Abstract
Arbuscular mycorrhizal fungi (AMF) are critical links in plant–soil continuum and play a critical role in soil carbon cycles. Soil respiration, one of the largest carbon fluxes in global carbon cycle, is sensitive to precipitation change in semiarid ecosystems. In this study, a field experiment with fungicide application and water addition was conducted during 2010–2013 in a semiarid steppe in Inner Mongolia, China, and soil respiration was continuously measured to investigate the influences of AMF on soil respiration under different precipitation regimes. Results showed that soil respiration was promoted by water addition treatment especially during drought seasons, which induced a nonlinear response of soil respiration to precipitation change. Fungicide application suppressed AMF root colonization without impacts on soil microbes. AMF suppression treatment accelerated soil respiration with 2.7, 28.5 and 37.6 g C m−2 across three seasons, which were mainly caused by the enhanced heterotrophic component. A steeper response of soil respiration rate to precipitation was found under fungicide application treatments, suggesting a greater dampening effect of AMF on soil carbon release as water availability increased. Our study highlighted the importance of AMF on soil carbon stabilization and sequestration in semiarid steppe ecosystems especially during wet seasons.
Plant roots may host many fungal species including mycorrhizal fungi, endophytes and pathogens1. More than 80% of terrestrial plant species form associations with arbuscular mycorrhizal fungi (AMF) as the symbiotic mycorrhiza2, which provide critical links in the plant–soil continuum3. It has been reported that AMF play important roles in supplying nutrients to host plants4,5 as well as determining and maintaining plant community structure and diversity6,7.
The impacts of AMF on soil carbon cycling are also receiving more and more attentions recently. Firstly, AMF are important components of soil microbial community and contribute to soil respiration. It has been reported that these fungi and their extraradical mycorrhizal hyphae (EMH) constitute as much as 20~30% of soil microbial biomass8; and their contribution to total soil respiration ranges from 6% to 25%9,10,11. Secondly, EMH can transfer carbon away from the rhizosphere soil with high respiratory activity, because they consist of a large and complex hyphae network in the soil. This property facilitates soil carbon sequestration12,13. Thirdly, AMF are important sources and components of the soil organic carbon (SOC) pool12,14. The fast turnover rate of their EMH leaves large amount of chitin15 and glomalin-related soil protein16 in the soil, both of which are resistant to decomposition17,18,19. Lastly, by entangling, enmeshing and binding, fungal hyphae facilitate the formation and stabilization of soil aggregates, especially macro-aggregates20,21,22,23,24, which could keep SOC from decomposition by other microbial communities and thus enhance carbon storage in soil17,25,26. Overall, AMF are one of the vital contributors to autotrophic soil respiration by consuming carbohydrates from plants10,11. On the other hand, they may also suppress soil carbon release and promote soil carbon accumulation by protecting SOC in macro-aggregates from the activity of soil microbes3,17. Until now, the balance between these opposite effects of the fungi on soil carbon sequestration and soil respiration is still unclear25.
Soil respiration, which consists of autotrophic and heterotrophic parts27,28, is the largest carbon fluxes from soil29. Generally, soil respiration is correlated positively to the mean annual temperature without consideration of the ecosystem and biome30,31. In a single ecosystem, the inter-annual variation of soil respiration is mainly regulated by precipitation change especially in arid and semiarid ecosystems31,32. The significant influences of precipitation change on soil respiration have been confirmed in many field manipulation experiments33,34. Precipitation increase could promote soil respiration by improving plant growth34 and microbial activity34,35. However, the impacts of AMF on precipitation-regulated-soil respiration are still unknown.
Semiarid steppes are the most widely spread grassland ecosystems in north China. They contribute 15.8% of the world carbon storage and more than 90% of them were stored in the soil36. In addition, more than 80% of the plant species form symbiotic mycorrhiza with AMF in this region37,38. Thus, the ecosystem provides us a perfect platform to study the roles of AMF on soil carbon release, which are important in evaluating soil carbon balance under different precipitation regimes. In this study, a field manipulative experiment with water addition and fungicide application treatments was conducted, and soil respiration rate during three growing seasons was measured continuously to evaluate the influences of AMF on soil respiration and its response to precipitation change.
Results
AMF suppression
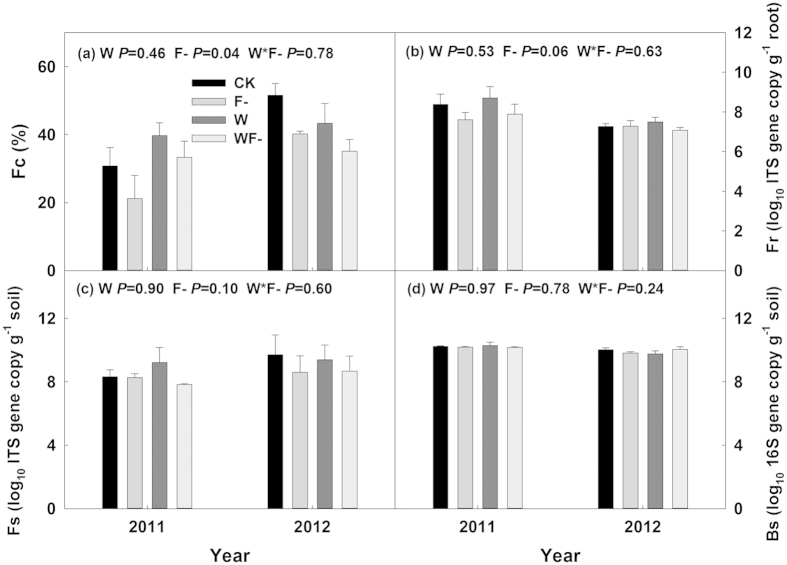
Fungicide application significantly reduced AMF root colonization by 15% (Fc; P = 0.04, Fig. 1a) and fungal concentration in roots by 6% (Fr; P = 0.06, Fig. 1b), but did not affect concentrations of fungi (Fs) and bacteria (Bs) in the soil (both P > 0.1, Fig. 1c,d). No significant water addition effect and its interaction with fungicide application were found on Fc, Fr, Fs and Bs (all P > 0.1, Fig. 1a–d).
Figure 1.
AMF root colonization (a, Fc), fungi in root (b, Fr), fungi (c, Fs) and bacteria (d, Bs) in soil under the four treatments. Results (P-values) of generalized linear mixed models (GLMM) were presented in each subplot. CK, control; F-, fungicide application; W, water addition; WF-, water addition plus fungicide application.
