Real-time PCR was performed on a TP700 real-time PCR machine (TaKaRa, Japan) using the SYBR Green master mix reagent (TaKaRa, Dalian, China). Ptr18S rRNA was used as the reference gene for internal standardization of real-time PCR data. The PCR primers used for qRT-PCR were listed in Supplementary Table S1.
Subcellular localization
The full-length of PtrWRKY19 was fused in frame with GFP cDNA and ligated into pCX-DG23. The 35S-PtrWRKY:GFP fusion construct was introduced into onion epidermal cells by particle bombardment (GJ-1000, SCIENTZ, China). GFP fluorescent images were examined with a confocal microscope (Olympus FV500) at 18 h after bombardment. The onion skin was stained with DAPI, and then photographed by the confocal microscopy (Leica TCS SP5).
Transcriptional repression in yeast
To determine the transcription activity of PtrWRKY19, the GAL4BD/UAS/LacZ transcient assays were performed in yeast cells. Yeast GaL4BD expression vectors were obtained from Clontech. The cDNAz encoding PtrWRKY19 was amplified by PCR and cloned into EcoRI and BamHI sites of pGBKT7 vector. Yeas strains harboring UAS-LacZ reporter construct were grown to late logarithmic phase, harvest by centrifugation and resuspended in 200 μl of breaking buffer containing 100 mM potassium phosphate, 0.5 mM dithiothreitol, protease inhibitor mixture, 0.2% Triton X-100, pH 7.8. The β-galactosidase activity was determined at least three independent clones using detection kit Galacto-Light (Tropix, Bedford, MA).
Gene cloning and vector construction
The cDNA fragment encoding PtrWRKY19 was amplified in PCR reaction mixture of 50 μl, which contained 2.5 μl cDNA, 10 × PCR buffer, 0.3 mM dNTP, 2 units of pfu DNA polymerase (Takara, Dalian, China) and 0.5 μM of each primer. The reaction program consisted of 34 cycles of 94 °C for 45 s, 54 °C for 45 s and 72 °C for 90 s, followed by a final extension of 72 °C for 10 min. The PCR product was purified and cloned into the plant binary vector pCXSN23 and transformed into E. coli strain DH5á and sequenced by BGI (Beijing, China). Primers used for gene cloning are provided in Supplementary Table S1.
Genomic DNA was isolated from mature leaves of poplar plants using cetyl trimethylammonium bromide method. According to the sequences deposited in the Populus genome database, an about 1.5 kb of upstream sequence of PtrWRKY19 was cloned and inserted into plant binary vector pCXGUS-P23. The PCR fragments were verified by DNA sequencing analysis by BGI (Beijing, China) in both directions.
These plant binary constructs were transformed into Agrobacterium tumefaciens EHA105 by the freeze-thaw method24.
Transformation of Arabidopsis and P. tomentosa Carr
A. tumefaciens strain EHA105 containing the PtrWRKY19:GUS promoter construct was used to transform A. thaliana (Col-0) plants via the floral dip method25. Transgenic lines were selected on MS media containing 40 mg.l−1 hygromycin and grown in a growth chamber under long-day conditions (23–25 °C with the 8 h/16 h dark/light photoperiod).
Transgenic poplar plants were generated by Agrobacterium-mediated transformation as described previously26. Recombinant Agrobacterium cells were used to infect poplar leaf discs and putative transgenic plants were selected on woody plant medium (WPM)27 supplemented with 9 mg l−1 hygromycin. Rooted plantlets were acclimatized in pots at 25 °C °C in a 14/10 h light/dark cycle and then transferred to the greenhouse for further studies.
Transient expression in tobacco leaves
The promoter fragment of the poplar C4H2 gene was amplified by PCR with the gene-specific primers described in Supplementary Table S1. These fragments were individually fused to the GUS reporter gene in the pCXGUS-P vector to generate reporter constructs. Agrobacterium cells carrying 35S-PtrWRKY19 construct were used as an effector. Co-expression experiments were performed according to the method described by Zhong et al.14. Agrobacterium strains EHA105 containing the PtrWRKY19-GUS or 35S-PtrWRKY19 construct, were co-infiltrated in fully expanded leaves of tobacco plants (Nicotiana benthamiana) using a 1 ml syringe. After agroinfiltration, plants were covered with a transparent plastic cover and transferred into a growth chamber at 25 °C with 16/8 h light/dark cycle for 2–3 days. Quantitative GUS assays were carried out on the infiltrated zone using 4-methylumbelliferyl-b-D-glucuronide as substrate28. GUS activities were estimated as the mean of three independent assays.
Histochemical staining for GUS activity
Histochemical staining of GUS activity was performed according to the method described previously29. Tissues of transgenic poplar plants were immersed into GUS reaction buffer [1 mM X-Gluc (5-bromo-4-chloro-3-indolyl-β- d-glucuronide), 100 mM phosphate buffer pH 7.0, 0.1% Triton X-100, 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 10 mM EDTA and 20% methanol]. After 2–4 h incubation in dark at 37 °C, stained samples were bleached with absolute ethyl alcohol: acetic acid =7:3 (v/v) and then photographed by light microscopy (Olympus ZX16).
Tissue sections and microscopy analysis
Inflorescence stem samples of Arabidopsis and the 5th internode on the stems of 6-month-old poplar plants were fixed in FAA buffer (formaldehyde:glacialacetic acid:50% ethanol, 1:1:18), and then embedded in paraffin. Specimens were cut into 8-μm-thick sections (Thermo Finesse 325 rotary microtome) and stained with 0.05% (w/v) toluidine blue O or phloroglucinol-HCl and observed under Zeiss Axio Scope (Zeiss, Oberkochen, Germany).
