Abstract
Female intrasexual competition is intense in cooperatively breeding species where offspring compete locally for resources and helpers. In mammals, females have been proposed to adjust prenatal investment according to the intensity of competition in the postnatal environment (a form of ‘predictive adaptive response’; PAR). We carried out a test of this hypothesis using ultrasound scanning of wild female banded mongooses in Uganda. In this species multiple females give birth together to a communal litter, and all females breed regularly from one year old. Total prenatal investment (size times the number of fetuses) increased with the number of potential female breeders in the group. This relationship was driven by fetus size rather than number. The response to competition was particularly strong in low weight females and when ecological conditions were poor. Increased prenatal investment did not trade off against maternal survival. In fact we found the opposite relationship: females with greater levels of prenatal investment had elevated postnatal maternal survival. Our results support the hypothesis that mammalian prenatal development is responsive to the intensity of postnatal competition. Understanding whether these responses are adaptive requires information on the long-term consequences of prenatal investment for offspring fitness.
Intrasexual competition is usually most severe among males, because males generally have higher variance in reproductive success than females1. This is manifested through conspicuous traits such as aggression and weaponry2. In cooperatively breeding species, female competition for reproduction is also intense, leading to overt and sometimes aggressive competition3. Because the cost of producing young is higher for females compared to males, theory suggests females will often resolve conflict without recourse to overt violence, for example, through the use of signals or threats4.
Recently, it has been suggested that females may compete over reproduction via maternal effects on offspring growth. In hyenas (Crocuta crocuta) and red squirrels (Tamiasciurus hudsonicus), for example, there is evidence that mothers prime their offspring to face competitive social environments through hormonal signaling (androgens or glucocorticoids[GCs]5,6). Experimental manipulations of population density in other taxa have also shown that offspring size is increased in response to adverse conditions (increased competition)7,8,9,10,11. These effects can be interpreted as a form of ‘predictive adaptive response’ (PAR), whereby mothers (or, potentially, offspring themselves) are hypothesized to adjust the developmental trajectory to ensure a match between offspring phenotype and the environment experienced postnatally or in later life12,13,14,15. However, no study of wild mammals has directly tested whether mothers adjust prenatal investment according to the postnatal environment, and in particular the intensity of reproductive competition.
We carried out this test in a wild cooperatively breeding mammal, the banded mongoose (Mungos mungo)16. Banded mongooses are small diurnal carnivores which live in stable groups of ~20 adults plus pups. Multiple females (mean = 3.5 females, range 1 to 13) give birth together in each breeding attempt, usually on the same day. Groups breed on average four times per year, experiencing considerable variation in environmental conditions (i.e. rainfall) which is strongly linked to invertebrate prey abundance17,18. Females compete postnatally using infanticide, but can escape infanticide through birth synchrony19. Offspring compete for access to lactating females and helpers (called “escorts”) who provision and protect pups after they emerge from the den. There is also evidence of prenatal maternal impacts on offspring competitiveness: mothers that are heavier at conception produce larger pups which have competitive advantage when competing for alloparental care; increasing pup survival20.
We carried out 360 ultrasound scans on 59 breeding females from 8 groups of banded mongooses to test (1) whether mothers adjust prenatal investment in response to reproductive competition, and (2) the consequences of variation in prenatal investment for mothers and offspring.
Methods
Study site
We studied a population of banded mongooses living on and around Mweya Peninsula, Queen Elizabeth National Park (QENP), Uganda (0°12′S, 27°54′E) between May 2000 and November 2013. For a detailed description of the climate, habitat and the population see Cant et al. 201318. Rainfall data was provided by Uganda Institute of Ecology Meteorological Station and, later, using a rain gauge.
Study population
All individuals in the population are known and individually marked with either colour-coded collars (7 g) or unique shave patterns (for details of trapping protocol and anesthesia are given elsewhere; Ketamine21; Isoflurane22). The identity of breeding females was determined from changes in body shape, ultrasound scans and palpation23,24. Each group was visited daily to determine accurate parturition dates. Since parturition can be determined precisely but conception cannot, we calculated the age of fetuses retrospectively assuming an average 60 day gestation (the mean period between peak mate guarding and birth23). Group size and the number of females were counted as the total number of individuals or females over 1 year old in each group for each communal litter. Individuals are habituated to step onto electronic scales to determine an accurate weight which allows regular weighing events without capture. Female weight at the time of conception was calculated using the closest weighing event prior (±10 days from conception) to the estimated conception date; if possible weights for all females within the same group came from the same weighing event.
