Abstract
Anaerobic digestion using mixed-culture with broader choice of pretreatments for hydrogen (H2) production was investigated. Pretreatment of wastewater sludge by five methods, such as heat, acid, base, microwave and chloroform was conducted using crude glycerol (CG) as substrate. Results for heat treatment (100 °C for 15 min) showed the highest H2 production across the pretreatment methods with 15.18 ± 0.26 mmol/L of medium at 30 °C in absence of complex media and nutrient solution. The heat-pretreated inoculum eliminated H2 consuming bacteria and produced twice as much as H2 as compared to other pretreatment methods. The fermentation conditions, such as CG concentration (1.23 to 24 g/L), percentage of inoculum size (InS) (1.23% to 24% v/v) along with initial pH (2.98 to 8.02) was tested using central composite design (CCD) with H2 production as response parameter. The maximum H2 production of 29.43 ± 0.71 mmol/L obtained at optimum conditions of 20 g/L CG, 20% InS and pH 7. Symbiotic correlation of pH over CG and InS had a significant (p-value: 0.0011) contribution to H2 production. The mixed-culture possessed better natural acclimatization activity for degrading CG, at substrate inhibition concentration and provided efficient inoculum conditions in comparison to mono- and co-culture systems. The heat pretreatment step used across mixed-culture system is simple, cheap and industrially applicable in comparison to mono-/co-culture systems for H2 production.
Keywords: crude glycerol; hydrogen; mixed-culture; secondary wastewater sludge; 1,3-propanediol
1. Introduction
Biodiesel and biohydrogen are considered as renewable, efficient and carbon dioxide (CO2)-free fuel of choice for the future [1,2]. Biodiesel production across the world is increasing rapidly and estimated to reach 20 billion liters in 2020 due to strong government policies and incentives across the world [3,4]. About 100 kg of crude glycerol (CG) is generated as waste by-product with every ton of biodiesel produced [5]. Sustainable production and commercialization of biodiesel depends on the demand and increased utilization of CG [3]. With presence of various impurities across CG, refining for glycerin is no longer cost-effective with decreasing market value for glycerin [4,6]. Value added utilization (valorization) of waste CG into biofuels or biochemical for additional market value represents a promising route with several advantages [7,8,9]. New valorization methods for the CG are exploited as a low cost, abundant feedstock, with increased substrate conversion efficiency and decreased operating costs in comparison to other organic wastes [1,6,7].
The present hydrogen (H2) production techniques by physico-chemical routes are fossil fuel dependent, expensive and release CO2 [10]. Microbial conversion of CG to H2 production is an attractive approach [6,11] and has gained advantage for high energy recovery potential [8,12]. H2 production with utilization of waste by-product will credit CG to reduce total production costs (by 13%–14%) of biodiesel fuels [3]. The presence of various impurities in CG is the major bottleneck for the production and recovery of value-added products [13]. However, the produced H2 can be easily separated from the fermentation media, requiring no additional purification steps and can be directly used as fuel [14], thus reducing the downstream processing cost in comparison to value-added products, such as 1,3-propanediol (1,3-PD) and ethanol requiring production at higher concentrations to minimize costly purification steps [10].
Microbial H2 production using organic wastes can be divided into two steps; dark fermentation and the photo-fermentation process [15]. Dark fermentation in absence of light has advantages in terms of a simple reactor set-up, and economical increased H2 production rate in comparison to photo-fermentation requiring complex set-up in the presence of a light source [15,16,17]. Conversion of complex organic wastes into simple low molecular weight volatile fatty acids, H2 and CO2 during acidogenesis/acidification step of conventional anaerobic digestion is known as dark fermentation [18,19].
Anaerobic digestion has been considered economical during treatment of complex organic wastes [18], and researchers are focused on developing the acidogenesis stage of H2 production using mixed-culture that could be affordable and accessible [20,21]. Anaerobic digestion in the presence of mixed-culture have broader choice of organic waste feedstocks, and are easy to operate and control the growth of cultures during H2 production [22].
Pretreatment steps to remove H2 consuming bacteria that coexist in mixed-culture is a necessary step and carried out using media enrichment, heat-shock, acid, alkali, chloroform and ultrasonication techniques [21,22,23,24]. Five pretreatment methods across acid, chemical, wet heat-, dry heat-shock, freezing and thawing were carried out for cattle manure sludge [25]. A comparative study across acid, heat and chloroform treatment on sewage sludge was carried out to enrich H2 producing bacteria and to eliminate methane production permanently [26]. Media enrichment is the only technique explored to date for activated sludge for H2 production by mixed-culture using CG [1,8,12]. Highest H2 yield by mixed-culture using vinasse (waste of sugarcane ethanol distillation columns) resulted in 3.66 mol H2/mol sucrose [15] and using CG resulted in around 0.90–0.96 mol H2/mol glycerol [1,8]. In this study the top five commonly appearing enrichment methods, such as heat, acid, base, microwave and chloroform were conducted to increase H2 yield using CG.
The cattle manure sludge, reactor waste, soil types etc. are commonly used as seed inoculum across mixed-culture studies; in this study wastewater sludge was selected for H2 production. The temperature of the wastewater sludge (30 °C) at the time of collection favors the growth of microorganisms. However, the optimum temperature for H2 producing microorganisms is around 37 °C and around 55 °C inhibits H2 consuming bacteria [8]. Initial experiments at different temperatures were evaluated in this study. Optimization of fermentation parameters using statistical tools such as Central Composite Design (CCD) narrow down experimental runs, suggest the optimum parameter range and identify the dominant parameter responsible for H2 production in fewer runs [4,15]. The parameters needed for optimization are the substrate concentration, media supplements, endo-nutrients, inoculum size and the fermentation pH [8,12,27]. Each of these input parameters are considered as important and play a dominant effect in determining the H2 production [8,12]. In this study, costly media supplements and endo-nutrients concentration was omitted to decrease the process cost by eliminating the use of expensive media components. H2 production was carried out using minimal medium in presence of CG as substrate.
The impurities in CG derived from restaurant and meat processing waste have increased inhibition effect on pure-/co-cultures in comparison to impurities in CG derived from pure substrates [28]. The mixed-culture nullifies the inhibition effect of impurity to utilize complex CG with synergistic effects that appears advantageous over pure and co-culture system during glycerol fermentation [1,22]. Utilization of CG as substrate during glycerol fermentation for increased H2 yield across pure-, co- and mixed-culture system was evaluated in this study.
Glycerol fermentation follows two possible pathways (oxidative and reductive) [10]. During the oxidative pathway glycerol is converted to H2 and various organic acids/alcohols. In the case of the reductive pathway, glycerol is reduced into 1,3-PD production [10,29]. A complete shift of glycerol fermentation from a reductive to oxidative pathway with decreased 1,3-PD production will increase H2 production [5,10]. Analysis of 1,3-PD as a response factor in the central composite design (CCD) will determine the behaviour of the mixed-culture for the possible pathway during glycerol fermentation.