Precipitation patterns, soil moisture and temperature
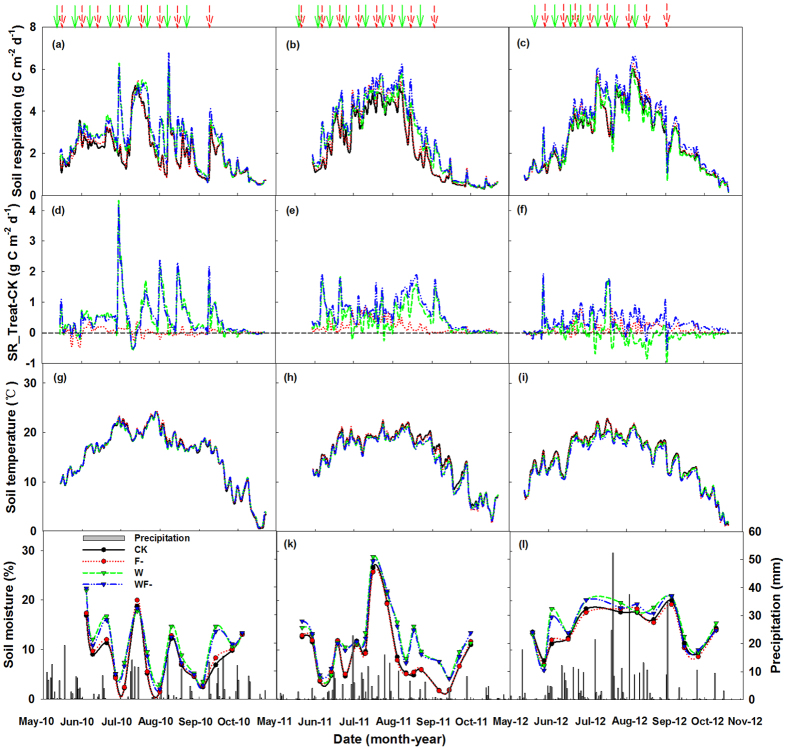
Precipitation during the growing seasons (May to Oct) was 295 mm in 2010, 225 mm in 2011 and 434 mm in 2012 (Fig. 2j–l). Seasonal precipitation of 2010 was similar to long-term (1980–2012) average precipitation (298 mm). However, the uneven distribution of rainfall with more than 60% of the precipitation occurring at the beginning (May) and the end (September and October) of the growing season resulted in severe water deficit during peak season (June to August) (Fig. 2j). Seasonal precipitation of 2011 was 24.5% lower than long-term average; and about 81% of the precipitation occurred during the peak season (Fig. 2k), which resulted in greater soil water availability than that of 2010. Seasonal distribution of wet year 2012 was similar to 2011 with 71% precipitation occurring during the peak season (Fig. 2l). Soil moisture dynamics showed close relationships with precipitation fluctuations during the three growing seasons (Fig. 2j–l). Soil temperature showed consistent seasonal dynamics with a peak in July and August during the three growing seasons (Fig. 2g–i).
Figure 2. Seasonal patterns of daily soil respiration rate.
(a–c), the differences in daily soil respiration rate between the treatments and the control (d–f), soil temperature (g–i), volumetric soil moisture and precipitation (j–l) during the growing seasons from 2010 to 2012. Arrows above the figure showed the time when water addition (short red arrow in dash line) and fungicide (solid green arrow) were applied. CK, control plots (solid black line); F-, fungicide application (dotted line in red); W, water addition (short dash line in green); WF-, water addition plus fungicide application (dash-dot-dot line in blue).
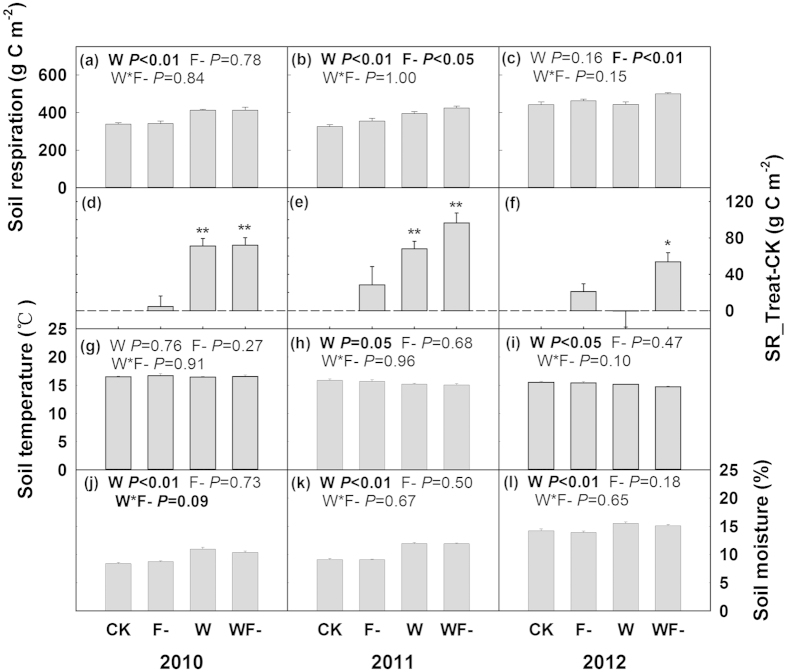
During the three growing seasons, water addition treatment decreased soil temperature by 2.7% with marginal significance (P = 0.07; Table 1 and Fig. 3g–i) and significantly enhanced soil moisture by 21% (P < 0.01; Table 1 and Fig. 3j–l). The enhancement effects were much higher in the relatively dry years. Water addition treatment enhanced soil moisture by 25% (P < 0.01) in 2010, by 31% (P < 0.01) in 2011 and 10.4% (P < 0.05) in 2012 (Fig. 3j–l), respectively. There was no significant variations in soil moisture or soil temperature caused by fungicide application (P > 0.05) and its interaction with water addition treatment (P > 0.05; Table 1).
