Abstract
Aquaporin-2, a member of the aquaporin family, is an arginine vasopressin-regulated water channel expressed in the renal collecting duct, and a promising marker of the concentrating and diluting ability of the kidney. The arginine vasopressin type-2 antagonist, tolvaptan, is a new-generation diuretic; it is especially indicated in patients with decompensated heart failure refractory to conventional diuretics. However, the ideal responders to tolvaptan have not yet been identified, and non-responders experience worse clinical courses despite treatment with tolvaptan. Urine aquaporin-2 has recently been demonstrated as a promising predictor of response to tolvaptan. We here validated aquaporin-2-guided tolvaptan therapy in patients with decompensated heart failure. Long-term efficacy of tolvaptan treatment in the responders defined by aquaporin-2 needs to be validated in the future prospective study.
Keywords: heart failure, congestion, chronic kidney disease, hyponatremia
1. Aquaporin-2 in Patients with Congestive Heart Failure (HF)
The aquaporin family has various activities in the human body, such as mediating water transport, and cell adhesion, migration, proliferation, and differentiation [1]. Of the aquaporin family members, aquaporin-2 has been characterized as an arginine vasopressin (AVP)-regulated water channel protein translocating between the apical plasma membrane and subapical vesicles in the principal cells of the collecting duct [2].
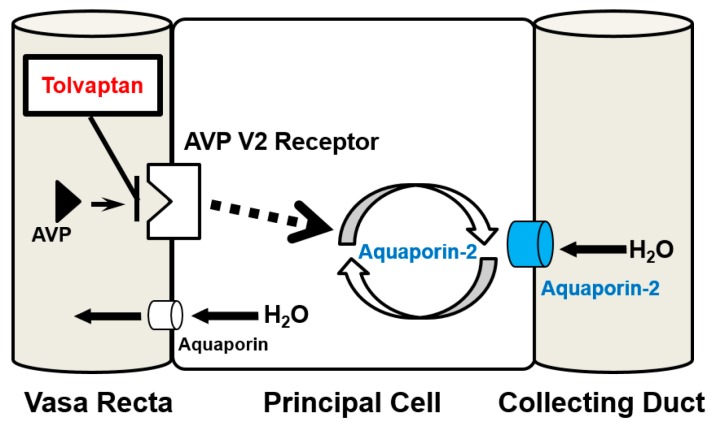
The mechanisms of aquaporin-2-mediated water retention have been studied for the past 20 years [3]. Secreted AVP binds to the AVP type-2 (V2) receptor located at the basolateral membrane of principal cells (Figure 1). Activation of the V2 receptor triggers the trafficking of aquaporin-2 from intracellular storage vesicles to the apical membrane by cAMP-dependent phosphorylation of aquaporin-2 [4,5]. Translocation of aquaporin-2 to the apical membrane increases water permeability, thereby increasing urine osmolality (U-OSM) [6].
Figure 1.
The relationship between arginine vasopressin, aquaporin-2, and tolvaptan in the collecting duct. AVP, arginine vasopressin.
Approximately 3% of aquaporin-2, in particular phosphorylated and translocated aquaporin-2, in the kidney tissue is excreted in urine [7]. Therefore, urine aquaporin-2 is considered a marker of collecting duct responsiveness to AVP [8].
Several techniques have been developed to quantify urine aquaporin-2, such as radioimmunoassay, Western blotting, and sandwich enzyme-linked immunosorbent assay [7,9,10], and urine aquaporin-2 has been quantified in various clinical conditions including pregnancy [11], liver cirrhosis [12], syndrome of inappropriate secretion of antidiuretic hormone [5], diabetes insipidus [4], and HF [13,14].
In patients with congestive HF, AVP is inappropriately secreted through the activation of a non-osmotic pathway owing to reduced effective circulating volume, despite peripheral water retention [15,16,17,18]. Secreted AVP stimulates the expression and translocation of aquaporin-2 at the apical membrane of the collecting duct [4,5], thereby facilitating water retention and successive hypervolemic hyponatremia (Figure 1) [15]. Therefore, urine aquaporin-2 level increases in patients with HF owing to inappropriately elevated serum AVP level. Plasma level of AVP increases in association with HF progression, accompanied by activation of the renin-angiotensin-aldosterone system and sympathetic nerve system, which facilitate water retention [15,16,17,19]. Many studies have demonstrated that hyponatremia is a predictor of morbidity and mortality in patients with HF [20,21,22,23].