To date, there is disagreement on the best pretreatment methods of sludge for enriching hydrogen-producing bacteria for maximum H2 yield [30]. In this study, enrichment of secondary wastewater sludge using different pretreatment techniques was carried out to obtain stable consortia of mixed-culture to use CG as sole substrate for H2 production. To optimize the fermentation parameters, such as CG concentration, inoculum size and fermentation pH, central composite design was used along with H2 as response factor. The present study also deals with a comparative platform for H2 production using pure-, co- and mixed-culture system with CG as substrate.
2. Results and Discussion
2.1. Hydrogen Production Using Different Pretreatment Methods
The pretreatment methods tested in this study for wastewater sludge for the inoculum enrichment for H2 production are presented in the Table 1. The heat treatment resulted in increased production (15.18 ± 0.26 mmol/L) in comparison to other methods using CG as substrate. The production using heat treatment at 30 °C (15.18 ± 0.26 mmol/L) was highest in comparison to 55 °C (4.57 ± 0.53 mmol/L) and 37 °C (12.76 ± 0.50 mmol/L). The same set-up of heat treatment was tested at 25 °C, however there was no increase in H2 production in comparison to 30 °C (data not shown). The fermentation temperature optimum for H2 production using pretreated wastewater sludge was highest at 30 °C in comparison to other temperatures as seen in Table 1. The highest H2 production of 15.18 ± 0.26 mmol/L-of medium was obtained for the heat pretreatment method at 30 °C. The lowest H2 production of 1.62 ± 0.91 mmol/L was observed in the case of chloroform treatment. H2 production increased with a decrease in the incubation temperature across all the pretreatment methods. The highest production of 15.18 ± 0.26 mmol/L in case of heat pretreatment of wastewater sludge at 30 °C was shortlisted for further optimization experiments using CCD.
Table 1.
Hydrogen production (mmol/L) for different pretreatment methods across different fermentation temperature conditions.
| Pretreatment Methods | Hydrogen Production (mmol/L) across Different Temperature Set-up | ||
|---|---|---|---|
| 55 °C | 37 °C | 30 °C | |
| Heat | 4.57 ± 0.53 | 12.76 ± 0.50 | 15.18 ± 0.26 |
| Acid | 1.94 ± 0.22 | 4.40 ± 0.51 | 7.05 ± 0.83 |
| Alkali | 4.22 ± 0.49 | 4.67 ± 0.59 | 10.02 ± 0.22 |
| Microwave | 3.98 ± 0.46 | 4.22 ± 0.49 | 6.09 ± 0.71 |
| Chloroform | 1.62 ± 0.91 | 2.71 ± 0.31 | 9.91 ± 0.68 |
2.2. Hydrogen Production during Optimization Studies
The H2 production across different CG concentrations, inoculum sizes and varying pH is represented in the Table 2. H2 production ranged from 5.11 ± 0.71 (obtained at CG: 20, InS: 6, pH: 4) to a maximum of 29.43 ± 0.71 mmol/L (obtained at CG: 20, InS: 20, pH: 7).
Table 2.
The experimental runs of central composite design across crude glycerol concentration (g/L), different inoculum size (%), varying pH and experimental runs in terms of hydrogen production (mmol/L) and 1,3-PD concentration (g/L).
| Run | Crude Glycerol (g/L) | Inoculum Size (%) | pH | Hydrogen Production (mmol/L) | 1,3-PD (g/L) |
|---|---|---|---|---|---|
| 1 | 20 | 6 | 4 | 5.11 ± 0.71 | 0.77 ± 0.04 |
| 2 | 6 | 6 | 7 | 17.62 ± 0.84 | 3.15 ± 0.26 |
| 3 | 13 | 13 | 5.5 | 17.28 ± 0.40 | 1.57 ± 0.23 |
| 4 | 13 | 13 | 5.5 | 18.81 ± 0.83 | 1.45 ± 0.30 |
| 5 | 6 | 6 | 4 | 5.21 ± 0.55 | 1.56 ± 0..56 |
| 6 | 1.23 | 13 | 5.5 | 9.86 ± 0.88 | 0.53 ± 0.62 |
| 7 | 20 | 20 | 7 | 29.43 ± 0.71 | 5.12 ± 0.59 |
| 8 | 13 | 13 | 5.5 | 17.28 ± 0.52 | 1.68 ± 0.76 |
| 9 | 20 | 6 | 7 | 21.84 ± 0.78 | 6.02 ± 0.56 |
| 10 | 6 | 20 | 4 | 8.06 ± 0.89 | 2.69 ± 0.75 |
| 11 | 20 | 20 | 4 | 8.41 ± 0.21 | 1.67 ± 0.62 |
| 12 | 13 | 13 | 5.5 | 18.44 ± 0.19 | 1.55 ± 0.66 |
| 13 | 13 | 13 | 2.98 | 8.51 ± 0.71 | 1.28 ± 0.58 |
| 14 | 24 | 13 | 5.5 | 10.51 ± 0.76 | 2.00 ± 0.67 |
| 15 | 13 | 13 | 5.5 | 19.28 ± 0.71 | 1.72 ± 0.77 |
| 16 | 13 | 1.23 | 5.5 | 9.94 ± 0.10 | 0.96 ± 0.64 |
| 17 | 6 | 20 | 7 | 17.12 ± 0.25 | 3.07 ± 0.66 |
| 18 | 13 | 24 | 5.5 | 12.26 ± 0.92 | 1.84 ± 0.73 |
| 19 | 13 | 13 | 8.02 | 14.55 ± 0.54 | 6.06 ± 0.59 |
| 20 | 13 | 13 | 5.5 | 18.68 ± 0.21 | 1.22 ± 0.62 |
The response surface quadratic model with p-value of 0.028 was significant, and pH with p-value of 0.0011 had a significant effect on H2 production. The model ANOVA results for H2 production are represented in the Table 3. The significant p-value of 0.0011 indicated the linear dominance of pH parameter on H2 production in comparison to CG and inoculum size.
Table 3.
The model ANOVA results for hydrogen production and 1,3-PD concentration.