Results
Isolation and characterization of PtrWRKY19
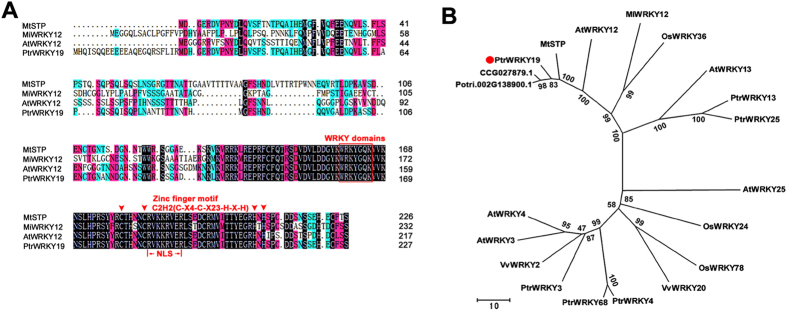
To isolate the orthologous gene of Arabidopsis AtWRKY12 from Populus trichocarpa, we used the AtWRKY12 amino acid sequence as a query sequence to blast in the P. trichocarpa genome database (http://www.phytozome.com/). A putative WRKY transcription factor, named PtrWRKY19 according to its structural feature30 was assembled in a contig and the full-length cDNA was amplified from total poplar leaf cDNA using the specific oligo-nucleotide primers (Supplementary Table S1). Sequence analysis showed that the cDNA sequence of PtrWRKY19 encodes a protein of 228 amino acid residues (Fig. 1A) with a predicted molecular mass of 26.4 kD and a calculated pI of 6.55. The deduced amino acid sequence of PtrWRKY19 contains a conserved WRKY domain at the C-terminal end along with a C2-H2 (C-X4-C-X23-H-X-H) type zinc-finger-like motif (Fig. 1A), which is the unique zinc ligands among WRKY domain belonging to group I and II31. In addition, a putative nuclear localization signal (NLS) was found in the C-terminal region of PtrWRKY19 protein (Fig. 1A).
Figure 1. Phylogenetic relationship of WRKY proteins.
(A) Multiple sequence alignment of PtrWRKY19 and other WRKY domain proteins. Identical amino acids are shaded in gray. The conserved WRKY domain and C2H2 zinc finger motif are marked by a line and triangles, respectively. (B) Phylogenetic tree of the deduced amino acid sequences of PtrWRKY19 and other WRKY proteins from Arabidopsis, rice, Medicago and grape. Phylogenetic analysis was performed by the neighbour-joining (NJ) method using MEGA version 5.0.4 Scale bar corresponds to 10 estimated amino acid substitutions per site.
A phylogenetic tree was constructed using the neighbor-joining method with the amino acid sequences of PtrWRKY19 and other plant WRKY proteins (Fig. 1B). PtrWRKY19 shared high similarity to CCG027879.1 (P. euphratica, 97.8%), Potri.002G138900.1 (P. trichocarpa, 89.8%), MtSTP (Medicag truncatula, 74.6%) and AtWRKY12 (Arabidopsis, 61.5%). Based on the classification methods reported previously30,31, PtrWRKY19 belongs to group IIc family (Fig. 1B), members of which contain only one WRKY domain and a C2-H2 type zinc-finger-like motif in the C-terminal.
Expression patterns of PtrWRKY19
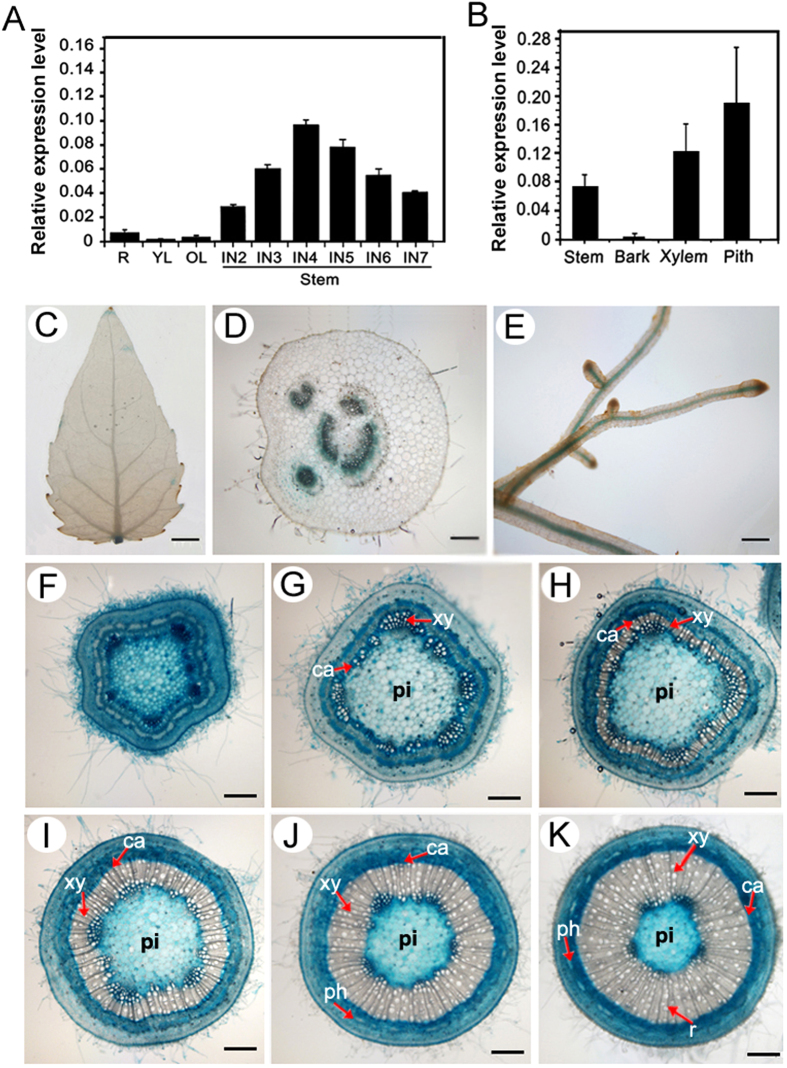
To determine the expression of PtrWRKY19 in different tissues of poplar plants, total RNA was isolated and mRNA was reversely transcribed into cDNA as a template for a real-time RT-PCR analysis. PtrWRKY19 was expressed in all the tissues examined, with highest expression in stems (Fig. 2A), especially in pith tissues (Fig. 2B). During internode development, PtrWRKY19 transcript level increased gradually with maturity and highest expression was found in the fourth internode (Fig. 2A).
Figure 2. Expression pattern of PtrWRKY19 in poplar.