Measuring fetus size and number
Number of fetuses was counted under anesthesia by palpitating the abdomen, and a cross-sectional ultrasound scan of each fetus was obtained using an ultrasound scanner (SIUI CTS-900V, UK) and ultrasound gel (Anagel, UK). Trapping females within the last few weeks of pregnancy was avoided and most trapping was conducted 3-4 weeks after oestrus. Previous study has shown no adverse effects of trapping and palpitating pregnant females24. The age of the fetus at the time of the ultrasound scan was calculated retrospectively from the litter birth date and the scan date, assuming a gestation length of 60 days (average female gestation length23).
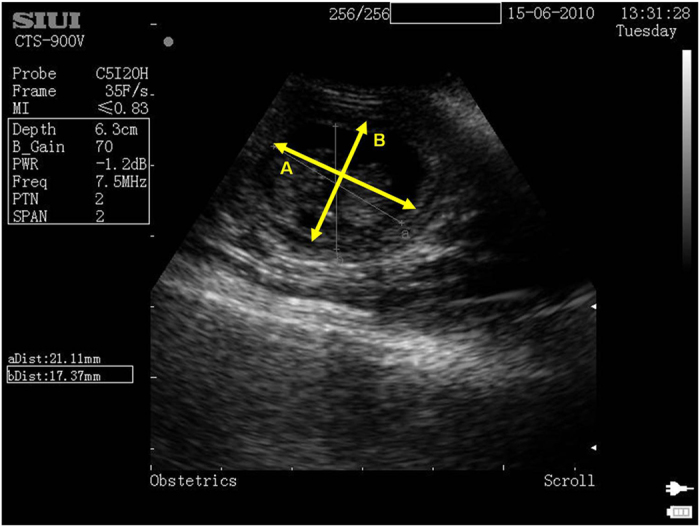
We used the cross-sectional area (mm2) of each fetus as measured from the ultrasound images as an estimate of fetus size. Fetuses were measured on average at 30 ± 7 (mean ± sd) days post conception when they are still roughly spherical in shape to minimize noise arising from different angles of the scan cross-section. The outline of a fetus was identified by the black pixilation of the fluid-filled amniotic sac and the white pixilation of the womb tissue and the amniotic sac membrane around the fetus. The mean of two perpendicular measurements of the diameter were taken using the computer software Image J (1.47c25) and used to calculate the elliptical area of the fetus (see Fig. 1).
Figure 1. Cross-sectional ultrasound scan of individual fetus with 2 perpendicular measurements A and B used to calculate the cross-sectional area (A/2 × B/2 × π).
Statistics
We analyzed fetus sizes and the number of fetuses using general linear mixed models (LMMs) and generalized linear mixed models (GLMMs) in R version 3.1.0 using lme4 package R1.1-626,27. GLMMs had either a poisson error structure with log-link function or binomial error structure with logit link function. Female, litter and group identities were included as random factors in analyses to account for the repeated sampling. Fixed terms included were female weight at conception, female age (months), number of adult females present in the group, group size and the total rainfall during gestation (ml). Because groups were trapped at different stages of pregnancy, fetus age (days) was included as a covariate when analyzing fetus size. Correlations between variables fitted in the same models as fixed effects were lower than the levels indicated by Freckleton28 to cause model fitting issues such as variance inflation in effect estimates (max r = 0.48). We obtained a minimal model via sequential removal of least significant factors, starting with 2-way interactions. Each factor was then added back into the minimum model in order to confirm removal was not contingent on the order of removal29.