2. Tolvaptan and Its Responsiveness
Tolvaptan, the only commercially available vasopressin V2 receptor antagonist, thus far, has been recently developed as a promising agent to treat patients with congestive HF refractory to conventional diuretics [24]. It has a unique therapeutic target: it blocks AVP-aquaporin-2 pathway, and increases the excretion of electrolyte-free water in urine (Figure 1). Many studies have demonstrated the short-term efficacy and safety of tolvaptan; it also ameliorates symptomatic congestion, normalizes hyponatremia to maintain hemodynamics and the sparing renal function, and terminates the vicious cycle of HF described above [25,26,27,28,29,30,31,32,33,34,35]. However, these benefits may not be expected in non-responders, whose urine volume does not increase despite the administration of tolvaptan [36]. Tolvaptan could not normalize serum sodium concentration, ameliorate symptomatic congestion, and improve renal function in non-responders. Moreover, indeterminate administration of tolvaptan to non-responders may result in a loss of the optimal timing of intensive treatment [37].
Tolvaptan was not found to be superior to placebo in terms of long-term survival rate in the Efficacy of Vasopressin Antagonist in HF Outcome Study with Tolvaptan (EVEREST) trial [38]. Considering that tolvaptan demonstrated improved survival rate over the placebo in patients with hyponatremia in this subanalysis [39], it may improve long-term prognosis only in a specific population. Therefore, it is necessary to identify the optimal population, i.e., responders [40,41].
3. Tolvaptan and Aquaporin-2
Several studies recently demonstrated that unresponsiveness to tolvaptan is associated with decreased renal function [42,43,44]. Older patients with chronic kidney disease have a trend of physiological insipidus mellitus, and have reduced urine concentrating ability. Tolvaptan may not be able to inhibit the already-extinct AVP-aquaporin-2 system in the collecting duct, and the residual function of the collecting duct is essential for response to tolvaptan [40,45]. Therefore, urine aquaporin-2 has been evaluated as a predictive “marker” of responsiveness to tolvaptan [46].
We recently demonstrated that higher urine aquaporin-2 level at baseline relative to plasma AVP was a novel predictor of responsiveness to tolvaptan [37], because urine aquaporin-2 level increases in association with AVP stimulation under the well-preserved function of the collecting duct. Higher level of urine aquaporin-2 is associated with elevated U-OSM in the responders [37]. Thus, U-OSM could be an alternative to urine aquaporin-2 for the prediction of responses to tolvaptan [40].
In contrast, urine aquaporin-2 levels in non-responders were not detectable, regardless of serum AVP level [37]. In general, expression level of aquaporin-2 decreases in patients with chronic kidney disease [47]. Sato et al. showed that aquaporin-2 is not expressed in the renal tissue of non-responders with diabetes nephropathy [48]. However, not all patients with chronic kidney disease have reduced urine excretion of aquaporin-2. Some patients have preserved function of the collecting duct despite decreased glomerular filtration ratio, and such patients are classified as responders to tolvaptan [37]. However, several studies reported that even patients with advanced chronic kidney disease responded to tolvaptan [43,44,45,49,50]. Therefore, urine aquaporin-2 could be a novel predictor to estimate the responders to tolvaptan especially among those with chronic kidney disease.