Quantitative real-time (qRT) PCR analysis of PtrWRKY19 transcript levels in various organs (IN, internode) of poplar. R: roots; YL: young leaves (the second leaf); OL: old leaves (the sixth leaf from the apex). (B) qRT-PCR analysis of PtrWRKY19 expression in different tissues of the stems at the 6th internode. Poplar 18S was used as an internal control. Bars represent ± SD. The PtrWRKY19 gene promoter-driven GUS expression vector was engendered and introduced into P. tomentosa Carr. Gus staining pattern of 4-week-old transgenic plants: (C) leaves; (D) petioles; (E) roots; (F–K) transverse sections of the stem at the different internodes (from 2nd to 7th internode). Scale bar: C = 0.5 cm; D, E = 2.5 mm; F-K = 200 μm. Cambial zone (Ca), Phloem (ph), Pith (pi), Ray (r), Xylem (Xy).
In order to further investigate the tissue-specific expression pattern of PtrWRKY19, a 1.5 kb promoter fragment of PtrWRKY19 was fused to a GUS reporter gene, and the recombinant gene was introduced into P. tomentosa Carr. Histochemical GUS staining showed weak GUS activity in leaves (Fig. 2C) and strong staining reactions in all vascular tissues of petioles and roots (Fig. 2D,E). In young stem tissues (2nd and 3rd internodes), strong GUS activity was observed in all tissues including sclerenchyma and parenchyma cells (Fig. 2F,G). In older stem internodes (from 4th to 7th), a strong GUS expression was detected in the phloem, cambium, developing xylem and stem pith cells, whereas mature xylem showed a faint GUS staining (Fig. 2H–K). In addition, the GUS gene under the control of the PtrWRKY19 promoter was transformed into A. thaliana. GUS activity was detected in all vascular tissues of transgenic plants, including veins, roots, tricomes, inflorescence stems and flowers (Supplementary Figure S1). These results indicate that PtrWRKY19 might be involved in SCW formation in stem tissues, especially in pith parenchyma cells.
PtrWRKY19 is localized in the nucleus and functions as a transcriptional repressor
The nuclear localization signal (NLS) prediction by WoLF PSORT software (http://wolfpsort.org/) showed that PtrWRKY19 contains a NLS sequence (RVKKRVER)32 (Fig. 1A). To determine whether PtrWRKY19 functioned as a transcription factor, we first analyzed its subcellular localization. PtrWRKY19 was fused to green fluorescent protein (GFP) under the control of the cauliflower mosaic virus (CaMV) 35S promoter. The fusion construct of 35S-PtrWRKY19::GFP and the control vector 35S-GFP were introduced into onion epidermal cells by particle bombardment. The green fluorescence of PtrWRKY19::GFP recombinant protein was exclusively detected in the nucleus of onion cells (Fig. 3A), suggesting that PtrWRKY19 localizes in the nucleus. In contrast, the 35S-GFP protein occurred throughout the cells including cytoplasm and the nucleus (Fig. 3A).
Figure 3. Subcellular localization and transactivation assays of PtrWRKY19.
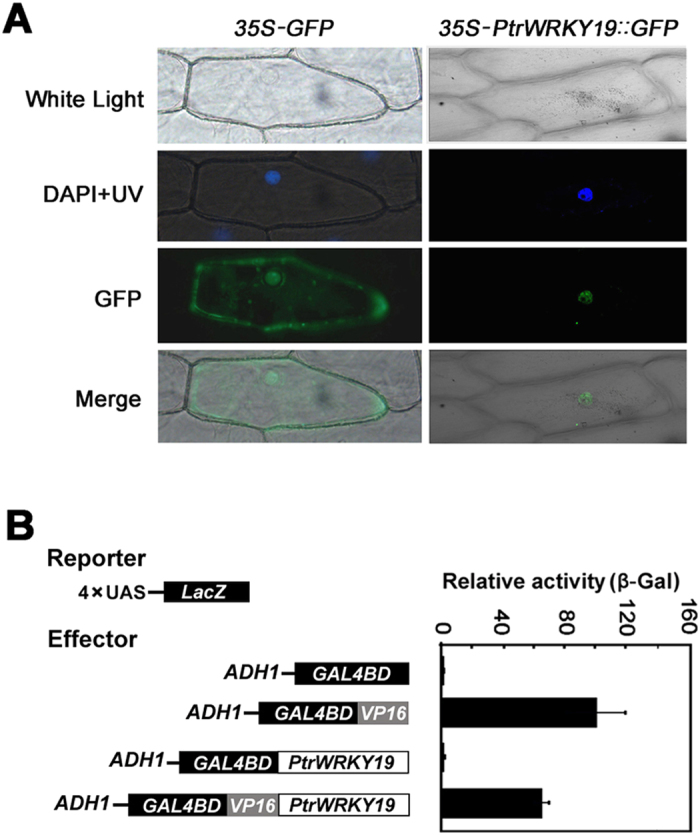
Onion epidermis was transformed with 35S-PtrWRKY19::GFP and 35S-GFP constructs by particle bombardment. The position of nucleus was ensured by DAPI staining and bright-field images were compared. In this experiment, 35S-GFP was used as control. (B) Transcriptional activation analysis of PtrWRKY19 analyzed by the chimeric reporter/effector assay in yeast on the plates with solid SD medium. Data represent mean of three biological repeats ± SD. GAL4DB null vector was used as a negative control and GAL4DB fused with VP16 was used a positive control.
To evaluate the transcriptional activity of PtrWRKY19 protein, the complete PtrWRKY19 was fused with GAL4:VP16 (Herpes simplex virus activation domain) protein. The activity of PtrWRKY19 as a transcription factor was determined by co-transfection of effector and various reporter constructs into yeast cells and measurement of reporter enzyme activity (Fig. 3B). The relative activity of reporter β-galactosidase in the existence of GAL4DB:VP16:PtrWRKY19 was distinctly reduced compared with that of control (the presence of GAL4DB:VP16 effector) (Fig. 3B), indicating that PtrWRKY19 is a transcriptional repressor.