To investigate if mothers adjust their prenatal investment in response to reproductive competition we estimated total prenatal investment by multiplying the average fetus size by the number of fetuses carried for each pregnancy. Variation in prenatal investment could be due to individual female adjustment in response to competition (a within-individual effect) or be the result of consistent differences between individuals. We tested the relative importance of within- versus between-individual effects using the method described by van de Pol & Wright30, which separates out the effect sizes in the fitted model attributable to variation within versus between individuals. To test the consequences of variation in prenatal investment for mothers and offspring we focused on pup survival to 3 months (y/n) using logistic regression, and pup weight (controlled age at capture <90 days) as well as female reproductive effort and survival. Maternity assignments for pups were based on 43 microsatellite loci as described in Sanderson et al.31. As individual fetus scans cannot be matched to pups an average fetus size was used in these analyses. Relative fetus size was calculated as the average fetus size in each female’s litter relative to average fetus size for all females within a breeding attempt. We tested whether prenatal investment predicted female participation in the next group litter (y/n) using a GLMM with binomial errors. We tested whether there was a trade-off between current investment in reproduction and female survival using Cox regression with backward selection of terms (Wald Chi-square). This analysis included total group size, number of females, and the average fetus size and number of fetuses as predictors, and to avoid repeat sampling used only the last reproductive event on record for each female. This analysis was conducted in SPSS 21.0.0.032.
Ethical Statement
Research was carried out under a permit from Uganda Wildlife Authority (UWA) and Uganda National Council for Science and Technology (UNCST), and all methods approved by UWA, UNCST and the Ethical Review panel of the University of Exeter. All methods were carried out in accordance with the Guidelines for the Treatment of Animals in Behavioural Research and Teaching published by the Association for the Study of Animal Behaviour33.
Results
(1) Do mothers adjust prenatal investment in response to reproductive competition?
The total prenatal investment (fetus size x number of fetuses carried) of females increased with the number of other adult females in the group during pregnancy, and with a female’s weight at conception (LMM, number of females, χ21 = 5.65, N = 142, P = 0.017, female weight: (LMM, χ21 = 12.60, N = 142, P < 0.001). This relationship was driven by fetus size rather than number: mean fetus size increased with the number of females in the group; increased more steeply in lighter females, and in breeding attempts featuring lower rainfall (LMM, 2 way interaction of female number with: weight, χ21 = 4.23, N = 360 scans, P = 0.040; rainfall, χ21 = 4.91, N = 360, P = 0.027; Fig. 2). Neither total group size nor female age influenced fetus size (see Supplementary Information (SI) Table S1). Within-female variation was a better predictor of fetal size in response to reproductive