| Source | p-Value | |
|---|---|---|
| Hydrogen | 1,3-Propanediol | |
| Model significant | 0.028 | <0.0001 |
| A-crude glycerol | 0.2701 | 0.0147 |
| B-inoculum size | 0.289 | 0.2138 |
| C-pH | 0.0011 | <0.0001 |
| AB | 0.4828 | 0.4875 |
| AC | 0.1949 | 0.0009 |
| BC | 0.9376 | 0.064 |
| A2 | 0.0824 | 0.5269 |
| B2 | 0.1334 | 0.3378 |
| C2 | 0.166 | <0.0001 |
The model equation that best represented the fitting data is shown below in (Equation (1)):
| Hydrogen = +18.18 + 1.31 × CG + 1.26 × InS + 5.08 × pH + 1.07 × CG × InS + 2.04 × CG × pH + 0.12 × InS × pH − 2.11 × CG × CG − 1.78 × InS × InS − 1.63 × pH × pH | (1) |
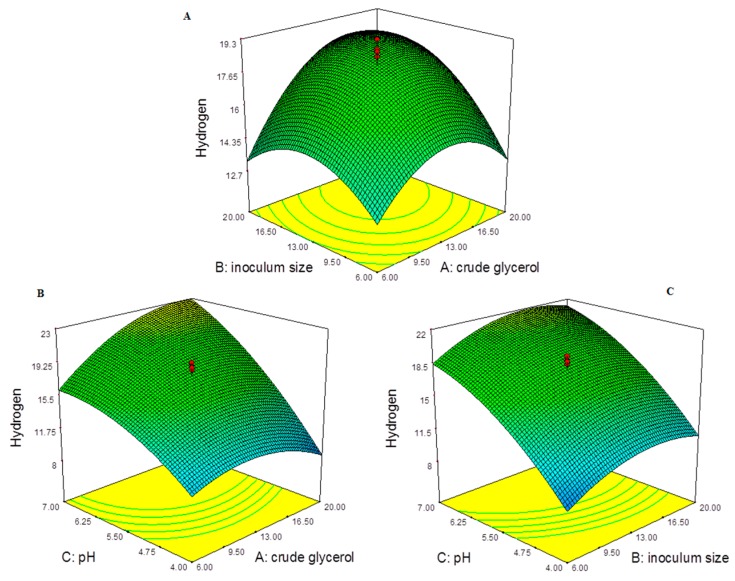
Across Equation (1), pH (5.08) had a positive value and was higher in comparison to other input parameters, indicating the influence of pH to be greater at higher value with dominating effect on H2 production. The H2 production response across the input parameters: CG, inoculum size and pH using response surface curve are represented in the Figure 1. At minimum concentration of CG with increasing inoculum size, the H2 production increased and decreased as seen in Figure 1A. The H2 production at minimum concentration of CG (6 g/L) and InS (6%) for run:2 and 5, varied from 5.21 ± 0.55 to 17.62 ± 0.84 mmol/L. In case of increased inoculum size of 20% at minimum concentration of CG (6 g/L), the H2 varied from 8.06 ± 0.89 to 17.12 ± 0.25 mmol/L. At maximum concentration of CG with increasing inoculum size, the H2 production increased. This is seen upon run:1 (CG: 20, InS: 6) with 5.11 ± 0.71 mmol/L increased to 29.43 ± 0.71 mmol/L of H2 production for run:7 at (CG: 20, InS: 20). The effect of increase in InS at maximum CG concentration had increasing effect on H2 production as seen in the Figure 1A. The H2 production at minimum concentration of CG (6 g/L) increased with increase in the pH as seen in the Figure 1B. This is seen in run:5 (CG: 6, pH: 4) with 5.21 ± 0.55 mmol/L increased to 17.12 ± 0.25 mmol/L of H2 production for run:17 at (CG: 6, pH: 7). This was even true at maximum concentration of CG for run:11 (CG: 20, pH: 4) with 8.41 ± 0.21 mmol/L increased to 29.43 ± 0.71 mmol/L for the run:7 (CG: 20, pH: 7). The effect of increase in pH across CG concentrations had a similar effect with increase in H2 production till pH nearing to optimum (pH 7.0) as seen in the Figure 1B. The relation of H2 production between CG and pH was also similar with pH and InS as seen with the response curve in the Figure 1C. For run:1 (InS: 6, pH: 4) with 5.11 ± 0.71 mmol/L increased to 21.84 ± 0.78 mmol/L of H2 production for run:9 at (InS: 6, pH: 7). Similar increase was true at maximum concentration of CG for run:11 (InS: 20, pH: 4) with 8.41 ± 0.21 mmol/L increased to 29.43 ± 0.71 mmol/L for the run:7 (InS: 20, pH: 7). The effect of increase in pH across inoculum size had similar effect with increase in H2 production till pH nearing to optimum (pH 7.0) as seen in the Figure 1C. The optimum fermentation conditions are dependent on substrate concentration, working pH and inoculum size for increased H2 production [2,5]. The fermentation pH had the dominant effect across the other input parameters and at neutral pH resulted in maximum H2 production at maximum CG (20 g/L) with 20% inoculum size.
Figure 1.
Hydrogen production (mmol/L) response across the input parameters: (A) across crude glycerol (g/L) and inoculum size (%); (B) across crude glycerol (g/L) and (C) inoculum size (%) and pH.
2.3. 1,3-Propanediol Production during Optimization Studies
The response of 1,3-PD production across different CG concentrations, inoculum sizes and varying pH is represented in Table 2. 1,3-PD production ranged from minimum of 0.53 ± 0.62 (run:6, CG: 1.23, InS: 13, pH: 5.5) to a maximum of 6.06 ± 0.59 g/L (run:19, CG: 13, InS: 13, pH: 8.02). The response surface quadratic model with p-value of <0.0001 was significant. The p-value of CG (0.0147) and pH (<0.0001) both had a significant effect on 1,3-PD production. The model equation that best represented the fitting data has been shown below in Equation (2):
| 1,3-Propanedion = +1.50 + 0.41 × CG + 0.18 × InS + 1.37 × pH − 0.13 × CG × InS + 0.84 × CG × pH − 0.38 × InS × pH + 0.08 × CG × CG + 0.14 × InS × InS + 0.94 × pH × pH | (2) |
Across Equation (2), akin to H2 production, the coefficient of pH (1.37) had a positive value and was higher in comparison to other input parameters. The significant p-value of <0.0011 indicated the linear dominance of pH along with CG (0.0147) parameter on 1,3-PD production as seen in Table 3.