Table 1. P-values of generalized linear mixed models (GLMM) showing the effects of block (random factor), water addition (W), fungicide application (F-), year (Y), and their interactions on soil dissolved organic carbon (DOC, mg kg−1 soil) and nitrogen (DON, mg kg−1 soil), soil microbial biomass carbon (MBC, mg kg−1 soil) and nitrogen (MBN, mg kg−1 soil) and microbial respiration (MR, mg CO2 kg−1 soil day−1), seasonal mean soil temperature (ST, °C) at 10 cm soil depth and soil moisture (SM, %) of the 0–10 cm soil layer and seasonal total soil respiration (SR, g C m−2).
| Source | DOC | DON | MBC | MBN | MR | ST | SM | SR |
|---|---|---|---|---|---|---|---|---|
| Block | 0.564 | 0.695 | 0.207 | 0.265 | 0.497 | 0.340 | 0.296 | 0.086 |
| W | 0.120 | 0.006 | 0.348 | 0.048 | 0.003 | 0.070 | <0.001 | <0.001 |
| F- | 0.137 | 0.074 | 0.991 | 0.626 | 0.059 | 0.290 | 0.222 | 0.013 |
| W × F- | 0.593 | 0.562 | 0.460 | 0.685 | 0.326 | 0.436 | 0.152 | 0.536 |
| Y | <0.001 | <0.001 | <0.001 | <0.001 | 0.011 | <0.001 | <0.001 | <0.001 |
| Y × W | 0.694 | 0.292 | 0.905 | 0.445 | <0.001 | 0.115 | <0.001 | <0.001 |
| Y × F- | 0.018 | 0.455 | 0.097 | 0.064 | 0.077 | 0.865 | 0.474 | 0.002 |
| Y × W × F- | 0.182 | 0.590 | 0.205 | 0.059 | 0.584 | 0.355 | 0.263 | 0.098 |
Figure 3. Seasonal accumulated soil respiration.
(a–c), the difference in seasonal soil respiration between the treatments and the control (d–f), seasonal mean soil temperature (g–i) and volumetric soil moisture (j–l) during the growing seasons from 2010 to 2012. Results (P-values) of two-way ANOVA were presented in each subplot (except d–f). * and ** on the bar (d–f) indicate significances from 0 at P < 0.05 and P < 0.01, respectively.
Seasonal and inter-seasonal variations of soil respiration
Soil respiration showed similar patterns during the three growing seasons, with a peak value during July-August and low rate at the beginning and end of the growing seasons (Fig. 2a–c). At the beginning and end of growing season in 2010, soil respiration was much higher compared with that in the same period of other seasons, because of higher precipitation during this period (Fig. 2a–c).
Total amounts of carbon release by soil respiration in the control plots were 299, 320 and 426 g C m−2 during the growing seasons in 2010–2012, respectively (Fig. 3a–c). Water addition treatment significantly improved soil respiration by 23% in 2010 and 20% in 2011 (both P < 0.01), while no obvious impacts in 2012 (P > 0.05, Fig. 3a–c). Fungicide application did not show significant impacts on soil respiration in 2010 (P > 0.05), while significantly promoted soil respiration by 8% in 2011 (P < 0.05) and by 9% in 2012 (P < 0.01, Fig. 2a–c). The increases of soil respiration induced by fungicide application mainly occurred on the peak seasons (Fig. 2d–f). There was no significant interaction between the effects of water addition and fungicide application on soil respiration (P > 0.05; Table 1 and Fig. 3a–c).
Soil respiration components
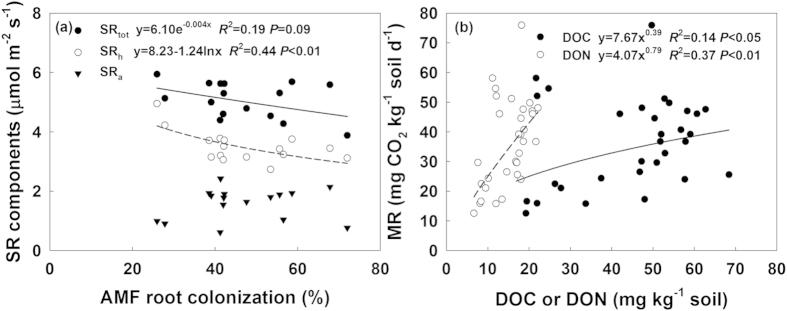
In 2013, seasonal mean soil respiration in the control plots was 4.6 μmol m−2 s−1, and consisted of 67% of heterotrophic and 33% of autotrophic parts (Fig. 4a–c). Both water addition and fungicide application increased soil respiration and its heterotrophic component significantly (both P < 0.05, Fig. 4a,b). However, the autotrophic part was not affected by both water and fungicide application treatments (both P > 0.05, Fig. 4c). There were no significant interactions between water addition effects and fungicide application observed on soil respiration and its components (all P > 0.05, Fig. 4a–c). Significantly negative relationships of soil respiration and its heterotrophic component with AMF root colonization were also found (Fig. 5a).
Figure 4. Seasonal dynamics of total soil respiration.
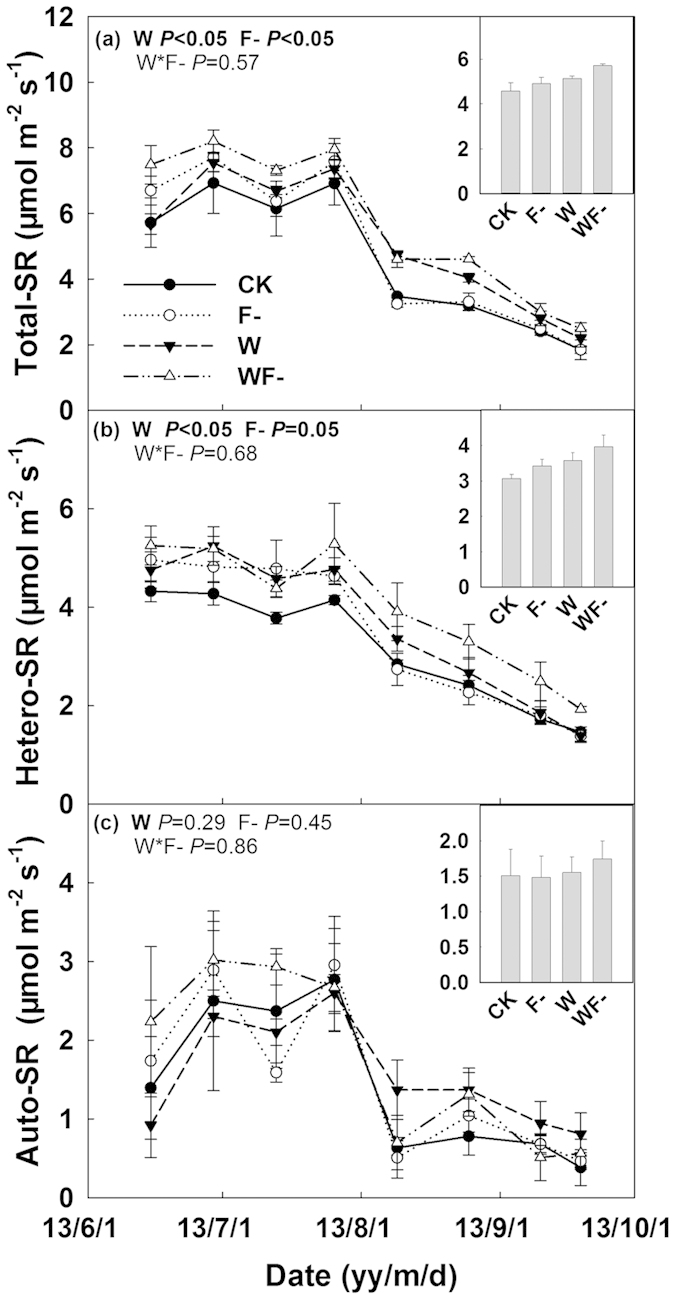
(total-SR, a), heterotrophic (hetero-SR, b) and autotrophic soil respiration (auto-SR, c) during the growing season in 2013. Results (P-values) of repeated-measures mixed model were presented in each subplot. The bar in the top right corner of each subplot indicates seasonal mean total SR, hetero-SR and auto-SR, respectively.