competition than between-female variation (LMM, within-female variation, χ21 = 4.51, N = 360, P = 0.034, between-female variation, χ21 = 3.38, N = 360, P = 0.066; SI Table S2). The number of fetuses was only influenced by female age, peaking at 4 years of age before declining (GLMM poisson, χ21 = 10.36, N = 361, P = 0.001). There was no significant relationship between fetus size and the number of fetuses (LMM, χ21 = 1.03, N = 581, P = 0.31). Thus individual females produced larger fetuses, but no fewer of them, when faced with competition from other female breeders.
Figure 2. Variation in prenatal investment as a function of the number of adult females in the group at conception.
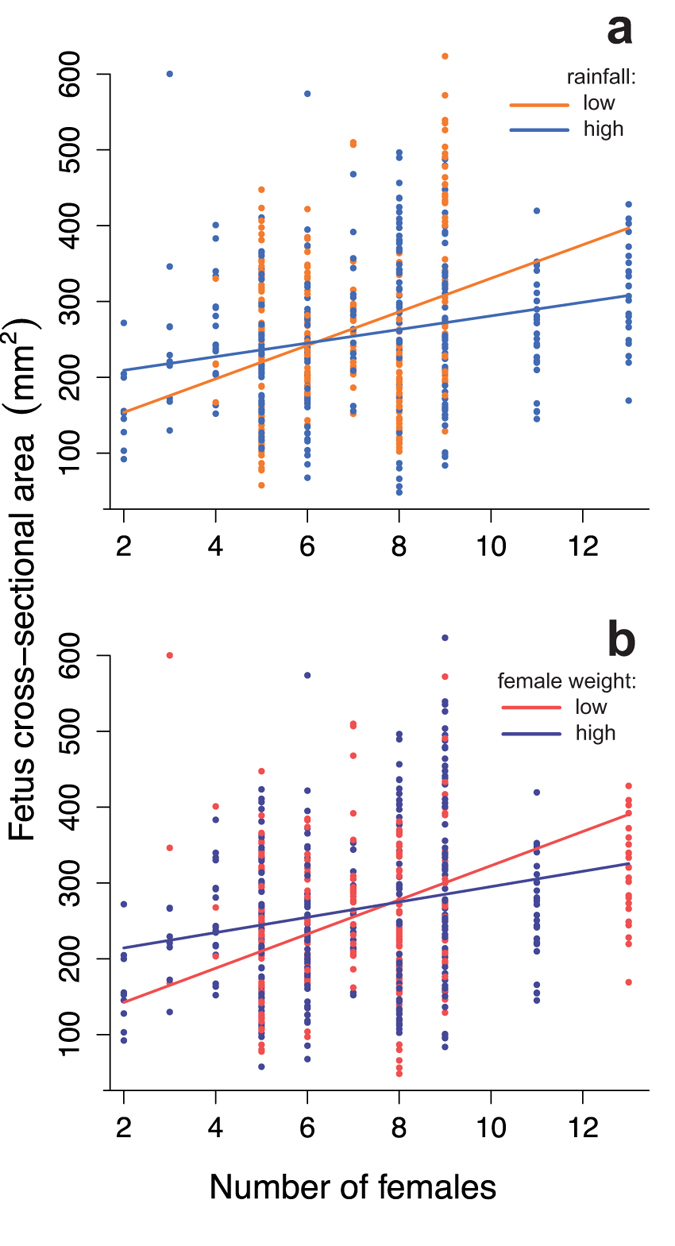
(a) Fetus cross-sectional area increases more sharply when rainfall is low (orange line) compared to high (light blue line); (b) Lighter females (red line) show the steepest increase in fetus size with female number compared to heavier females (dark blue line). Female weight (mean ± sd = 1447 ± 201 g) and rainfall (mean ± sd = 128.3 ± 40.9 ml) are continuous variables that have been categorized for illustrative purposes using the 25% and 75% quartiles.
(2) What are the consequences of variation in prenatal investment for mothers and offspring?
Female reproductive success (number of assigned pups at emergence) increased with the number of fetuses during gestation, (GLMM poisson, χ21 = 5.44, N = 153 females, P = 0.02; SI Table S3). However, larger fetuses did not translate into a greater number of assigned pups (GLMM poisson, χ21 = 0.76, N = 151 pups, P = 0.38). Fetus size also did not influence pup weight at 3 months (LMM, χ21 = 0.37, N = 115 pups, P = 0.54; SI Table S4), nor survival to 3 months (GLMM, binomial, χ21 = 0.12, N = 131 pups, P = 0.72). Relative fetus size (measured relative to other scanned females in a particular breeding attempt) also did not predict a female’s share of total group reproductive success (GLMM binomial, χ21 = 1.14, N = 153, P = 0.29) nor pup survival to 3 months (GLMM binomial, χ21 = 1.09, N = 131, P = 0.30). Thus, we found no evidence that the production of larger fetuses translated into improved success in postnatal reproductive competition, at least in the short term.
Finally, we found no evidence of a cost of prenatal investment to mothers in terms of future survival or reproduction. In fact, higher total prenatal investment was associated with higher post-scan survival of mothers (Cox regression, Wald χ21 = 6.57, N = 360, P = 0.010; Fig. 3). Again this relationship was driven by fetus size rather than number (SI Table S5). Females that invested more prenatally were not less likely to reproduce in the next breeding attempt (GLMM binomial, χ21 = 0.35, N = 164, P = 0.061; SI Table S6). Thus we found no evidence of a survival cost to mothers of elevated prenatal investment, nor did mothers compensate for high prenatal investment by reducing reproductive effort in the next breeding attempt.
Figure 3. Maternal survival as a function of prenatal investment.
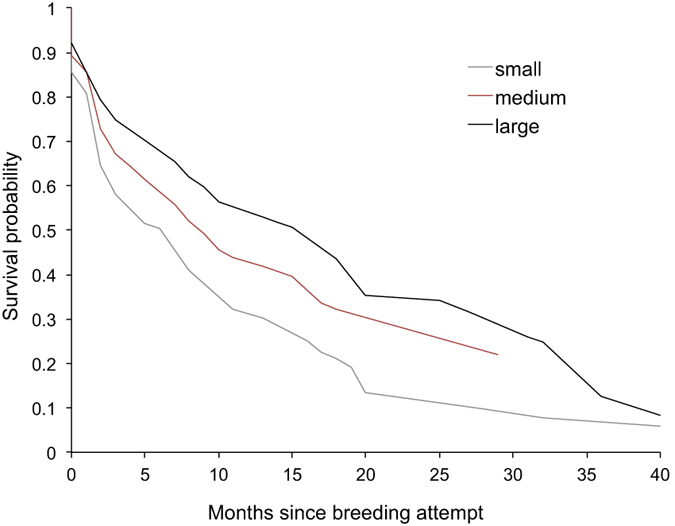
Mothers that invested more prenatally survived longer. Fetus size (mean ± sd = 247.90 ± 100.88 mm2) has been categorized for illustrative purposes using the 25% (179.54 mm2), mean and 75% (319.09 mm2) quartiles.