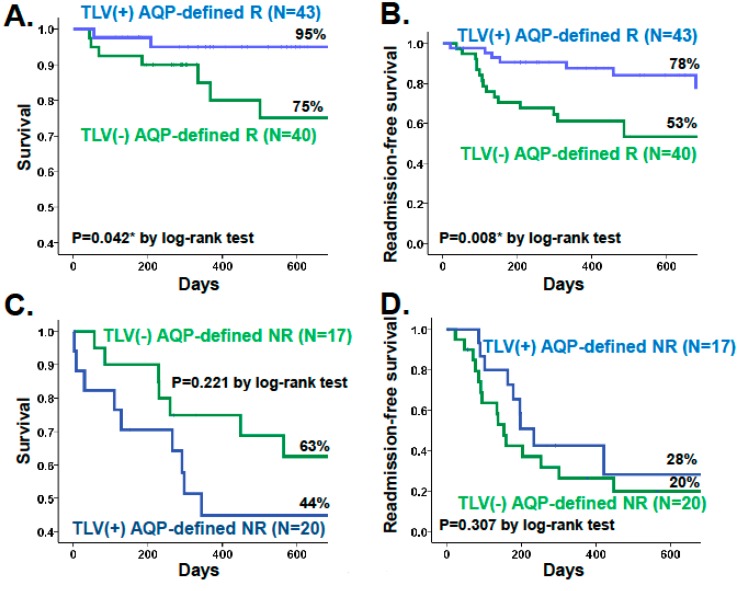
Long-term improvements in survival and re-admission-free survival rate were observed in the responders receiving tolvaptan, who were defined by urine aquaporin-2, although the study was retrospective (Figure 2A,B) [37]. In this study, potential response was defined as baseline urine aquaporin-2/serum AVP level >1.4 × 103 L/gCre, and those receiving tolvaptan were compared with propensity score-matched control group. In contrast, non-responders, whose urine aquaporin-2/erum AVP level <1.4 × 103 L/gCre, could neither achieve improvement in survival nor in re-admission-free survival rate despite tolvaptan administration, compared with propensity score-matched non-responders without tolvaptan (Figure 2A,B). Tolvaptan ameliorated symptomatic congestion, normalized hyponatremia, improved renal function, and reduced the dose of diuretics in the aquaporin-defined responders. These effects may improve patients’ prognosis during long-term tolvaptan treatment. Prospective randomized trials are warranted to evaluate long-term improvement in the prognosis of the aquaporin-defined responders receiving tolvaptan.
Figure 2.
Kaplan-Meier curves showing survival and readmission-free survival rate stratified by the administration of tolvaptan in responders (A,B) and non-responders (C,D). R, responder; NR, non-responder; TLV, tolvaptan, AQP, aquaporin.
Measurement of urine aquaporin-2 is also useful in confirming the response to tolvaptan in a timely fashion. Martin et al. showed that urine aquaporin-2 level decreased accompanied by an increase in urine volume from 2 h after the administration of tolvaptan [14]. Udelson et al. demonstrated a decrease in urine osmolality at 4 h after tolvaptan administration, which indicates decreased aquaporin-2 activity [31]. The decreased level of urine aquaporin-2 is restored the next morning, but does not reach the baseline level, which may indicate prolonged blockade of V2 receptor by tolvaptan for >24 h [51]. In contrast, urine aquaporin-2 remained undetectable during tolvaptan treatment in the non-responders [51]. Persistent undetectable level of urine aquaporin-2 regardless of tolvaptan administration indicates lack of response to tolvaptan.
4. Conclusions
In conclusion, urine aquaporin-2 is a novel predictor of responsiveness to tolvaptan, and the responders achieved amelioration of symptomatic congestion, normalization of hyponatremia, and improvement in renal function during tolvaptan treatment. Long-term prognosis in the aquaporin-defined responders during tolvaptan treatment should be confirmed in a prospective randomized trial in the future.
Abbreviations
- HF
heart failure
- AVP
arginine vasopressin
- U-OSM
urine osmolality
Acknowledgments
This work was supported by a Grants-in-aid from Japan Society for the Promotion of Science.
Author Contributions
Koichiro Kinugawa desinged and reviewd the study; Teruhiko Imamura desgined the study and wrote the paper
Conflicts of Interest
The authors declare no conflict of interest. This work was supported by a Grants-in-aid from Japan Society for the Promotion of Science.