Figure 5. Relationships of (a) total-SR and its components with AMF root colonization (Fc), and (b) soil dissolved organic carbon (DOC) and nitrogen (DON) with microbial respiration (MR).
Soil aggregates, dissolved organic carbon and nitrogen, microbial biomass carbon and nitrogen, microbial respiration
The proportion of soil macro-aggregates, micro-aggregates and silt in the control plots were 49.8%, 47.7% and 2.4%, respectively (Fig. 6a). Fungicide application significantly decreased the proportion of macro-aggregates by 8.2% and increased that of silt by 56%, respectively (both P < 0.05; Fig. 6a). However, there was no significant water addition effect on all aggregates composition (P > 0.05; Fig. 6a). A significant negative relationship was found between proportion of silt in soil and AMF root colonization (Fig. 6b).
Figure 6.
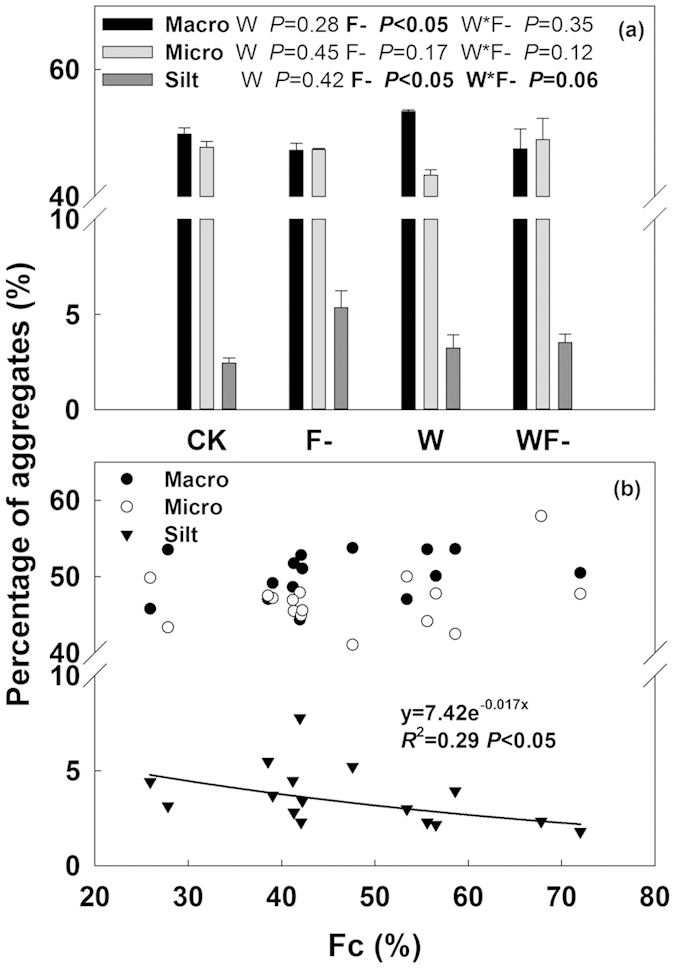
(a) Percentage composition of soil macro-aggregates (>250 μm in diameter), micro-aggregates (50~250 μm in diameter) and silt (<50 μm in diameter) under four treatments in 2013. Results (P-values) of two-way ANOVA were presented. (b) Relationships between AMF root colonization (Fc) and different aggregates components.
Water addition treatment increased soil dissolved matter especially DON by 26% (P < 0.01; Table S1 and 1). Fungicide application also increased DON by 14% marginally significantly (P = 0.07; Table 1 and S1). Treatments did not cause significant changes in soil microbial biomass except an increase in MBN by 20% in water addition plots (P < 0.05; Table 1 and S1). In the laboratory incubation, microbial respiration (MR) from the soils of water addition and fungicide application plots was 54% (P < 0.01) and 26% (P = 0.06) higher than that of the control plots, respectively (Table 1 and S1). Significantly positive relationships of MR with DOC and DON were found, respectively (Fig. 5b).
Responses of seasonal soil respiration to precipitation and soil moisture
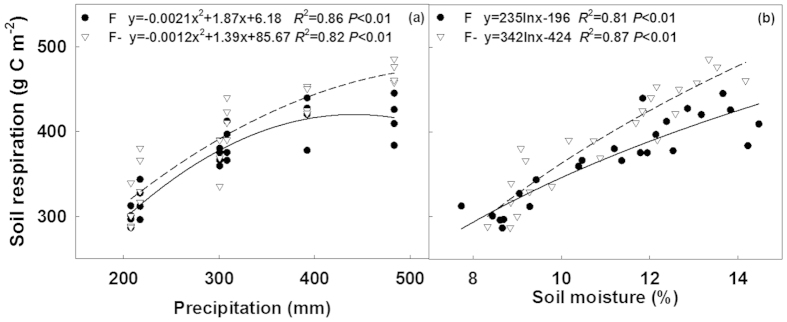
Non-linear responses of seasonal soil respiration to precipitation were found in both AMF control plots (F, including non-fungicide CK and W treatments) and AMF suppression plots (F-, including fungicide F- and WF- treatments), respectively (Fig. 7a). In the F plots, increased rate of soil respiration showed an obvious decrease when seasonal precipitation was above 300 mm, which was close to the long-term mean precipitation (Fig. 7a). Comparatively, the change rate of soil respiration to precipitation was relatively stable in the F- plots, which resulted in significantly higher soil respiration rate with increasing precipitation than that in the F plots (Fig. 7a). Logarithmic relationships between seasonal total soil respiration and mean soil moisture were also found across the three seasons (Fig. 7b).