PtrWRKY19 can complement the Arabidopsis wrky12 mutant
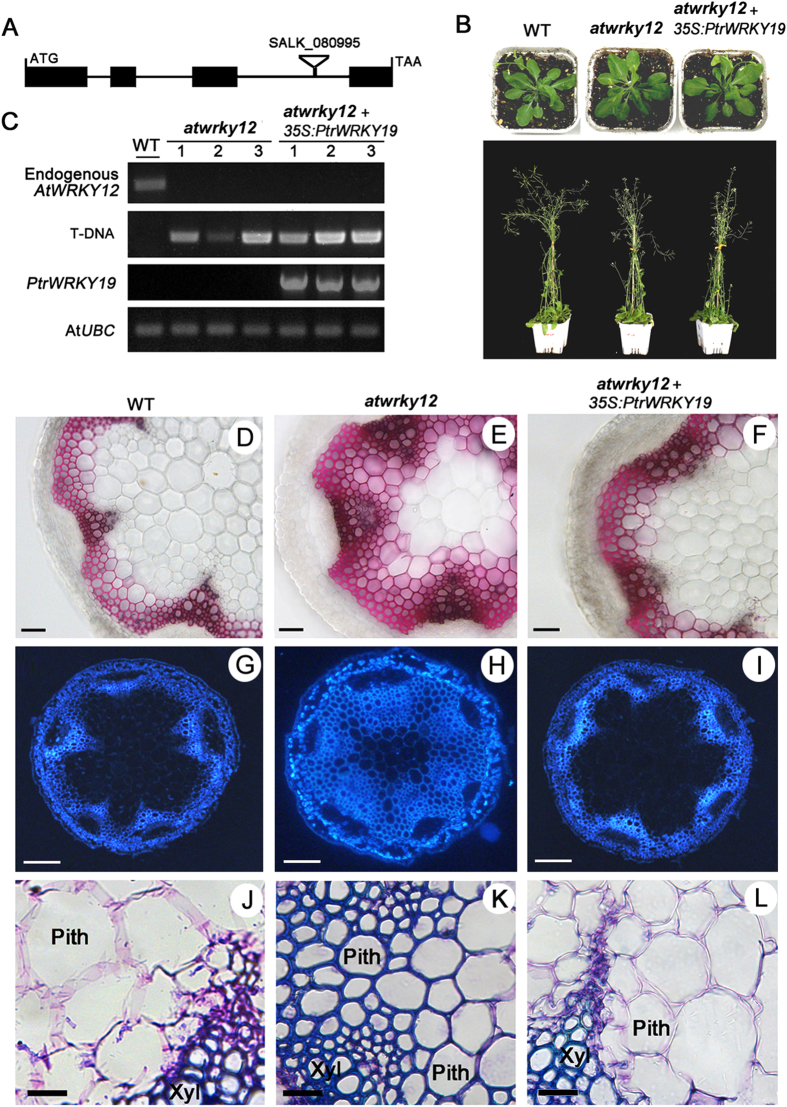
A previous study has demonstrated that loss-of-function of AtWRKY12 in Arabidopsis or its ortholog in M. truncatula results in SCW thickening in pith cells involved in ectopic deposition of lignin, xylan, and cellulose19. To confirm whether PtrWRKY19 is a functional ortholog of AtWRKY12 in poplar, the PtrWRKY19 gene under the control of the CaMV 35S promoter was transformed into homozygous AtWRKY12 mutant plants (SALK_080995), atwrky12 (Fig. 4A). More than twenty independent transgenic lines were obtained. Most of them showed a phenotype that resembled wild-type plants (Fig. 4B) and only a few (3 lines) displayed retarded growth (data not shown). RT-PCR analysis further confirmed the expression of PtrWRKY19 in atwrky12 mutant and transgenic plants (Fig. 4C). Determination of dry weights showed that a significant increase in biomass density was found in atwrky12 mutant lines compared to the wild-type (WT), whereas no obvious reduction did seem to occur in transgenic plants overexpressing PtrWRKY19 (Supplementary Figure S2).
Figure 4. Complementation of the Arabidopsis wrky12 mutant with PtrWRKY19.
(A) AtWRKY12 gene structure and T-DNA insertion site. (B) Visible phenotypes of WT, wrky12 mutant and wrky12 mutant transformed with 35S-PtrWRKY19 gene. (C) Expression levels of AtWRKY12 and PtrWRKY19 in WT, wrky12 mutant and transgenic plants by PCR analysis. AtUBC gene was used as the control. (D–F) Phloroglucinol staining of the stems from WT, wrky12 mutant and wrky12 mutant complemented with 35S-PtrWRKY19 gene, respectively. (G–I) UV autofluorescence of cross-sections of the stems from WT, wrky12 mutant and wrky12 mutant complemented with 35S-PtrWRKY19 gene, respectively. (J–L) Light microscopy of pith cell walls by toluidine bule staining. Xyl, Xylem. Scale bar represent 50 μm (D–F); 100 μm (G–I); 20 μm (J–L).
Moreover, stem sections of WT, atwrky12, and atwrky12+35S-PtrWRKY19 were treated with phloroglucinol-HCl. The red color showed that ectopic composition of lignin was detected in the pith parenchyma cells of the atwrky12 mutant (Fig. 4E). In contrast, no staining signal was found in the pith cells of WT and atwrky12+35S-PtrWRKY19 plants (Fig. 4D,F). The lignin pattern of stem sections was visualized by UV autofluorescence. Strong signals for lignin were observed in the xylem and pith cells of the atwrky12 mutant (Fig. 4H), whereas lignin autofluorescence was only detected in the xylem cells of WT and atwrky12+35S-PtrWRKY19 lines (Fig. 4G,I). In addition, toluidine blue staining also showed that the lignified pith cell walls in the mutant were obviously thicker than in the WT and atwrky12+35S-PtrWRKY19 plants (Fig. 4J–L). These results showed that PtrWRKY19 is an ortholog of AtWRKY12 in poplar and functions as a negative regulator of SCW development in pith cells.