Discussion
Female banded mongooses produced larger, but no fewer, offspring when there were more adult females in the group. Since all adult females breed in most breeding attempts, this is consistent with the hypothesis that females strategically up-regulate prenatal investment in the face of elevated postnatal reproductive competition. Such responses may be particularly likely to evolve in breeding systems where females co-breed regularly. Females showed steeper increases in prenatal investment when ecological conditions were harsh, and when they were in relatively poor body condition, two factors which are expected to exacerbate the intensity of postnatal competition among offspring34. We found no evidence that increased prenatal investment incurred future costs to females in terms of reproduction or survival. On the contrary, females that invested more prenatally showed improved future survival (Fig. 3). A positive relationship between current reproductive investment and future survival is expected where females vary considerably in quality or access to resources, since high quality females may be able to divert more resources to offspring production without compromising their somatic function (the ‘big house big car’ effect35,36).
Increasing fetus size in response to increased social competition is a subtle way in which females could compete over reproduction within social groups without risking the costs of fighting or killing offspring3,4. However, we found no detectable benefit (in terms of short-term reproductive success) associated with increased investment in fetus size. Neither absolute fetus size nor fetus size relative to other co-breeders predicted the number of offspring that survived to emerge from the den. The lack of any detectable advantage to elevated prenatal investment is surprising, and may reflect a high level of noise associated with high pup mortality due to intra- or intergroup infanticide and predation18,19. It may also be that the benefits of increased prenatal investment are realised later in the life of the offspring. Studies of human famine and laboratory rodents, for example, suggest that early life environments can influence health and fitness across the lifespan, not just in the short term13.
Our findings offer an interesting contrast to studies of social birds and fish, in which dominant females produce smaller eggs or a larger number of eggs when there are many helpers in the group37,38,39,40. In banded mongooses, all group members contribute to rearing young, but prenatal investment did not vary with the potential number of helpers (measured by total group size). Our findings suggest that the intensity of reproductive competition, rather than the availability of helpers, is the main determinant of variation in prenatal investment in this species. Larger pups have better access to adult group members who provide parental care and, upon emergence, aggressively defend access to the best helpers or ‘escorts’41. Where postnatal competition among offspring has characteristics of contest competition, the best response to competition will be to invest more resources per offspring prenatally, rather than to produce more of them42,43. Producing a larger number of offspring could also bring benefits, but at the unavoidable cost of intensified competition among littermates.
Our study complements previous studies which suggest that mothers use hormones to influence the development of their offspring in utero to improve their success in the postnatal environment, a form of PAR13,44. The PAR hypothesis has been criticized because long term forecasts of environmental conditions are inherently unreliable14,15. In cooperative breeders, however, the quality of the postnatal environment is largely determined by the number of breeders competing for reproduction and the number of helpers available to offspring. These features of social groups remain stable over the course of offspring development, from gestation to nutritional independence, so are highly predictable. Cooperative birds and mammals, including humans, are thus likely candidates to evolve PARs. We found evidence that female banded mongooses respond to reproductive competition by adjusting prenatal investment, consistent with the PAR hypothesis, but we did not find evidence that this response is adaptive. To test the PAR hypothesis fully will require study of the consequences of variation in prenatal investment across the lifetime of offspring in animals exposed to natural predators and pathogens.
Additional Information
How to cite this article: Inzani, E. L. et al. Female reproductive competition explains variation in prenatal investment in wild banded mongooses. Sci. Rep. 6, 20013; doi: 10.1038/srep20013 (2016).