Figure 7. Nonlinear responses of seasonal soil respiration in the F and F- plots to precipitation.
(a) and soil moisture (b). F (AMF control) represented the treatments without fungicide application and included the CK and W treatments, while F- (AMF suppression) represented the treatments with fungicide application and included F- and WF- treatments.
Discussion
Water addition treatment significantly enhanced soil respiration except in the wet year of 2012, which induced a non-linear response of soil respiration to precipitation change and soil water content (Fig. 7a,b). Such non-linear relationship was also observed in other water manipulation experiments33,34,39. In semiarid ecosystems, both plant growth40 and microbial activity41 are sensitive to soil moisture, which induce a great response of soil respiration to water addition. In our study, enhanced aboveground net primary production (30%, unpublished data) and microbial respiration (54%, Table S1 and 1) by water addition were observed, and might act as the major contributors to increases in soil respiration. Enhanced microbial respiration in water addition plots was closely related to improved soil dissolved matter (especially DON). Otherwise, the sensitivity of soil respiration to water addition also depended on ambient precipitation and no significant effects of water addition were observed in the wet year of 2012 (Fig. 3l). Similar results have also been reported in studies carried out in Sonoran desert42 and a mesic heathland ecosystem43. Generally, more precipitation means more raining days and lower temperature. Immediate decreases in soil temperature and corresponding sharp declines of soil respiration rate after large rainfall (including water addition) events were observed in the experiment especially during the wet 2012, which might limit soil respiration rate and its response to water addition in further (Fig. 2f). Our results suggested that water addition would increase soil respiration, but its effects depend on the ambient water condition, which results in a nonlinear response of soil respiration to precipitation change.
Fungicide application decreased AMF colonization and fungi concentration in roots, without altering soil microbial biomass (MBC and MBN) as well as their composition (fungi and bacteria) of microbes in soil. These results were consistent to previous studies in this region38 and tall-grass prairies in north America3,6,44,45, and confirmed that benomyl was effective on suppressing AMF colonization in plants with minor side effects on soil microbial community.
AMF suppression treatment increased soil respiration especially under wet condition. In many greenhouse experiments, however, soil respiration in non-AMF treatment was much lower than that in AMF-inoculated treatment46,47,48. The presence of AMF could enhance plant productivity and thus promote soil respiration especially its autotrophic component46,47,48. While in the field, we did not observed significant changes in plant productivity and autotrophic soil respiration. Unchanged plant productivity has also been reported by other AMF suppression experiment3,38. In this study, enhanced soil respiration in fungicide application treatments mainly stemmed from the increase in heterotrophic component of soil respiration (Fig. 4a,b), which was proven by evidences observed in the field soil respiration separation and laboratory soil incubation experiment (Table S1 and 1 and Fig. 4). Heterotrophic soil respiration, results from soil organic carbon decomposition, was determined by both soil microbial activity and substrate availability. Previous studies have shown that AMF hyphae can promote formation of soil macro-aggregates and reduce SOC decomposition3,20,21,22,26. A significant decrease in soil macro-aggregate component and a remarkable increase in silt component after four-year fungicide application were observed in our experiment (Fig. 6a). The breakdown of soil macro-aggregates would promote release of soil organic matter, inducing higher soil organic matter availability (DON) and microbial activity (MR). Therefore, in spite of unchanged soil microbial biomass, the higher accessibility of substrate under fungicide application treatments triggered soil carbon release and its response to increasing precipitation, especially when precipitation was beyond the long-term average (Fig. 7).
In summary, soil respiration was triggered by water addition especially during drought season, exhibited nonlinear relationship with precipitation change. AMF symbiosis could regulate the response of soil respiration to precipitation in this Inner Mongolia steppe ecosystem. AMF suppression treatment accelerated carbon release by soil respiration up to 9% during the experiment period, which was mainly stemmed from significant increase of heterotrophic part of soil respiration. Protection of AMF on soil carbon in macro-aggregates from heterotrophic decomposition might become more important with increasing water availability. Other factors of global climate change such as elevated CO24,49, nitrogen deposition49,50 and land use change51 have also showed to impact the species and abundance of AMF, which would eventually influence belowground carbon processes and stabilization of ecosystem carbon balance. Therefore, further studies, especially global change multiple-factor experiments, are needed to fully understand the feedback relationship between AMF with ecosystem carbon dynamic and the incorporate that into ecosystem carbon cycle researches.
Materials and Methods
Study site and experimental design
Our study site located at the Xilin River Basin, Inner Mongolia, China (116°42′ E, 43°38′ N, 1250 m a.s.l.). The site has been fenced since 1999 and no grazing or other disturbance thereafter. The site is a typical steppe and dominated by perennial grasses, such as Leymus chinensis and Stipa grandis, etc. Long-term (1980–2012) mean annual temperature of the study area is 0.3 °C, annual precipitation is 346 mm with more than 80% occurring during the growing season (May-October).
The experiment was carried out from early May 2010 as a two-factor random block design with water addition and fungicide application treatments. Sixteen 2 m × 2 m plots were grouped into four blocks with 1 m interval between plots. The plots were assigned randomly into four treatments in each block, including control (CK), water addition (W), fungicide application (F-) and water addition plus fungicide application (WF-). In the water addition plots, 120 mm precipitation was added with 8 times using a hand sprinkler biweekly from mid-May to early September in each season. In the fungicide treated plots, benomyl was applied to suppress AMF biweekly with 8 times but one week before the water addition. Benomyl solution (16 g benomyl dissolved in 8 L water) was applied manually to each fungicide application plot including F- and WF-, while plots of CK and W received equivalent amount of water at the same time. In this study, benomyl was chosen to suppress AMF, which is a systemic fungicide and its function only takes effect when metabolized into some anti-fungal catabolites in vivo of plants. Previous studies have reported its efficient in reducing AMF infection in roots but with fewest side effects on soil directly3,6,38,44,45. In order to minimize edge effects, all the measurements and samplings were conducted in the central zone with a 20 cm buffer zone around the plots.