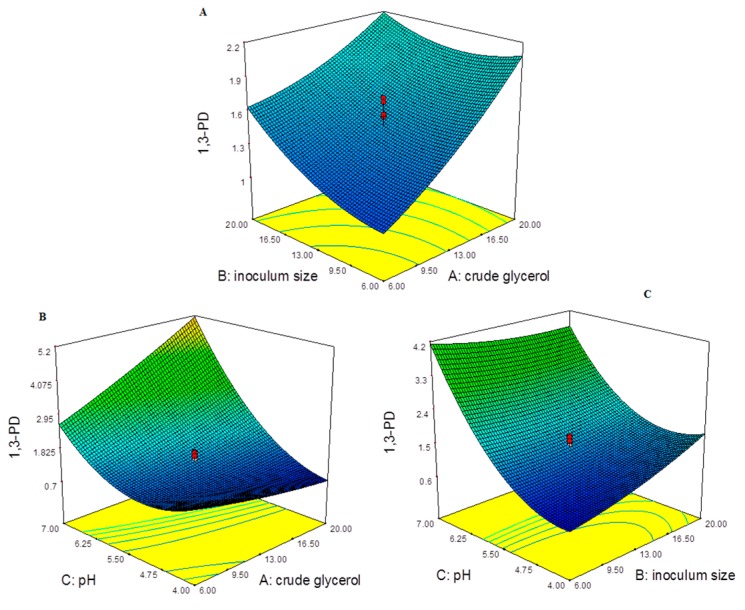
The 1,3-PD production response across the input parameters: CG, inoculum size and pH using response surface curve are represented in Figure 2. At maximum concentration of CG with increasing inoculum size, 1,3-PD production was less than maximum response (6.06 g/L) as seen in Figure 2A. The 1,3-PD production at maximum concentration of CG (20 g/L) with increasing InS from 6%–20% for run:1 and 11, ranged between 0.77 ± 0.04 to 1.67 ± 0.62 g/L. The response of 1,3-PD increased with increase in CG concentration at every interval of InS as seen in the Figure 2A. The 1,3-PD production at maximum concentration of CG (20 g/L) increased with increase in the fermentation pH as seen in the Figure 2B. This is seen in run:1 (CG: 6, pH: 4) with 0.77 ± 0.04 g/L increased to 5.12 ± 0.59 and 6.02 ± 0.56 g/L for run:7 and 9 at (CG: 20, pH: 7). The production of 1,3-PD reached maximum in the pH range (7–8), which was also dependent on the CG concentration. The relation of 1,3-PD production between CG and pH was also similar with pH and InS as seen with the response curve in the Figure 2C. In the case of run:10 and 11 (InS: 20, pH: 4) the range was from 1.67 ± 0.62 to 2.69 ± 0.75 g/L and increased in a range from 3.07 ± 0.66 to 5.12 ± 0.59 g/L of 1,3-PD production for run:7 and 17 at (InS: 20, pH: 7). The production of 1,3-PD reached maximum in the pH range (7–8), which was also dependent on the InS. The decreased production of 1,3-PD during glycerol fermentation increased the H2 production [2,10]. The maximum H2 production of 29.43 ± 0.71 mmol/L was observed at 5.12 ± 0.59 g/L of 1,3-PD production, which was lower in comparison to maximum production of 1,3-PD (6.06 ± 0.59 g/L) resulting in decreased H2 production with 14.55 ± 0.54 mmol/L.
Figure 2.
1,3-PD production (g/L) response across the input parameters: (A) crude glycerol (g/L) and inoculum size (%); (B) across crude glycerol (g/L) and (C) inoculum size (%) and pH.
Enrichment methods using acid, chemical, wet heat-, dry heat-shock, freezing and thawing was carried out for cattle manure sludge, and increased H2 production using acid pretreatment was 1.9–9.8 times greater compared to control sludge [25]. A comparative study was made across acid, heat and chloroform treatment on sewage sludge during immobilized H2 production in a mixed-culture under anaerobic conditions. Chloroform treatment inhibited hydrogen consuming bacteria, avoided fast conversion of H2 to acetic acid and repeated culture showed H2 production till 15 days [26]. Heat pretreatment was the best among other pretreatments (acid and base) for enriching H2 producing bacteria from anaerobic sludge [31]. The increased H2 production depends on the source of the seed sludge and the pretreatment method. Heat treatment is a simple, effective and practical method in comparison to other methods for enriching H2-producing bacteria from different seed source [30,31,32,33]. The effect of heat pretreatment depends on the seed inoculum to eliminate H2 consumers and also on the kind of substrate used during fermentation [32]. In this study, the heat-pretreated inoculum possessed higher natural acclimatization activity for degrading CG and produced twice as much as H2 in comparison to other pretreatment methods.
The results obtained across pretreatment methods over different fermentation temperatures, helped to narrow down the input parameters for the CCD model. The input parameters using CG concentration ranged from 1.23 to 24 g/L, inoculum size from 1.23% to 24% and pH from 2.98 to 8.02, respectively along with responses of H2 and 1,3-PD production represented in the Table 2. The central point from CCD model at CG (13 g/L), InS (13%) and pH (5.5) resulted in the production of H2 reaching only 18.81 ± 0.83 mmol/L in comparison to 15.18 ± 0.26 at CG (10 g/L). The increase in concentration of CG with increasing inoculum size increased H2 production, however the effect of initial pH was dominant and had a significant effect (p-value: 0.0011) in increasing H2 production. At maximum concentration of CG (20 g/L) for run:1 and 11, with increase in inoculum size from six to 20% resulted in marginal H2 production from 5.11 ± 0.71 to 8.41 ± 0.21 mmol/L. However, at the same values of CG and InS, when the pH was adjusted from 4 to 7, the H2 production increased with 29.43 ± 0.71 mmol/L. Further increase in the initial pH to 8.02 at CG (13 g/L) and InS (13%) resulted in decreased H2 production reaching only 14.55 ± 0.54 mmol/L. Anaerobic digestion for H2 production depends on growth limitation of methanogens, performance of H2 production and shift from acidogenesis to solventogenesis depends on the pH condition [8]. The optimum pH for H2 production is effective, when the methanogenic bacteria get repressed at pH 5.5–6 and also with heat-shock pretreatment (100 °C) [33]. The conditions of initial pH: 7 in presence of CG (20 g/L) and InS (20%) was optimum for the growth of H2 producers in mixed-culture, which resulted in increased H2 production reaching 29.43 ± 0.71 mmol/L.
The H2 yield comparison along with seed inoculum, experimental condition, and type of design used across mixed-culture studies is represented in Table 4. The results obtained with hot air oven pretreatment for anaerobic granule sludge using CG (22.19 g/L) in presence of endo-nutrient (2.89 mL/L) resulted in H2 production of 1.37 mmol/L h [27]. The conversion of CG into H2 with heat-treated mixed-culture at substrate concentration of 3 g/L in presence of nutrient solutions resulted in 0.31 mol H2/mol glycerol [32]. Enrichment of activated sludge using minimal medium in presence of CG (15 g/L) resulted in an H2 yield ranging from 0.66 to 0.96 mol H2/mol of glycerol [1,8]. The highest H2 yield of 1.41 mol/mol of glycerol was obtained from enrichment of activated sludge using complex modified HM 100 medium using CG (1 g/L) [12]. The optimal condition in this study resulted in H2 production rate of around 1.23 mmol/L h and with 72% of substrate utilization at H2 yield of 0.82 mol/mol of glycerol. The results obtained in this study are slightly higher in presence of minimal medium at 20 g/L, and is comparable to studies (as seen in Table 4) using complex medium, nutrient solutions, and working at very low CG concentrations. The heat treatment method used in this study is simple, low cost and industrially applicable in comparison to using costly medium enrichment pretreatment methods. This study provides dual environment benefits with wastewater treatment along with utilization of CG into generation of H2 for possible domestic renewable energy source for the biodiesel industry.
Table 4.