Supplementary Material
Acknowledgments
We are grateful to UWA and UNCST for permission to conduct our research. We thank N. Guma, M. Driciru and the Wardens and staff of Queen Elizabeth National Park for continuous logistical support. We thank F. Mwanguhya, S. Kyambulima, K. Mwesige, R. Businge, S. Ahabyona for assistance in the field. We thank three anonymous reviewers for comments on the manuscript. The study was funded by an ERC grant to MAC (grant number 309249).
Footnotes
Author Contributions M.C., E.V., S.H. and E.I. designed research; E.V. and S.H. collected data; M.C. and G.K. supervised field project; E.I., F.T.H.M. and E.V. analysed data; H.N. and J.S. carried out genetic analysis; M.C., E.I. and E.V. drafted the paper; all authors contributed to the final version.
References
- Andersson M. Sexual Selection. Princeton, NJ. Princeton University Press (1994). [Google Scholar]
- Clutton-Brock T. Sexual selection in males and females. Science. 318(5858), 1882–1885 (2007). [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T. & Huchard E. Social competition and its consequences in female mammals. J. Zool. 289(3), 151–171 (2013). [Google Scholar]
- Cant M. A. & Young A. J. Resolving social conflict among females without overt aggression. Philos. T. Roy. Soc. B. 368(1631), 20130076 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dloniak S. M., French J. A. & Holekamp K. E. Rank-related maternal effects of androgens on behaviour in wild spotted hyenas. Nature. 440(7088), 1190–1193 (2006). [DOI] [PubMed] [Google Scholar]
- Dantzer B. et al. Density Triggers Maternal Hormones That Increase Adaptive Offspring Growth in a Wild Mammal. Science 340, 1215–1217 (2013). [DOI] [PubMed] [Google Scholar]
- Allen R. M., Buckley Y. M. & Marshall D. J. Offspring size plasticity in response to intraspecific competition: An adaptive maternal effect across life‐history stages. The Am. Natur. 171(2), 225–237 (2008). [DOI] [PubMed] [Google Scholar]
- Burns C. W. Effects of crowding and different food levels on growth and reproductive investment of Daphnia. Oecol. 101(2), 234–244 (1995). [DOI] [PubMed] [Google Scholar]
- Cleuvers M., Goser B. & Ratte H.-T. Life-Strategy Shift by Intraspecific Interaction in Daphnia magna: Change in Reproduction from Quantity to Quality. Oecologia. 110(3), 337–345 (1997). [DOI] [PubMed] [Google Scholar]
- Kindsvater H. K. & Otto S. P. The evolution of offspring size across life-history stages. Am. Natur. 184(5), 543–555 (2014). [DOI] [PubMed] [Google Scholar]
- Meylan S., Clobert J. & Sinervo B. Adaptive significance of maternal induction of density‐dependent phenotypes. Oikos. 116(4), 650–661 (2007). [Google Scholar]
- Bateson P., Gluckman P. & Hanson M. The biology of developmental plasticity and the Predictive Adaptive Response hypothesis. J. Physiol. 592(11), 2357–2368 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P. D. & Hanson M. A. Living with the past: evolution, development, and patterns of disease. Science. 305(5691), 1733–1736 (2004). [DOI] [PubMed] [Google Scholar]
- Rickard I. J. & Lummaa V. The Predictive Adaptive Response and metabolic syndrome: Challenges for the hypothesis. Trends Endocrin. Met. 18(3), 94–99 (2007). [DOI] [PubMed] [Google Scholar]
- Wells J. C. Flaws in the theory of predictive adaptive responses. Trends Endocrin. Met. 18(9), 331–337 (2007). [DOI] [PubMed] [Google Scholar]
- Cant M. A. Cooperative breeding systems In The Evolution of Parental Care (eds. Royle N. J. et al.) 206–225 (Oxford University Press, 2012). [Google Scholar]
- Rood J. P. Population dynamics and food habits of the banded mongoose. East Afr. Wildl. J. 13, 89–111 (1975). [Google Scholar]
- Cant M. A., Vitikainen E. & Nichols H. J. Demography and social evolution of banded mongooses. Adv. Stud. Behav. 45, 407–445 (2013). [Google Scholar]
- Cant M. A., Nichols H. J., Johnstone R. A. & Hodge S. J. Policing of reproduction by hidden threats in a cooperative mammal. Proc. Nat. Acad. Sci. USA 111, 326–330 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge S. J. et al. Maternal weight, offspring competition and the evolution of communal breeding. Behav. Ecol. 20, 729–735 (2009). [Google Scholar]
- Hodge S. J. Counting the costs: the evolution of male-biased care in the cooperative breeding banded mongoose. Anim. Behav. 74, 911–919 (2007). [Google Scholar]
- Jordan N. R., Mwanguhya F., Kyabulima S., Rüedi P. & Cant M. A. Scent marking within and between groups of wild banded mongooses. J. Zool. 280(1), 72–83 (2010). [Google Scholar]
- Cant M. A. Social control of reproduction in banded mongooses. Anim. Behav. 59, 147–158 (2000). [DOI] [PubMed] [Google Scholar]
- Gilchrist J. S., Otali E. & Mwanguhya F. Why breed communally? Factors affecting fecundity in a communal breeding mammal: the banded mongoose (Mungos mungo). Behav. Ecol. Sociobiol. 57, 119–131 (2004). [Google Scholar]
- Schneider C. A., Rasband W. S. & Eliceiri K. W. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9, 671–675 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D., Mächler M., Bolker B. M. & Walker S. C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. arXiv:1406 (2012). (Date of access:01/06/2013). [Google Scholar]
- R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/ (2013).