Continuous soil respiration and environmental factors measurements
Soil respiration and environmental factors were measured continuously during the growing seasons of 2010–2012. At the beginning of the experiment, one PVC collar (20.3 cm in diameter and 8 cm in height) was inserted into the soil to a depth of 3 cm in each plot. Any live plants inside the collars were removed immediately once found. A continuous soil respiration measurement system was installed at middle May of 2010. The system consists of 16 automatic chambers (Truwel Inc., Beijing, China) attached to a multiplexer (Truwel Inc.), an infrared gas analyzer (LI-840, LI-COR Inc., Lincoln, USA), an air pump (LI-COR Inc.), and a power supply system (Dahe Inc., Beijing, China). Soil respiration rate in each plot was measured every two hours during the growing seasons. Each measurement lasted 150 s including 30 s for the closing and opening of the chamber. Concentrations of CO2, H2O and air temperature inside the chamber were measured every 2 s and recorded using CR5000 data-logger (Campbell Scientific Inc., Logan, IL, USA). Soil respiration rates were calculated based on the slope of the linear regression of CO2 concentration with time.
Soil temperature at a depth of 10 cm was also continuously measured every 2-hours by type E thermocouple (REOTEMP Inc., San Diego, USA) and recorded in CR5000 data-logger. Volumetric soil moisture of 0–10 cm was measured weekly using a portable TDR-200 soil moisture probe (Spectrum Technologies Inc., Plainfield, USA). Precipitation data were recorded in a weather station about 300 m away from the experiment site.
Separation of soil respiration components
A supplementary experiment by mini-trenching method32,52 was conducted to separate soil respiration in 2013. Briefly, a shallow PVC collar (10 cm in diameter and 6 cm in depth) and a deep collar (10 cm in diameter and 40 cm in depth) were inserted into the soil in each plot both with 3 cm above the ground in April 2013. Soil respiration measured from the two collars could be represented as total and heterotrophic soil respiration. Their difference was the autotrophic part32,49. Soil respiration in this season was measured biweekly from early May to September using a portable LI-8100 automated soil flux system (LI-COR Inc.).
Like other soil respiration partitioning methods used in grasslands such as clipping and trenching, the mini-trenching method may also overestimate heterotrophic soil respiration for the dead root decomposition or underestimate it due to the less roots exudates27,28,32. However, compared with other partitioning approaches27,28, this method is simple, economical and space efficient32, and meets the goal of our experiment. In order to avoid disturbances caused by collar installation, only data after two month of the installation were used in analysis, when the constituents of soil respiration tend to be steady with about 70% as heterotrophic soil respiration.
Soil microbial biomass, microbial respiration and dissolved organic matter
In August 2012 and 2013, three soil core samples (10 cm in depth and 7 cm in diameter) were collected from each plot and mixed into one sample for the laboratorial analyses. Soil microbial biomass carbon (MBC) and nitrogen (MBN) were determined using fumigation-extraction method41,53. Soil dissolved organic carbon (DOC) and nitrogen (DON) were estimated by measuring the extractable carbon and nitrogen contents in the unfumigated samples41,53. Microbial respiration (MR) was determined by measuring alkali absorption of CO2 emitted at 25 oC for 4 days followed by titrating the remaining OH− with a standard acid41.
AMF root colonization
In the peak seasons, three soil cores (10 cm in depth and 7 cm in diameter) were collected from each plot, and live roots were collected and mixed into one sample. The root samples were cleared in 10% KOH at 90 °C in a water bath for 40 min. After washed and drown into 2% HCL solution for 5 min, roots were stained in acid fuchsin-Lactic acid-Glycerin stain solution at 90 °C for 20 min. 25 root segments with length of 20 mm were examined microscopically to assess the percentage of the root length colonized by AMF (Fc)54.
Root fungi, soil fungi and bacteria
Total roots genomic DNA were extracted from 100 mg of roots using the DNeasy plant DNA extraction kit (Tiangen Biotech CO., Beijing, China) to examine fungi in roots (Fr). Total soil genomic DNA was also extracted from 250 mg of soil samples using the MoBio Soil DNA isolation kit (MoBio Laboratories, Inc., Carlsbad, USA) to determine fungal (Fs) and bacterial (Bs) concentration in soil. Both the DNA was eluted twice in 50 μL of buffer TE (10 mM Tris-HCl, 1 mM EDTA, pH = 8.0) and was stored at −20 °C until real-time PCR amplification.
Real-time PCR assays were conducted using StepOnePlusTM Instrument (Applied Biosystems). Each 20 μL of the amplification reaction solution contained the following reagents: 10 μL of SYBR Premix Ex Taq (Takara Bio Inc., Dalian, China), 0.4 μL of each primer (10 mmol mL−1), 0.4 μL of ROX Reference Dye (50 × ), 6.8 μL of H2O, and 2 μL of template DNA. The primer pair ITS1F and ITS4 was adopted in PCR for all fungal groups55, the primer pair 27F and 533R was used for PCR amplification of all bacteria group56. The PCR testing followed the procedures of 30 s at 95 °C, 40 cycles at 95 °C for 10 s, 30 s at 56 °C for annealing, 72 °C for 1 min, and 80 °C for 10 s. The concentrations were all represented by the logarithm of gene copy number per gram roots or soil.
Soil aggregates
Water-stable aggregates were separated into four different size classes (>2000, 2000~250, 250~50 and <50 μm in diameter) using wet-sieving method57, which involved three nested sieves of 2000 μm, 250 μm and 50 μm in pore size, respectively. The <50 μm and 50~250 μm size classes were referred to as silt and micro-aggregates, respectively; while the 250~2000 and >2000 μm size classes were referred to as macro-aggregates57.
Statistical analysis
The original and derived variables were analyzed with generalized linear mixed models (GLMM) to test the treatment effects on AMF root colonization (Fc), fungi in root (Fr), fungal concentration in soil (Fs), bacterial concentration in soil (Bs), seasonal soil respiration (SR), soil temperature (ST) and moisture (SM), soil dissolved organic carbon (DOC) and nitrogen (DON), microbial biomass carbon (MBC) and nitrogen (MBN) and microbial respiration (MR). Water addition, fungicide treatment, year and their interactions were considered as fixed effects; block was considered as a random effect58. These analyses were performed using ASReml 3.0 (VSN International Ltd., Hemel Hempstead, U.K.). Two-way ANOVA were conducted to test treatment effects on soil aggregates in 2013. Repeated-measures mixed model were conducted to test the water addition and fungicide application effects on SR components. Regression analysis was conducted to determine the relationships of Fc with soil aggregates and SR components, inter-annual precipitation with SR, seasonal mean SM with SR, DOC and DON with MR, respectively. These statistical analyses were performed using SAS 9.1 (SAS Institute Inc., Cary, USA).