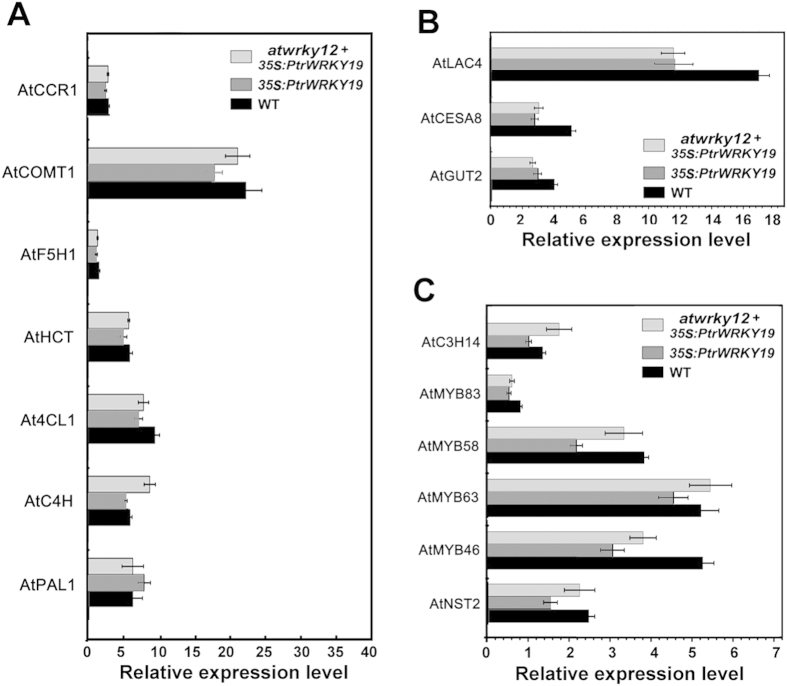
To elucidate the transcriptional regulatory mechanism of PtrWRKY19 underlying the control of SCW development of pith cells, the expression levels of genes involved in SCW biosynthesis in WT, 35S-PtrWRKY19 and atwrky12+35S-PtrWRKY19 plants were determined. As shown in Fig. 5A,B, overexpression of PtrWRKY19 in wild-type and atwrky12 background did not seem to repress the expression of lignin biosysnthetic genes (AtCCR1, AtCOMT1, AtF5H1, AtHCT, At4CL1, AtC4H), but down-reglated the expression of xylan biosynthetic gene (AtGUT2), laccase gene (AtLAC4) and cellulose synthase gene (AtCES8), compared to the wild-type plants. To investigate whether overexpression of PtrWRKY19 regulated expression of the transcription factor genes involved in SCW formation, transcript levels of some NAC and MYB genes in transgenic plants were tested by qRT-PCR. The results revealed that expression of these transcriptional regulators was repressed in 35S-PtrWRKY19 plants compared to the wild-type control (Fig. 5C). But in atwrky12 background, overexpression of PtrWRKY19 did not result in decreased expression of these genes.
Figure 5. Transcript levels of SCW biosynthesis-related genes in WT, 35S-PtrWRKY19 and wrky12 mutant complemented with 35S-PtrWRKY19 gene.
Transcript levels of structural genes in lignin biosynthesis pathway. COMT, Caffeic acid O-methyltransferase; PAL, phenylalanine ammonialyase; HCT, hydroxycinnamoyl transferase; F3H, Flavanone 3-hydroxylase. HCT, p-hydro-xycinnamoyl-CoA; C4H, cinnamate 4-hydroxylase; CCR, cinnamoyl-CoA reductase; 4CL, 4-coumarate:CoA-ligase; CAD, cinnamyl alcohol dehydrogenase. (B) Expression levels of cellulose, xylan biosynthetic genes. LAC, Laccase; CesA, cellulose synthase; GUT, glucuronosyl transferase. (C) Transcript levels of SCW regulatory genes. The AtUBC gene was used as the internal reference. Data points shown are means from three biological replicates (individual lines). Each bar indicates ± SD of triplicate experiments.
Overexpression of PtrWRKY19 in P. tomentosa Carr
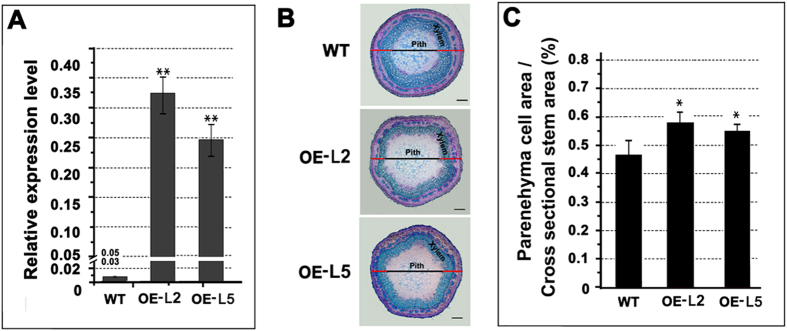
To further investigate whether PtrWRKY19 is involved in the regulation of SCW development in pith cells of poplar, the 35S-PtrWRKY19 fusion gene was introduced into Chinese white poplar (P. tomentosa Carr.) A. tumefaciens-mediated transformation. More than ten hygromycin-resistant putative transformants were obtained and PCR analysis was used to confirm the integration of the transgenes in transformed plants (Supplementary Figure S3). Expression of PtrWRKY19 in transgenic plants were determined by qRT-PCR and these lines (L2 and L5) with significantly high transcript level of PtrWRKY19 were chosen for further analysis (Fig. 6A). No phenotypic changes were observed in all generated transgenic plants compared with the wild type (Supplementary Figure S4). Histological analyses of xylem were performed using the natural autofluorescence of phenolic compounds under UV-light. Less lignin autofluorescence was detected in cross-sections of 35S-PtrWRKY19 lines as compared with that of the control (Supplementary Figure S5). Toluidine blue staining showed that although stem cross-sectional area of transgenic 35S-PtrWRKY19 plants was similar to that of the wild type, the cross-sectional area of parenchyma cells in pith significantly increase in transgenic 35S-PtrWRKY19 plants (Fig. 6B,C), indicating that secondary wall thickening was repressed in xylem of transgenic plants overexpressing PtrWRKY19 compared to wild-type plants.
Figure 6. Effect of PtrWRKY19 overexpression in transgenic poplars.
(A) Quantitative RT-PCR analysis of PtrWRKY19 in the wild type and two independent 35S-PtrWRKY19 lines. Poplar 18S was used as an internal control. (B) Transverse sections of the stems from the wild type and transgenic plants. (C) Parenehyma cell area measurements of the stems. Values are means ±SD for n = 4. Asterisks above the bars indicate values determined by Student’s t test to be significantly different from control (**P < 0.01).