- Freckleton R. P. Dealing with collinearity in behavioural and ecological data: model averaging and the problems of measurement error. Behav. Ecol. Sociobiol. 65(1), 91–101 (2011). [Google Scholar]
- Crawley M. J. The R book. Chichester. UK, Wiley (2007). [Google Scholar]
- van de Pol M. & Wright J. A simple method for distinguishing within-versus between-subject effects using mixed models. Anim. Behav. 77(3), 753–758 (2009). [Google Scholar]
- Sanderson J., Wang J., Vitikainen E. I. K., Cant M. A. & Nichols H. J. Banded mongooses avoid inbreeding when mating with members of the same natal group. Molec. Ecol (2015). 10.1111/mec.13253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows. Version 21.0. Armonk, NY, IBM Corp (2012).
- Association for the Study of Animal Behaviour. Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 71, 245–253 (2006). [Google Scholar]
- Douhard M. et al. Fitness consequences of environmental conditions at different life stages in a long-lived vertebrate. Proc. Roy. Soc. Lond. B: Biol. Sci. 281(1785), 20140276 (2014). 10.1098/rspb.2014.0276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Noordwijk A. J. & de Jong G. Acquisition and allocation of resources: their influence on variation in life history tactics. Amer. Nat. 137–142 (1986). [Google Scholar]
- Reznick D., Nunney L. & Tessier A. Big houses, big cars, superfleas and the costs of reproduction. Trends Ecol. & Evol. 15(10), 421–425 (2000). [DOI] [PubMed] [Google Scholar]
- Russell A. F., Langmore N. E., Cockburn A., Astheimer L. B. & Kilner R. M. Reduced egg investment can conceal helper effects in cooperatively breeding birds. Science. 317(5840), 941–944 (2007). [DOI] [PubMed] [Google Scholar]
- Canestrari D., Marcos J. M. & Baglione V. Helpers at the nest compensate for reduced maternal investment in egg size in carrion crows. J. Evol. Biol. 24(9), 1870–1878 (2011). [DOI] [PubMed] [Google Scholar]
- Taborsky B., Skubic E. & Bruintjes R. Mothers adjust egg size to helper number in a cooperatively breeding cichlid. Behav. Ecol. 18(4), 652–657 (2007). [Google Scholar]
- Koenig W. D., Walters E. L. & Haydock J. Helpers and egg investment in the cooperatively breeding acorn woodpecker: testing the concealed helper effects hypothesis. Behav. Ecol. Sociobiol. 63(11), 1659–1665 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist J. S. Aggressive monopolization of mobile carers by young of a cooperative breeder. Proc. Roy. Soc. B-Biol. Sci. 275, 2491–2498 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G. A. Assessment strategy and the evolution of fighting behaviour. J. Theor. Biol. 47, 223–243 (1974). [DOI] [PubMed] [Google Scholar]
- Grinsted L., Breuker C. J. & Bilde T. Cooperative breeding favors maternal investment in size over number of eggs in spiders. Evol. 68, 1961–1973 (2014). [DOI] [PubMed] [Google Scholar]
- Bateson P. et al. Developmental plasticity and human health. Nature. 430(6998), 419–421 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