Additional Information
How to cite this article: Zhang, B. et al. Arbuscular mycorrhizal fungi regulate soil respiration and its response to precipitation change in a semiarid steppe. Sci. Rep. 6, 19990; doi: 10.1038/srep19990 (2016).
Supplementary Material
Acknowledgments
We appreciated Dennis Baldocchi and Bernhard Schmidt’s helpful comments on an early version of this manuscript. This study was supported in part by the “Strategic Priority Research Program-Climate Change: Carbon Budget and Relevant Issues” of the Chinese Academy of Sciences (XDA05050402), the National Natural Science Foundation of China (31430016 and 31170453), and a Selected Young Scientist Program supported by the State Key Laboratory of Vegetation and Environment Change. We also thank China Scholarship Council for its finacial support of Dr. SP Chen.
Footnotes
Author Contributions S.C., Y.L. and X.H. designed the experiment; B.Z., S.L., T.R., Z.Y. and H.Z. carried out the field work and laboratory analyses; B.Z., S.C. and Y.L. performed the data analysis; B.Z., S.C. and Y.L. drafted the manuscript.
References
- Wehner J. et al. Determinants of root-associated fungal communities within Asteraceae in a semi-arid grassland. J Ecol 102, 425–436 (2014). [Google Scholar]
- Fitter A., Heinemeyer A. & Staddon P. The impact of elevated CO2 and global climate change on arbuscular mycorrhizas: a mycocentric approach. New Phytol 147, 179–187 (2000). [Google Scholar]
- Wilson G. W. T., Rice C. W., Rillig M. C., Springer A. & Hartnett D. C. Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: results from long-term field experiments. Ecol Lett 12, 452–461 (2009). [DOI] [PubMed] [Google Scholar]
- Cheng L. et al. Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 337, 1084–1087 (2012). [DOI] [PubMed] [Google Scholar]
- Kiers E. T. et al. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333, 880–882 (2011). [DOI] [PubMed] [Google Scholar]
- Hartnett D. C. & Wilson G. W. T. Mycorrhizae influence plant community structure and diversity in tallgrass prairie. Ecology 80, 1187–1195 (1999). [Google Scholar]
- Heijden M. G. A. v. d. et al. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396, 69–72 (1998). [Google Scholar]
- Leake J. et al. Networks of power and influence: the role of mycorrhizal mycelium in controlling plant communities and agroecosystem functioning. Can J Bot 82, 1016–1045 (2004). [Google Scholar]
- Moyano F. E., Kutsch W. L. & Schulze E.-D. Response of mycorrhizal, rhizosphere and soil basal respiration to temperature and photosynthesis in a barley field. Soil Biol Biochem 39, 843–853 (2007). [Google Scholar]
- Heinemeyer A., Hartley I. P. & Evans S. P., Carreira De La Fuente, J. A. & Ineson, P. Forest soil CO2 flux: uncovering the contribution and environmental responses of ectomycorrhizas. Global Change Biol 13, 1786–1797 (2007). [Google Scholar]
- Nottingham A. T., Turner B. L., Winter K., van der Heijden M. G. A. & Tanner E. V. J. Arbuscular mycorrhizal mycelial respiration in a moist tropical forest. New Phytol 186, 957–967 (2010). [DOI] [PubMed] [Google Scholar]
- Treseder K. K. & Allen M. F. Mycorrhizal fungi have a potential role in soil carbon storage under elevated CO2 and nitrogen deposition. New Phytol 147, 189–200 (2000). [Google Scholar]
- Miller R. M., Wilson G. W. & Johnson N. C. Arbuscular mycorrhizae and grassland ecosystems in Biocomplexity of Plant-Fungal Interactions (ed Southworth D.) 59–83 (Wiley-Blackwell, 2012). [Google Scholar]
- Clemmensen K. E. et al. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 339, 1615–1618 (2013). [DOI] [PubMed] [Google Scholar]
- Staddon P. L., Ramsey C. B., Ostle N., Ineson P. & Fitter A. H. Rapid turnover of hyphae of mycorrhizal fungi determined by AMS microanalysis of 14C. Science 300, 1138–1140 (2003). [DOI] [PubMed] [Google Scholar]
- Wright S. & Upadhyaya A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant soil 198, 97–107 (1998). [Google Scholar]
- Zhu Y. & Miller R. M. Carbon cycling by arbuscular mycorrhizal fungi in soil–plant systems. Trends Plant Sci 8, 407–409 (2003). [DOI] [PubMed] [Google Scholar]
- Singh B. K., Bardgett R. D., Smith P. & Reay D. S. Microorganisms and climate change: terrestrial feedbacks and mitigation options. Nat Rev Microbiol 8, 779–790 (2010). [DOI] [PubMed] [Google Scholar]
- Rillig M. C., Wright S. F., Nichols K. A., Schmidt W. F. & Torn M. S. Large contribution of arbuscular mycorrhizal fungi to soil carbon pools in tropical forest soils. Plant Soil 233, 167–177 (2001). [Google Scholar]
- Tisdall J. M., Smith S. E. & Rengasamy P. Aggregation of soil by fungal hyphae. Aust J Soil Res 35, 55–60 (1997). [Google Scholar]
- Miller R. M. & Jastrow J. D. The role of mycorrhizal fungi in soil conservation in Mycorrhizae in Sustainable Agriculture (eds G. J. Bethlenfalvay & R. G. Linderman) 29–44 (ASA Special Publication, 1992). [Google Scholar]
- Rillig M. C. & Mummey D. L. Mycorrhizas and soil structure. New Phytol 171, 41–53 (2006). [DOI] [PubMed] [Google Scholar]
- Leifheit E. F., Veresoglou S. D., Lehmann A., Morris E. K. & Rillig M. C. Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation—a meta-analysis. Plant Soil 374, 523–537 (2014). [Google Scholar]
- Rillig M. C. et al. Plant root and mycorrhizal fungal traits for understanding soil aggregation. New Phytol 205, 1385–1388 (2015). [DOI] [PubMed] [Google Scholar]
- King G. M. Enhancing soil carbon storage for carbon remediation: potential contributions and constraints by microbes. Trends microbiol 19, 75–84 (2011). [DOI] [PubMed] [Google Scholar]
- van der Heijden M. G., Martin F. M., Selosse M. A. & Sanders I. R. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol 205, 1406–1423 (2015). [DOI] [PubMed] [Google Scholar]
- Hanson P. J., Edwards N. T., Garten C. T. & Andrews J. A. Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 48, 115–146 (2000). [Google Scholar]
- Kuzyakov Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil biol Biochem 38, 425–448 (2006). [Google Scholar]