Hydrogen yield comparison across mixed-culture studies using crude glycerol as substrate.
| Seed Inoculum | Pretreatment | Substrate | Experimental Condition | Experimental Design | H2 Yield (mol H2/mol glycerol) | Ref. |
|---|---|---|---|---|---|---|
| Anaerobic granule from an upflow anaerobic sludge blanket (UASB) reactor | 105 °C for 2 h in hot-air oven | CG derived from fried chicken oil waste | CG at 22.19 g/L, supplement with endo-nutrients, initial pH 5.5 at 35 °C with 150 rpm | CCD on CG, sludge and endo-nutrient concentration | 0.30 | [27] |
| Wheat soil | 105 °C for 2 h | CG derived from transesterification of soybean oil | CG at 3 g/L, supplement with nutrient solution, initial pH 6.2 at 30 °C | No design | 0.31 | [32] |
| Wastewater sludge | Media enrichment | CG derived from transesterification of rapeseed, sunflower and soy | CG at 15 g/L along with Minimal medium to dilute CG with pH 6.8 at 37 °C with 120 rpm | Plackett-Burman on CG concentration, temperature and initial pH | 0.66 to 0.96 | [1,8] |
| Activated sludge | Media enrichment | CG derived from transesterification of canola oil and restaurant fats | CG at 1 g/L along with Modified HM 100 medium to dilute CG with pH 6.5 at 40 °C with 120 rpm | Plackett-Burman with five independent variables | 1.41 | [12] |
| Wastewater sludge | 100 °C for 15 min in an Isotemp Standard Lab Ovens | CG derived from transesterification of meat processing plants and used grease from restaurants | CG at 20 g/L along with Modified basal medium to dilute CG with pH 6.5 at 37 °C with 150 rpm | CCD on CG concentration, inoculum size and fermentation pH | 0.82 | This study |
2.4. Hydrogen Production across Pure-, Co- and Mixed-Culture System
To determine CG substrate inhibition effect at higher concentration, H2 production across the mono-/co- and mixed-culture systems was carried out. The co-culture system of two defined microorganisms possesses increased substrate inhibition effect in comparison to single pure culture [4,11]. Two strains Enterobacter aerogenes and Clostridium butyricum, commonly used for H2 production using CG [13] were selected along with mixed-culture. The optimum condition for mixed-culture (CG: 20 g/L, InS: 20%) was carried out using mono- (C. butyricum) and co-culture system comprising (E. aerogenes and C. butyricum) for H2 production. The comparison across mono-, co- and mixed-culture system for the microorganisms used, growth media, incubation condition, incubation time along with cumulative H2 production and yield is presented in Table 5.
Table 5.
Comparison of pure-, co- and mixed-culture hydrogen production processes in terms of inoculum development, hydrogen yield and by-product (1,3-PD) concentration.
| Hydrogen Production Steps | Hydrogen Production System | ||
|---|---|---|---|
| Mono-Culture | Co-Culture | Mixed-Culture | |
| Microorganisms used | Clostridium butyricum | Enterobacter aerogenes + Clostridium butyricum | Seed wastewater sludge |
| Growth media | Glucose, casein peptone, KH2PO4, MgSO4·7H2O, yeast extract and l-cysteine | Glucose, casein peptone, KH2PO4, MgSO4·7H2O, yeast extract and l-cysteine | Without growth media |
| Incubation conditions | 37 °C at 18 h | 37 °C at 18 h | 100 °C for 15 min |
| Incubation time | 18 h | 18 h | 15 min |
| Cumulative Hydrogen production (mmol/L) | 13.92 ± 0.62 | 24.85 ± 0.92 | 29.43 ± 0.71 |
| Hydrogen Yield (mol H2/mol glycerol) | 0.39 | 0.69 | 0.82 |
| 1,3-PD concentration (g/L) | 6.54 ± 0.38 | 6.01 ± 0.62 | 5.12 ± 0.59 |
The media pH conditions for mono- and co-culture was around 6.5, which is optimum for H2 production [2]. The cumulative H2 production (mmol/L) for mono- (13.92 ± 0.62) and co-culture system (24.85 ± 0.92) was lower in comparison to mixed-culture system (29.43 ± 0.71) and was also true in case of H2 yield (as seen in Table 5). Cost distribution in case of pure culture with techno-economic analysis using CG by Saurabh et al., suggested alternative options for reduction of process cost [14]. One such alternative option can be use of mixed-culture system over pure- and co-culture systems to minimize the process cost in terms of growth media and incubation conditions. The complex growth media components in case of pure culture require glucose, casein peptone, KH2PO4, MgSO4·7H2O, yeast extract and l-cysteine. However, using a heat pretreatment approach for selecting H2 producing organisms requires no growth media components in comparison to enrichment techniques with complex modified HM 100 medium used [12]. The incubation time for pure-/co-culture system varies from 12–18 h depending upon the microorganism selected and is also similar to time required during enrichment technique. However, in the case of the mixed-culture, the heat pretreatment option can be carried out in only 15 min. The mixed-culture system in comparison to mono- and co-culture system in terms of growth media, incubation condition and time holds an additional advantage along with increased H2 production. In addition to increased H2 production, the mixed-culture also possesses the ability to work at substrate inhibitor concentration, which determines glycerol degrading syntrophic H2 producing microorganisms. The consortium of mixed-culture obtained from the wastewater sludge was able to produce maximum H2, even at substrate inhibition concentration (20 g/L). The increase in concentration from 15 to 20 g/L for E. aerogenes and C. butyricum during mono- and co-culture studies resulted in decreased H2 production, indicating substrate inhibition concentration at 20 g/L [2,5,10]. The wastewater sludge is the largest depositor of different kinds of microorganisms, possessing increased substrate inhibition along with increased glycerol degrading ability. The pure- and co-culture at substrate inhibition concentration (20 g/L) tends to produce 1,3-PD at higher concentration (6.54 ± 0.38 and 6.01 ± 0.62 g/L) with decreased H2 production (as seen in Table 5). The substrate inhibition concentration on pure- and co-culture shifts the metabolic pathway towards reductive pathway for 1,3-PD production instead of H2 production through the oxidative pathway. The higher the concentration of CG in the fermentation media, the higher is the production of 1,3-PD using pure- and co-culture systems (as seen in Table 5) [2,10]. The mixed-culture overcomes the substrate inhibition concentration of CG with increased H2 yield in comparison to pure- and co-culture system as seen from the Table 5. The microbial community analysis after the heat pretreatment belonged mostly to Clostridium family, and showed dominance over non-hydrogen producing microorganism [32]. However, to understand the population dynamics of mixed-culture system and its ability to work at higher CG concentration in the absence of complex media components are investigations for the future.
3. Materials and Methods
3.1. Crude Glycerol as Substrate
CG was supplied by Rothsay® (Winnipeg, MB, Canada) that uses inedible fat containing waste from meat processing plants and used grease from restaurants for biodiesel production [5]. The CG contained (w/w): up to 23.6% of glycerol, 35.9% carbon and 3.2% nitrogen, 3.06% ash, 5.75% moisture and 67.56% matter organic non-glycerol [2]. This is the first time that CG derived from the processing of animal fat and restaurant waste was used for anaerobic digestion for H2 production using mixed-culture.
3.2. Seed Inoculum
The wastewater sludge was collected from Quebec Urban Community (QUC) wastewater treatment plant (WWTP) (Quebec, QC, Canada). The secondary sludge sample was collected in pre-cleaned high-density polyethylene (HDPE) containers and stored at 4 ± 1 °C, until further use. The characteristics of secondary sludge comprised in (g/L): total solids (TS) (9.15 ± 0.13), suspended solids (SS) (7.22 ± 0.35), total chemical oxygen demand (TCOD) (6.11 ± 1.4), total organic carbon (TOC) (411.82 ± 0.66), ammonia-nitrogen (0.21 ± 0.01) and pH (6.14 ± 0.41) as analyzed by [34].