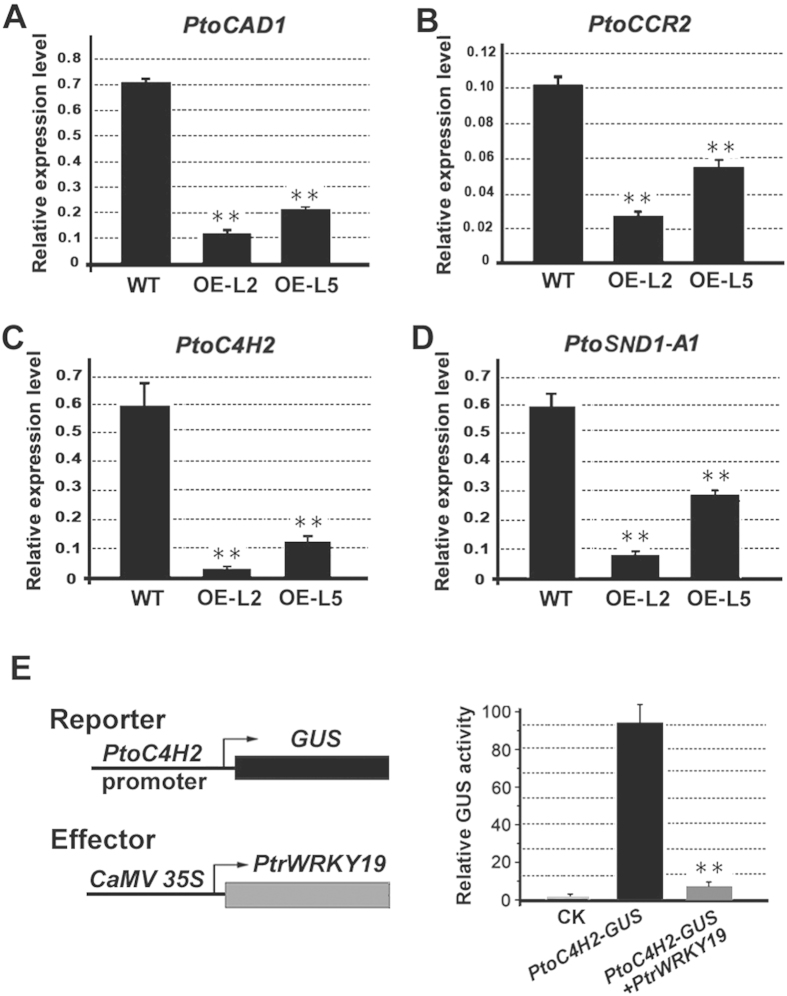
To test the prediction that PtrWRKY19 is involved in pith SCW formation in poplar, the expression of lignin biosynthetic genes in transgenic 35S-PtrWRKY19 plants was examined by qRT-PCR. Reduced transcript levels were observed for the PtoCAD1, PtoCCR2 and PtoC4H2 genes (Fig. 7A–C), suggesting an effect on the lignin biosynthesis genes. In previous reports, WRKY12 and its orthologs negatively regulate the expression of NST2, which acts as master regulators play a critical role in switching on and off secondary wall biosynthesis33, and represses cell wall biosynthetic genes in pith cells19,20. We also determined the level of PtoSND1-A1 encoding NAC secondary wall thickening pro-moting factor in poplar and found that PtrWRKY19 overexpression reduced significantly (P < 0.01) transcript level of PtoSND1-A1 (Fig. 7D).
Figure 7. PtrWRKY19 protein represses the expression of lignin pathway genes and SCW-related genes in transgenic plants.
(A–D) Quantitative RT-PCR was used to analysis expression levels of PtoCAD1, PtoCCR2, PtoC4H2 and PtoSND1-A1, respectively. (E) PtrWRKY19 binds to the PtoC4H2 promoter. Schematic of the PtoC4H2-GUS reporter (top) and PtrWRKY19::GFP effectors (bottom). Transcription level of the PtoC4H2 promoter reporter by PtrWRKY19 was assessed by GUS activity. PtoC4H2-GFP was used as a control. Error bars represent ±SD from 3 independent experiments. **P < 0.01, Student’s t test.
Previous studies showed that WRKY transcription factors specifically bind to W-box elements in their target gene promoters34. To determine the relationship between PtrWRKY19 and its target gene promoters, the PtoC4H2 promoter with at least two predicted W-box elements (Supplementary Figure S6) were transiently expression in tobacco leaves using a chimeric reporter/effector assay. In this assay, the effector construct containing PtrWRKY19 driven by the CaMV 35S promoter was cotransformed with the reporter construct harboring the β-glucuronidase (GUS) reporter gene driven by the full-length PtoC4H2 promoter (Fig. 7E). Compared with the GUS activity in tobacco cells carrying the PtoC4H2-GUS gene, co-expression of the 35S-PtrWRKY19 and PtoC4H2-GUS gene resulted in significantly reduced (P < 0.01) by approximately 12-fold (Fig. 7E), suggesting that PtrWRKY19 acts as a negative regulator of SCW formation in pith cells in poplar.
Discussion
In vascular plant, stems provide mechanical strength to support the plant’s weight, and conduct the transport of water, hormones and minerals. The stem has three simple cell types: the parenchyma, collenchyma, and sclerenchymacells. Parenchyma cells have thin primary walls and occur in pith, cortex and epidermis. In contrast, sclerenchyma cells, such as xylem and phloem, have thick lignified secondary walls35. In dicotyledonous plants, the pith parenchyma cells, which are encircled by a ring of xylem with secondary walls, have only primary cell walls, indicating that there exists a conserved mechanism for inhibiting secondary growth in pith cells. In this study, we have identified a transcriptional regulator PtrWRKY19 from poplar, the amino acid sequence of which shows homology to the conserved WRKY domain of various WRKY transcription factors in responsible for the parenchymatous nature of the pith cells (Fig. 1A). The heterologous expression of PtrWRKY19 in atwrky12 mutant plants successfully complemented the phenotype caused by the loss-of-function of AtWRKY12 (Fig. 4), which is a negative regulator of SCW formation in pith parenchyma19. Therefore, these results suggested that PtrWRKY19 is involved in negatively regulating secondary wall development in pith tissues of poplar.