- Raich J. W. & Schlesinger W. H. The global carbon-dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 44, 81–99 (1992). [Google Scholar]
- Bahn M. et al. Soil respiration at mean annual temperature predicts annual total across vegetation types and biomes. Biogeosciences 7, 2147–2157 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raich J. W., Potter C. S. & Bhagawati D. Interannual variability in global soil respiration, 1980-94. Global Change Biol 8, 800–812 (2002). [Google Scholar]
- Zhou X., Wan S. & Luo Y. Source components and interannual variability of soil CO2 efflux under experimental warming and clipping in a grassland ecosystem. Global Change Biol 13, 761–775 (2007). [Google Scholar]
- Yan L., Chen S., Huang J. & Lin G. Differential responses of auto- and heterotrophic soil respiration to water and nitrogen addition in a semiarid temperate steppe. Global Change Biol 16, 2345–2357 (2010). [Google Scholar]
- Talmon Y., Sternberg M. & GrÜNzweig J. M. Impact of rainfall manipulations and biotic controls on soil respiration in Mediterranean and desert ecosystems along an aridity gradient. Global Change Biol 17, 1108–1118 (2011). [Google Scholar]
- Dungait J. A. J., Hopkins D. W., Gregory A. S. & Whitmore A. P. Soil organic matter turnover is governed by accessibility not recalcitrance. Global Change Biol 18, 1781–1796 (2012). [Google Scholar]
- Ni J. Carbon storage in grasslands of China. J Arid Environ 50, 205–218 (2002). [Google Scholar]
- Bao Y. & Yan W. Arbuscular mycorrhizae and their structural types on common plants in grasslands of mid-western Inner Mongolia. Biodiversity Science 12, 501–508 (2004). [Google Scholar]
- Yang G. et al. The interaction between arbuscular mycorrhizal fungi and soil phosphorus availability influences plant community productivity and ecosystem stability. J Ecol 102, 1072–1082 (2014). [Google Scholar]
- Thomey M. L. et al. Effect of precipitation variability on net primary production and soil respiration in a Chihuahuan Desert grassland. Global Change Biol 17, 1505–1515 (2011). [Google Scholar]
- Potts D. L. et al. Antecedent moisture and seasonal precipitation influence the response of canopy-scale carbon and water exchange to rainfall pulses in a semi-arid grassland. New Phytol 170, 849–860 (2006). [DOI] [PubMed] [Google Scholar]
- Liu W. X., Zhang Z. & Wan S. Q. Predominant role of water in regulating soil and microbial respiration and their responses to climate change in a semiarid grassland. Global Change Biol 15, 184–195 (2009). [Google Scholar]
- Cable J. M., Ogle K., Williams D. G., Weltzin J. F. & Huxman T. E. Soil texture drives responses of soil respiration to precipitation pulses in the Sonoran Desert: Implications for climate change. Ecosystems 11, 961–979 (2008). [Google Scholar]
- Sowerby A., Emmett B. A., Tietema A. & Beier C. Contrasting effects of repeated summer drought on soil carbon efflux in hydric and mesic heathland soils. Global Change Biol 14, 2388–2404 (2008). [Google Scholar]
- Fitter A. H. & Nichols R. The use of benomyl to control infection by vesicular arbuscular mycorrhizal fungi. New Phytol 110, 201–206 (1988). [Google Scholar]
- Cahill J. F., Elle E., Smith G. R. & Shore B. H. Disruption of a belowground mutualism alters interactions between plants and their floral visitors. Ecology 89, 1791–1801 (2008). [DOI] [PubMed] [Google Scholar]
- Langley J. A., Johnson N. C. & Koch G. W. Mycorrhizal status influences the rate but not the temperature sensitivity of soil respiration. Plant Soil 277, 335–344 (2005). [Google Scholar]
- Atkin O. K. et al. Temperature dependence of respiration in roots colonized by arbuscular mycorrhizal fungi. New Phytol 182, 188–199 (2009). [DOI] [PubMed] [Google Scholar]
- Cavagnaro T. R., Barrios-Masias F. H. & Jackson L. E. Arbuscular mycorrhizas and their role in plant growth, nitrogen interception and soil gas efflux in an organic production system. Plant Soil 353, 181–194 (2012). [Google Scholar]
- Antoninka A., Reich P. B. & Johnson N. C. Seven years of carbon dioxide enrichment, nitrogen fertilization and plant diversity influence arbuscular mycorrhizal fungi in a grassland ecosystem. New Phytol 192, 200–214 (2011). [DOI] [PubMed] [Google Scholar]
- Egerton-Warburton L. M. & Allen E. B. Shifts in arbuscular mycorrhizal communities along an anthropogenic nitrogen deposition gradient. Ecol Appl 10, 484–496 (2000). [Google Scholar]
- Liu Y. J. et al. Direct and indirect influences of 8 yr of nitrogen and phosphorus fertilization on Glomeromycota in an alpine meadow ecosystem. New Phytol 194, 523–535 (2012). [DOI] [PubMed] [Google Scholar]
- Heinemeyer A., Tortorella D., Petrovicova B. & Gelsomino A. Partitioning of soil CO2 flux components in a temperate grassland ecosystem. Eur J Soil Sci 63, 249–260 (2012). [Google Scholar]
- Vance E., Brookes P. & Jenkinson D. An extraction method for measuring soil microbial biomass C. Soil biol Biochem 19, 703–707 (1987). [Google Scholar]
- Berch S. M. & Kendrick B. Vesicular-arbuscular mycorrhizae of southern Ontario Fferns and fern-allies. Mycologia 74, 769–776 (1982). [Google Scholar]
- Manter D. K. & Vivanco J. M. Use of the ITS primers, ITS1F and ITS4, to characterize fungal abundance and diversity in mixed-template samples by qPCR and length heterogeneity analysis. J Microbial Meth 71, 7–14 (2007). [DOI] [PubMed] [Google Scholar]
- Watanabe K., Kodama Y. & Harayama S. Design and evaluation of PCR primers to amplify bacterial 16S ribosomal DNA fragments used for community fingerprinting. J Microbial Meth 44, 253–262 (2001). [DOI] [PubMed] [Google Scholar]
- Elliott E. T. Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Sci Soc Am J 50, 627–633 (1986). [Google Scholar]
- Butler D. G., Cullis B. R., Gilmour A. R. & Gogel B. J. ASReml-R reference manual (release 3): mixed models for S language environments. Available at: http://www.vsni.co.uk/downloads/asreml/release3/asreml-R. (2009) (Date of access: 22/7/2014).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.