3.3. Evaluation of Different Pretreatment Methods for Preparation of Seed Inoculum
Different pretreatment techniques for wastewater sludge to enhance and optimize the H2 production efficiency using CG as substrate were investigated. The top five pretreatment techniques, such as heat, acid, alkali, microwave, and chloroform use on wastewater to inhibit the H2 consuming bacteria and screen H2 producing mixed-culture were selected.
The pretreatment conditions employed were as follows: (a) Heat pretreatment (heat-shock): 50 mL of wastewater sludge was transferred into a 150 mL serum bottle, sparged with N2 gas for 4 min to create anaerobic condition, sealed with pre-inserted septa (Headspace 20 mm Crimp Seals with Septa, Thermo Scientific™, Pittsburgh, PA, USA) kept at 100 °C for 15 min in an Isotemp Standard Lab Ovens (Fisher Scientific™, Pittsburgh, PA, USA) and later cooled prior to inoculation for H2 production [35]; (b) Acid pretreatment: The pH of wastewater sludge (50 mL) was adjusted to 3.0 using 0.1 M HCl, kept at room temperature for 24 h, later pH was re-adjusted to 6.5 (working pH) using 0.1 M NaOH, transferred to serum bottle, sealed and sparged with N2 gas, prior to use [35]; (c) Alkali pretreatment: Similar to preparation steps of acid treatment, pH of wastewater sludge was adjusted to 12 using 0.1 M NaOH at room temperature 24 h and re-adjusted to working pH 6.5 [35]; (d) Microwave pretreatment: The wastewater sludge of around 50 mL (2 × 25 mL) was put in the microwave (MARS microwave extractor, CEM Corporation, Matthews, NC, USA) at conditions pressure (120 psi) for 2 min at 560–600 W maintained at 90 °C, the treated wastewater was collected, sparged and sealed prior to use [36]; (e) Chloroform pretreatment: The wastewater sludge (50 mL) was mixed with chloroform (0.05% v/v), transferred to serum bottle, incubated for 24 h, sparged and sealed prior to inoculation [37].
3.4. Hydrogen Production Using Modified Basal Media
A fermentation media containing 1% (w/v) CG, 2% casein peptone, 0.2% KH2PO4, 0.05% MgSO4·7H2O and 0.05% yeast extract was maintained at a pH = 6.5 and transferred to serum bottles with a working volume of 50 mL. The headspace of the bottles was purged with pure N2 gas for 4 min to create anaerobic conditions and later sealed with pre-inserted septa (headspace 20 mm crimp seals with septa, Thermo Scientific™) followed by sterilization in an autoclave (Tuttnauer 3870-Heidolph) [5]. The pretreated sludge with 5% (v/v) inoculum size was transferred into the culture broth using a sterile syringe (All-Plastic Norm-Ject™ Syringes, Thermo Scientific™) along with control experiments without any pretreatment of sludge were performed simultaneously. The serum bottles were incubated in an orbital incubator shaker (INFORS HT-multitron standard) at 150 rpm at different temperatures (30, 37 and 55 °C) for five days. All batch experiments in the study were performed in triplicates, presented values are the average of triplicates and error bars represent the standard deviation (±) values.
3.5. Investigating Process Parameters Using Statistical Model
The best pretreatment method resulting in increased H2 production was later selected for optimization experiments. Central composite design (CCD) was used to investigate the effects of substrate (CG) concentration (g/L), inoculum size (InS) (%) and fermentation pH using Design-Expert 7 software (Stat-Ease Inc., Minneapolis, MN, USA), which resulted in 20 set of experiments. The central composite design matrix comprising of varied CG concentration along with different inoculum size and pH is given in Table 2. Across the 20 experiments, the central point of the model with CG (13 g/L), InS (13%) and pH (5.5) appeared 6 times.
Each set of experiment was performed at exact CG concentration along with media supplement (casein polypeptone, KH2PO4, yeast extract and MgSO4·7H2O) was mixed in distilled water to make-up the required volume (final volume (50 mL) minus inoculum size). The required volume was pH adjusted, transferred to serum bottle, purged, sealed and sterilized. After inoculation, serum bottles were incubated in an orbital shaker at 150 rpm for 5 days at 30 °C. Each experimental run was carried out in triplicates. The gas sample (1 mL) for every 24 h was collected from the headspace using a gas tight syringe in vacuumed sample vials for H2 analysis by gas chromatography (GC). The aqueous sample at the end of fermentation was analyzed for glycerol and end 1,3-PD concentration by GC, as described later.
In the response surface methodology (RSM), the H2 production (mmol/L) and 1,3-propanediol (g/L) was chosen as the response variable. The interaction and relation between the input and the responses variables was determined by design matrix evaluation considering the significance p-value, R2 values for the models tested and final model equation in terms of factors was obtained from the analysis of variance (ANOVA) [2,38].
3.6. Comparative Study of Hydrogen Production across Pure-, Co- and Mixed-Culture
The optimized conditions of mixed-culture obtained from CCD model were used for a comparative study across pure culture of Enterobacter aerogenes, Clostridium butyricum and co-culture of these two bacteria with mixed-culture for H2 production. The anaerobic growth of E. aerogenes and C. butyricum were carried out in presence of basal media (minimal media) containing glucose (10 g/L), peptone (20 g/L), KH2PO4 (2 g/L), yeast extract (0.5 g/L), MgSO4·7H2O (0.5 g/L) and l-cysteine-HCl·H2O (1 g/L) included in case of C. butyricum [2,5,10]. The media preparation, sterilization and incubation were the same as explained in the previous section (H2 production using modified basal media).
3.7. Analytical Techniques
3.7.1. Analysis of Hydrogen by GC
The collected gas sample using gas tight syringe in vacuumed sample vials at the end of fermentation was analyzed by gas chromatography (Agilent technology, Santa Clara, CA, USA) fitted with a 3 m PoraPLOT Q® column (Agilent technology, Santa Clara, CA, USA) and equipped with a thermal conductivity detector (TCD). The GC set-up with injector, column temperature and detector temperature set at 100 °C and carrier gas nitrogen was used at a flow rate of 3.5 mL/min [10]. The volume of gas produced was calculated and converted to mmol, considering the temperature and atmospheric pressure during the experimental runs [38].
3.7.2. Analysis of End-Metabolites/by-Products by GC-FID
The concentrations of 1,3-propanediol and glycerol were analysed on ZB-WAX plus column fitted with flame ionization detector (FID) detector in a gas chromatography (GC) (7890B GC-Agilent, Santa Clara, CA, USA) set-up. The GC condition at a flow rate of 1 mL/min using helium carrier gas at a temperature profile of 80–240 °C under 8.4 min method run time was developed [5].