The WRKY family proteins are well known for their involvement in diverse physiological and developmental processes, especially in the regulation of plant stress tolerance31,36,37. Recently, it has been demonstrated that WRKY transcription factors also play important regulatory roles in regulating the biosynthesis of many phenolic compounds, such as flavonoids38. For example, the Arabidopsis WRKY transcription factor TTG2 has been reported to be involved in condensed tannins and mucilage production in the seed coat, in a TTG1-dependent way39. A novel function of the WRKY transcription factors is to inhibit pith secondary wall formation19,20. The Arabidopsis AtWRKY12 gene was predominantly expressed in pith cells and atwrky12 loss-of-function mutants showed induced secondary wall formation of pith cells19. Similarly, in monocotyledonous species M. lutarioriparius, MlWRKY12 was expressed in vascular bundle sheath, sclerenchyma and parenchyma tissues and heterologous expression experiments showed that MlWRKY12 can complement Arabidopsis atwrky12 mutant20. In grapevine, VvWRKY2 was specifically expressed in cells undergoing ligification in young stems. Transgenic tobacco plants over-expressing VvWRKY2 exhibited altered expression of genes involved in lignin biosynthesis pathway and cell wall formation40. In this present reports, poplar PtrWRKY19 exhibited typical features of WRKY transcription factors (Fig. 1A) and was highly expressed in stems, especially in pith cells (Fig. 2B). GUS activity analysis revealed that PtrWRKY19 was also expressed in vascular tissues of roots and petioles (Fig. 2D,E), inconsistent with that of AtWRKY12 which specifically expressed in pith and cortex cells of stems but not in roots19. When PtrWRKY19 was constitutively expressed in Arabidopsis and wrky12 mutant plants, transgenic plants exhibited normal phenotypes compared with the wild type (Fig. 4B). Quantitative RT-PCR analysis indicated that s few SCW biosynthetic genes were down-regulated in transgenic plants overexpressing PtrWRKY19, suggesting that PtrWRKY19 is a negative factor for SCW formation in pith cells.
WRKY proteins can act as transcriptional activators or repressors41. Among the 4 characterized WRKY proteins involved in regulating SCW formation in pith cells, Arabidopsis AtWRKY12, Miscanthus MlWRKY12 and M. truncatula MtSTP are transcriptional repressors19,20, whereas only AtWRKY13 shows transactivation activity42. Interestingly, all of these proteins belong to group II WRKYs, which contain one WRKY motif and one C2H2-type zinc-finger motif (Fig. 1). PtrWRKY19, an ortholog of AtWRKY12, had similar function in SCW formation in pith cells in poplar. In vivo assay showed that PtrWRKY19 had transcriptional repressor activity in yeast cells (Fig. 3). In addition, a putative WRKY transcription factor Potri.002G138900.1, which shares high similarity to PtrWRKY19 (Fig. 1B), was found in the poplar genome. However, it remains unclear whether there exist other homologs of PtrWRKY19 in the genome.
Almost all WRKY transcription factors bind preferentially to W-box elements containing a “TGAC” core sequence in their target gene promoters31. In Arabidopsis, direct binding of AtWRKY12 to the NST2 promoter and repression of NST2 expression by WRKY12 were confirmed by EMSA and in planta transgenic experiments19. Similar results were obtained in transgenic 35S:AtWRKY13 plants by performed in vivo chromatin immunoprecipitation (ChIP) assays18. In transgenic poplar overexpressing PtrWRKY19, the expression levels of lignin biosynthetic genes and NAC transcription factor were reduced (Fig. 7A–D). Transient expression assays showed that PtoC4H2 with several W-box elements was s downstream target of PtrWRKY19 and its expression was repressed by PtrWRKY19 (Fig. 7E). These results indicated that there exists a conserved mechanism in regulating SCW formation in pith cells in herbaceous and woody plants.
In A. thaliana and M. truncatula, loss of WRKY expression in mutants resulted in derepression of NAC and MYB46 transcription factors and this activation turned on SCW formation in pith cells in which parenchyma cells normally have only thin primary walls19. The secondary thickening of pith cells in Araibdopsis wrky12 mutant results in an approximately 50% increase in biomass density in stem tissue19. In this study, interestingly, secondary wall thickening in xylem of transgenic poplar plants overexpressing PtrWRKY19 appeared to be repressed and led to an significant increase in cross-sectional area of pith parenchyma cells compared to wild-type plants (Fig. 6B,C). In addition, we also found that in transgenic 35S:PtrWRKY19 plants, more parenchyma cells in pith resulted in lower dry weight than the wild-type (Data not shown). Further studies will investigate whether knock-down or silencing of PtrWRKY19 will result in an increase in biomass produce of transgenic poplar plants.
Additional Information
How to cite this article: Yang, L. et al. PtrWRKY19, a novel WRKY transcription factor, contributes to the regulation of pith secondary wall formation in Populus trichocarpa. Sci. Rep. 6, 18643; doi: 10.1038/srep18643 (2016).
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31300990, 31370672, 31171620), the Natural Science Foundation Project of CQ (CSTC2013JJB8007), the Fundamental Research Funds for the Central Universities (XDJK2014a005) and the “One Hundred Talents Program” of the Chinese Academy of Sciences.
Footnotes
Author Contributions L.Y. and K.M.L. designed the research. Y.L., X.Z., F.Y. and D.F. performed gene cloning, vector construction, transgenic plants generation, and drafted the manuscript. D.F. and F.Y. performed phenotype analysis and qRT-PCR. L.Y. and K.M.L. modified the manuscript. All authors reviewed the manuscript.