4. Conclusions
The enrichment of H2 producing bacteria using five pretreatment methods (by acid, base, heat, chloroform and microwave respectively) were evaluated for H2 production from wastewater sludge using CG as substrate. The heat treatment resulted in eliminating H2 consuming bacteria with highest hydrogen production (15.18 ± 0.26 mmol/L) at 30 °C across the pretreatment methods. The CCD model was significant with (p-value: 0.028 and <0.0001) to identify the optimal CG concentration (20 g/L), inoculum size (20%) and pH (7) that positively influenced for increased H2 production (29.43 ± 0.71 mmol/L) with decreased by-product (1,3-PD: 5.12 ± 0.59 g/L) production. High correlation of pH, CG and InS resulted with model significant p-value of 0.028. The pH (p-value: 0.0011) had the highest impact on the H2 production in comparison to other parameters (InS and CG conc.). In the present study, under optimized conditions, the mixed-culture reported maximum H2 production and also provided efficient inoculum conditions in comparison to mono- and co-culture system. The heat pretreatment step used in this mixed-culture system is simple, cheap and industrially applicable in comparison to pure-/co-culture system for H2 production.
Acknowledgments
Financial support from NSERC (No. 284111, Discovery; No. 476649-14, Collaborative Research and Development Grant) and INRS-ETE has been acknowledged.
Author Contributions
Vinayak Laxman Pachapur planned the experiments, carried out data analysis and prepared the manuscript; Prianka Kutty performed the experiments and analysed the samples; Satinder Kaur Brar and Antonio Avalos Ramirez corrected the manuscript and supervised the research work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Varrone C., Rosa S., Fiocchetti F., Giussani B., Izzo G., Massini G., Marone A., Signorini A., Wang A. Enrichment of activated sludge for enhanced hydrogen production from crude glycerol. Int. J. Hydrog. Energy. 2013;38:1319–1331. doi: 10.1016/j.ijhydene.2012.11.069. [DOI] [Google Scholar]
- 2.Pachapur V.L., Sarma S.J., Brar S.K., Le Bihan Y., Buelna G., Verma M. Biohydrogen production by co-fermentation of crude glycerol and apple pomace hydrolysate using co-culture of Enterobacter aerogenes and Clostridium butyricum. Bioresour. Technol. 2015;193:297–306. doi: 10.1016/j.biortech.2015.06.095. [DOI] [PubMed] [Google Scholar]
- 3.Ayoub M., Abdullah A.Z. Critical review on the current scenario and significance of crude glycerol resulting from biodiesel industry towards more sustainable renewable energy industry. Renew. Sustain. Energy Rev. 2012;16:2671–2686. doi: 10.1016/j.rser.2012.01.054. [DOI] [Google Scholar]
- 4.Pachapur V.L., Sarma S.J., Brar S.K., Le Bihan Y., Buelna G., Verma M. Biological hydrogen production using co-culture versus mono-culture system. Environ. Technol. Rev. 2015;4:55–70. doi: 10.1080/21622515.2015.1068381. [DOI] [Google Scholar]
- 5.Pachapur V., Sarma S., Brar S., Bihan Y., Buelna G., Verma M. Hydrogen production from biodiesel industry waste by using a co-culture of Enterobacter aerogenes and Clostridium butyricum. Biofuels. 2015 doi: 10.1080/17597269.2015.1122471. [DOI] [PubMed] [Google Scholar]
- 6.Jitrwung R., Yargeau V. Biohydrogen and bioethanol production from biodiesel-based glycerol by Enterobacter aerogenes in a continuous stir tank reactor. Int. J. Mol. Sci. 2015;16:10650–10664. doi: 10.3390/ijms160510650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amaral P.F.F., Ferreira T.F., Fontes G.C., Coelho M.A.Z. Glycerol valorization: New biotechnological routes. Food Bioprod. Process. 2009;87:179–186. doi: 10.1016/j.fbp.2009.03.008. [DOI] [Google Scholar]
- 8.Varrone C., Giussani B., Izzo G., Massini G., Marone A., Signorini A., Wang A. Statistical optimization of biohydrogen and ethanol production from crude glycerol by microbial mixed culture. Int. J. Hydrog. Energy. 2012;37:16479–16488. doi: 10.1016/j.ijhydene.2012.02.106. [DOI] [Google Scholar]
- 9.Varrone C., Heggeset T., Le S., Haugen T., Markussen S., Skiadas I., Gavala H. Comparison of different strategies for selection/adaptation of mixed microbial cultures able to ferment crude glycerol derived from second-generation biodiesel. Biomed Res. Int. 2015 doi: 10.1155/2015/932934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pachapur V.L., Sarma S.J., Brar S.K., Le Bihan Y., Buelna G., Soccol C.R. Evidence of metabolic shift on hydrogen, ethanol and 1,3-propanediol production from crude glycerol by nitrogen sparging under micro-aerobic conditions using co-culture of Enterobacter aerogenes and Clostridium butyricum. Int. J. Hydrog. Energy. 2015;40:8669–8676. doi: 10.1016/j.ijhydene.2015.05.024. [DOI] [Google Scholar]
- 11.Laxman Pachapur V., Jyoti Sarma S., Kaur Brar S., Le Bihan Y., Ricardo Soccol C., Buelna G., Verma M. Co-culture strategies for increased biohydrogen production. Int. J. Energy Res. 2015;39:1479–1504. doi: 10.1002/er.3364. [DOI] [Google Scholar]
- 12.Mangayil R., Aho T., Karp M., Santala V. Improved bioconversion of crude glycerol to hydrogen by statistical optimization of media components. Renew. Energy. 2015;75:583–589. doi: 10.1016/j.renene.2014.10.051. [DOI] [Google Scholar]
- 13.Sarma S.J., Brar S.K., Sydney E.B., Le Bihan Y., Buelna G., Soccol C.R. Microbial hydrogen production by bioconversion of crude glycerol: A review. Int. J. Hydrog. Energy. 2012;37:6473–6490. doi: 10.1016/j.ijhydene.2012.01.050. [DOI] [Google Scholar]
- 14.Sarma S.J., Brar S.K., Le Bihan Y., Buelna G. Bio-hydrogen production by biodiesel-derived crude glycerol bioconversion: A techno-economic evaluation. Bioprocess. Biosyst. Eng. 2013;36:1–10. doi: 10.1007/s00449-012-0755-8. [DOI] [PubMed] [Google Scholar]
- 15.Sydney E.B., Larroche C., Novak A.C., Nouaille R., Sarma S.J., Brar S.K., Letti L.A.J., Soccol V.T., Soccol C.R. Economic process to produce biohydrogen and volatile fatty acids by a mixed culture using vinasse from sugarcane ethanol industry as nutrient source. Bioresour. Technol. 2014;159:380–386. doi: 10.1016/j.biortech.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 16.Romão B., Batista F., Ferreira J., Costa H., Resende M., Cardoso V. Biohydrogen production through dark fermentation by a microbial consortium using whey permeate as substrate. Appl. Biochem. Biotechnol. 2014;172:3670–3685. doi: 10.1007/s12010-014-0778-5. [DOI] [PubMed] [Google Scholar]
- 17.Phowan P., Danvirutai P. Hydrogen production from cassava pulp hydrolysate by mixed seed cultures: Effects of initial pH, substrate and biomass concentrations. Biomass Bioenergy. 2014;64:1–10. doi: 10.1016/j.biombioe.2014.03.057. [DOI] [Google Scholar]
- 18.Jürgensen L., Ehimen E.A., Born J., Holm-Nielsen J.B. Hydrogen production using an anaerobic baffled reactor–mass balances for pathway analysis and gas composition profiles. Int. J. Hydrog. Energy. 2015;40:12154–12161. doi: 10.1016/j.ijhydene.2015.07.068. [DOI] [Google Scholar]
- 19.Ali Shah F., Mahmood Q., Maroof Shah M., Pervez A., Ahmad Asad S. Microbial ecology of anaerobic digesters: The key players of anaerobiosis. Sci. World J. 2014;21 doi: 10.1155/2014/183752. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Yang Z., Xu X., Guo R., Fan X., Zhao X. Accelerated methanogenesis from effluents of hydrogen-producing stage in anaerobic digestion by mixed cultures enriched with acetate and nano-sized magnetite particles. Bioresour. Technol. 2015;190:132–139. doi: 10.1016/j.biortech.2015.04.057. [DOI] [PubMed] [Google Scholar]
- 21.Chandrasekhar K., Lee Y.-J., Lee D.-W. Biohydrogen production: Strategies to improve process efficiency through microbial routes. Int. J. Mol. Sci. 2015;16:8266–8293. doi: 10.3390/ijms16048266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin Y., Hu J., Wang J. Gamma irradiation as a pretreatment method for enriching hydrogen-producing bacteria from digested sludge. Int. J. Hydrog. Energy. 2014;39:13543–13549. doi: 10.1016/j.ijhydene.2014.01.147. [DOI] [Google Scholar]
- 23.Pradhan N., Dipasquale L., d'Ippolito G., Panico A., Lens P.N., Esposito G., Fontana A. Hydrogen production by the thermophilic bacterium Thermotoga neapolitana. Int. J. Mol. Sci. 2015;16:12578–12600. doi: 10.3390/ijms160612578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandra R., Nikhil G., Mohan S.V. Single-stage operation of hybrid dark-photo fermentation to enhance biohydrogen production through regulation of system redox condition: Evaluation with real-field wastewater. Int. J. Mol. Sci. 2015;16:9540–9556. doi: 10.3390/ijms16059540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheong D.-Y., Hansen C.L. Bacterial stress enrichment enhances anaerobic hydrogen production in cattle manure sludge. Appl. Microbiol. Biotechnol. 2006;72:635–643. doi: 10.1007/s00253-006-0313-x. [DOI] [PubMed] [Google Scholar]
- 26.Hu B., Chen S. Pretreatment of methanogenic granules for immobilized hydrogen fermentation. Int. J. Hydrog. Energy. 2007;32:3266–3273. doi: 10.1016/j.ijhydene.2007.03.005. [DOI] [Google Scholar]
- 27.Sittijunda S., Reungsang A. Biohydrogen production from waste glycerol and sludge by anaerobic mixed cultures. Int. J. Hydrog. Energy. 2012;37:13789–13796. doi: 10.1016/j.ijhydene.2012.03.126. [DOI] [Google Scholar]
- 28.Sarma S.J., Brar S.K., Le Bihan Y., Buelna G., Soccol C.R. Hydrogen production from meat processing and restaurant waste derived crude glycerol by anaerobic fermentation and utilization of the spent broth. J. Chem. Technol. Biotechnol. 2013;88:2264–2271. doi: 10.1002/jctb.4099. [DOI] [Google Scholar]
- 29.Wilkens E., Ringel A.K., Hortig D., Willke T., Vorlop K.-D. High-level production of 1,3-propanediol from crude glycerol by Clostridium butyricum akr102a. Appl. Microbiol. Biotechnol. 2012;93:1057–1063. doi: 10.1007/s00253-011-3595-6. [DOI] [PubMed] [Google Scholar]
- 30.Wang J., Wan W. Comparison of different pretreatment methods for enriching hydrogen-producing bacteria from digested sludge. Int. J. Hydrog. Energy. 2008;33:2934–2941. doi: 10.1016/j.ijhydene.2008.03.048. [DOI] [Google Scholar]
- 31.Mu Y., Yu H.-Q., Wang G. Evaluation of three methods for enriching h 2-producing cultures from anaerobic sludge. Enzym. Microb. Technol. 2007;40:947–953. doi: 10.1016/j.enzmictec.2006.07.033. [DOI] [Google Scholar]
- 32.Selembo P.A., Perez J.M., Lloyd W.A., Logan B.E. Enhanced hydrogen and 1,3-propanediol production from glycerol by fermentation using mixed cultures. Biotechnol. Bioeng. 2009;104:1098–1106. doi: 10.1002/bit.22487. [DOI] [PubMed] [Google Scholar]
- 33.Rossi D.M., Da Costa J.B., de Souza E.A., Peralba M.D.C.R., Samios D., Ayub M.A.Z. Comparison of different pretreatment methods for hydrogen production using environmental microbial consortia on residual glycerol from biodiesel. Int. J. Hydrog. Energy. 2011;36:4814–4819. doi: 10.1016/j.ijhydene.2011.01.005. [DOI] [Google Scholar]
- 34.Puicharla R., Mohapatra D., Brar S., Drogui P., Auger S., Surampalli R. A persistent antibiotic partitioning and co-relation with metals in wastewater treatment plant—chlortetracycline. J. Environ. Chem. Eng. 2014;2:1596–1603. doi: 10.1016/j.jece.2014.06.001. [DOI] [Google Scholar]
- 35.Chen C.C., Lin C.Y., Lin M.C. Acid–base enrichment enhances anaerobic hydrogen production process. Appl. Microbiol. Biotechnol. 2002;58:224–228. doi: 10.1007/s002530100814. [DOI] [PubMed] [Google Scholar]
- 36.Guo L., Li X.M., Bo X., Yang Q., Zeng G.M., Liao D.X., Liu J.J. Impacts of sterilization, microwave and ultrasonication pretreatment on hydrogen producing using waste sludge. Bioresour. Technol. 2008;99:3651–3658. doi: 10.1016/j.biortech.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 37.Hu B., Chen S. Biological hydrogen production using chloroform-treated methanogenic granules. Appl. Biochem. Biotechnol. 2008;148:83–95. doi: 10.1007/s12010-007-8048-4. [DOI] [PubMed] [Google Scholar]
- 38.Sarma S.J., Dhillon G.S., Brar S.K., Le Bihan Y., Buelna G., Verma M. Investigation of the effect of different crude glycerol components on hydrogen production by Enterobacter aerogenes nrrl b-407. Renew. Energy. 2013;60:566–571. doi: 10.1016/j.renene.2013.06.007. [DOI] [Google Scholar]