References
- Carpita N. C. & Gibeaut D. M. Structural models of primary-cell walls in flowering plants-consistency of molecular-structure with the physical-properties of the walls during growth. Plant J 3, 1–30 (1993). [DOI] [PubMed] [Google Scholar]
- Zhong R. & Ye Z. H. Regulation of cell wall biosynthesis. Curr Opin Plant Biol 10, 564–572 (2007). [DOI] [PubMed] [Google Scholar]
- Geitmann A. Mechanical modeling and structural analysis of the primary plant cell wall. Curr Opin Plant Biol 13, 693–699 (2010). [DOI] [PubMed] [Google Scholar]
- Hamant O. & Traas J. The mechanics behind plant development. New Phytol 185, 369–385 (2010). [DOI] [PubMed] [Google Scholar]
- Lee K. J. D., Marcus S. E. & Knox J. P. Cell wall biology: perspectives from cell wall imaging. Mol Plant 4, 212–219 (2011). [DOI] [PubMed] [Google Scholar]
- Wang H. Z. & Dixon R. A. On-off switches for secondary cell wall biosynthesis. Mol Plant 5, 297–303 (2012). [DOI] [PubMed] [Google Scholar]
- Pauly M. & Keegstra K. Plant cell wall polymers as precursors for biofuels. Curr Opin Plant Biol 13, 305–312 (2010). [DOI] [PubMed] [Google Scholar]
- Zhong R., Richardson E. A. & Ye Z. H. The MYB46 Transcription factor is a direct target of SND1 and regulates secondary wall biosynthesis in Arabidopsis. Plant Cell 19, 2776–2792 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R. & Ye Z. H. Complexity of the transcriptional network controlling secondary wall biosynthesis. Plant Sci 229, 193–207 (2014). [DOI] [PubMed] [Google Scholar]
- Zhong R., Lee C., Zhou J., McCarthy R. L. & Ye Z. H. A battery of transcription factors involved in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell 20, 2763–2782 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzlaff A. et al. Characterisation of a pine MYB that regulates lignification. Plant J 36, 743–754 (2003). [DOI] [PubMed] [Google Scholar]
- Goicoechea M. et al. EgMYB2, a new transcriptional activator from Eucalyptus xylem, regulates secondary cell wall formation and lignin biosynthesis. Plang J 43, 553–567 (2005). [DOI] [PubMed] [Google Scholar]
- McCarthy R. L. et al. The Poplar MYB Transcription factors, PtrMYB3 and PtrMYB20, are involved in the regulation of secondary wall biosynthesis. Plant Cell Physiol 51, 1084–1090 (2010). [DOI] [PubMed] [Google Scholar]
- Zhong R., McCarthy R. L., Lee C. & Ye Z.-H. Dissection of the transcriptional program regulating secondary wall biosynthesis during wood formation in poplar. Plant Physiol 157, 1452–1468 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner-Lange S. et al. Disruption of Arabidopsis thaliana MYB26 results in male sterility due to non-dehiscent anthers. Plant J 34, 519–528 (2003). [DOI] [PubMed] [Google Scholar]
- Mitsuda N., Seki M., Shinozaki K. & Ohme-Takagi M. The NAC transcription factors NST1 and NST2 of Arabidopsis regulate secondary wall thickenings and are required for anther dehiscence. Plant Cell 17, 2993–3006 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. et al. Arabidopsis MYB26/MALE STERILE35 regulates secondary thickening in the endothecium and is essential for anther dehiscence. Plant Cell 19, 534–548 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Tian Z. & Yu D. WRKY13 acts in stem development in Arabidopsis thaliana. Plant Sci 236, 205–213 (2015). [DOI] [PubMed] [Google Scholar]
- Wang H. et al. Mutation of WRKY transcription factors initiates pith secondary wall formation and increases stem biomass in dicotyledonous plants. Proc Natl Acad Sci USA 107, 22338–22343 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. et al. MlWRKY12, a novel Miscanthus transcription factor, participates in pith secondary cell wall formation and promotes flowering. Plant Sci 212, 1–9 (2013). [DOI] [PubMed] [Google Scholar]
- Zhao G. et al. Molecular evolution of the H6 subtype influenza a viruses from poultry in eastern China from 2002 to 2010. Virology J 8, 470 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P. et al. WoLF PSORT: protein localization predictor. Nucleic Acids Res 35, W585–587 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Songkumarn P., Liu J. & Wang G. L. A versatile zero background T-vector system for gene cloning and functional genomics. Plant Physiol 150, 1111–1121 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofgen R. & Willmitzer L. Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res 16, 9877–9877 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Henriques R., Lin S.-S., Niu Q.-W. & Chua N.-H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoco 1, 641–646 (2006). [DOI] [PubMed] [Google Scholar]
- Jia Z., Sun Y., Yuan L., Tian Q. & Luo K. The chitinase gene (Bbchit1) from Beauveria bassiana enhances resistance to Cytospora chrysosperma In Populus tomentosa Carr. Biotechnol Lett 32, 1325–1332 (2010). [DOI] [PubMed] [Google Scholar]
- Lloyd G. & McCown B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb Proc Intl Plant Prop Soc 30, 421–427 (1980). [Google Scholar]
- Jefferson R. A., Kavanagh T. A. & Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6, 3901–3907 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A. & Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6, 3901 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y. et al. Genome-wide identification and characterization of the Populus WRKY transcription factor family and analysis of their expression in response to biotic and abiotic stresses. J Exp Bot 65, 6629–6644 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T., Rushton P. J., Robatzek S. & Somssich I. E. The WRKY superfamily of plant transcription factors. Trends Plant Sci 5, 199–206 (2000). [DOI] [PubMed] [Google Scholar]
- Lan A. et al. A salicylic acid-induced rice (Oryza sativa L.) transcription factor OsWRKY77 is involved in disease resistance of Arabidopsis thaliana. Plant Biol (Stuttg) 15, 452–461 (2013). [DOI] [PubMed] [Google Scholar]
- Zhong R., Lee C., Zhou J., McCarthy R. L. & Ye Z.-H. A battery of transcription factors involved in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell 20, 2763–2782 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulker B. & Somssich I. E. WRKY transcription factors: from DNA binding towards biological function. Curr Opin Plant Biol 7, 491–498 (2004). [DOI] [PubMed] [Google Scholar]
- Demura T. & Fukuda H. Transcriptional regulation in wood formation. Trends Plant Sci 12, 64–70 (2007). [DOI] [PubMed] [Google Scholar]
- Chen L. et al. The role of WRKY transcription factors in plant abiotic stresses. Biochim Biophys Acta 1819, 120–128 (2012). [DOI] [PubMed] [Google Scholar]
- Bakshi M. & Oelmuller R. WRKY transcription factors: Jack of many trades in plants. Plant Signal Behav 9, e27700 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naoumkina M. A., He X. & Dixon R. A. Elicitor-induced transcription factors for metabolic reprogramming of secondary metabolism in Medicago truncatula. BMC Plant Biol 8, 132 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. S., Kolevski B. & Smyth D. R. TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. Plant Cell 14, 1359–1375 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaumie S. et al. The grapevine transcription factor WRKY2 influences the lignin pathway and xylem development in tobacco. Plant Mol Biol 72, 215–234 (2010). [DOI] [PubMed] [Google Scholar]
- Pandey S. P. & Somssich I. E. The role of WRKY transcription factors in plant immunity. Plant Physiol 150, 1648–1655 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Tian Z. X. & Yu D. Q. WRKY13 acts in stem development in Arabidopsis thaliana. Plant Sci 236, 205–213 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.