References
- 1.Ishibashi K., Kondo S., Hara S., Morishita Y. The evolutionary aspects of aquaporin family. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;300:R566–R576. doi: 10.1152/ajpregu.90464.2008. [DOI] [PubMed] [Google Scholar]
- 2.Park E.J., Kwon T.H. A minireview on vasopressin-regulated aquaporin-2 in kidney collecting duct cells. Electrol. Blood Press. 2015;13:1–6. doi: 10.5049/EBP.2015.13.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishikawa S.E. Hyponatremia associated with heart failure: Pathological role of vasopressin-dependent impaired water excretion. J. Clin. Med. 2015;4:933–947. doi: 10.3390/jcm4050933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanno K., Sasaki S., Hirata Y., Ishikawa S., Fushimi K., Nakanishi S., Bichet D.G., Marumo F. Urinary excretion of aquaporin-2 in patients with diabetes insipidus. N. Engl. J. Med. 1995;332:1540–1545. doi: 10.1056/NEJM199506083322303. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa S., Saito T., Fukagawa A., Higashiyama M., Nakamura T., Kusaka I., Nagasaka S., Honda K., Saito T. Close association of urinary excretion of aquaporin-2 with appropriate and inappropriate arginine vasopressin-dependent antidiuresis in hyponatremia in elderly subjects. J. Clin. Endocrinol. Metab. 2001;86:1665–1671. doi: 10.1210/jc.86.4.1665. [DOI] [PubMed] [Google Scholar]
- 6.Robertson G.L., Mahr E.A., Athar S., Sinha T. Development and clinical application of a new method for the radioimmunoassay of arginine vasopressin in human plasma. J. Clin. Investig. 1973;52:2340–2352. doi: 10.1172/JCI107423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rai T., Sekine K., Kanno K., Hata K., Miura M., Mizushima A., Marumo F., Sasaki S. Urinary excretion of aquaporin-2 water channel protein in human and rat. J. Am. Soc. Nephrol. 1997;8:1357–1362. doi: 10.1681/ASN.V891357. [DOI] [PubMed] [Google Scholar]
- 8.Elliot S., Goldsmith P., Knepper M., Haughey M., Olson B. Urinary excretion of aquaporin-2 in humans: A potential marker of collecting duct responsiveness to vasopressin. J. Am. Soc. Nephrol. 1996;7:403–409. doi: 10.1681/ASN.V73403. [DOI] [PubMed] [Google Scholar]
- 9.Umenishi F., Summer S.N., Cadnapaphornchai M., Schrier R.W. Comparison of three methods to quantify urinary aquaporin-2 protein. Kidney Int. 2002;62:2288–2293. doi: 10.1046/j.1523-1755.2002.00686.x. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki S., Ohmoto Y., Mori T., Iwata F., Muraguchi M. Daily variance of urinary excretion of AQP2 determined by sandwich elisa method. Clin. Exp. Nephrol. 2012;16:406–410. doi: 10.1007/s10157-011-0574-2. [DOI] [PubMed] [Google Scholar]
- 11.Buemi M., D'Anna R., di Pasquale G., Floccari F., Ruello A., Aloisi C., Leonardi I., Frisina N., Corica F. Urinary excretion of aquaporin-2 water channel during pregnancy. Cell. Physiol. Biochem. 2001;11:203–208. doi: 10.1159/000047807. [DOI] [PubMed] [Google Scholar]
- 12.Ivarsen P., Frokiaer J., Aagaard N.K., Hansen E.F., Bendtsen F., Nielsen S., Vilstrup H. Increased urinary excretion of aquaporin 2 in patients with liver cirrhosis. Gut. 2003;52:1194–1199. doi: 10.1136/gut.52.8.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funayama H., Nakamura T., Saito T., Yoshimura A., Saito M., Kawakami M., Ishikawa S.E. Urinary excretion of aquaporin-2 water channel exaggerated dependent upon vasopressin in congestive heart failure. Kidney Int. 2004;66:1387–1392. doi: 10.1111/j.1523-1755.2004.00902.x. [DOI] [PubMed] [Google Scholar]
- 14.Martin P.Y., Abraham W.T., Lieming X., Olson B.R., Oren R.M., Ohara M., Schrier R.W. Selective V2-receptor vasopressin antagonism decreases urinary aquaporin-2 excretion in patients with chronic heart failure. J. Am. Soc. Nephrol. 1999;10:2165–2170. doi: 10.1681/ASN.V10102165. [DOI] [PubMed] [Google Scholar]
- 15.Imamura T., Kinugawa K., Hatano M., Fujino T., Inaba T., Maki H., Kinoshita O., Nawata K., Kyo S., Ono M., et al. Low cardiac output stimulates vasopressin release in patients with stage D heart failure. Circ. J. 2014;78:2259–2267. doi: 10.1253/circj.CJ-14-0368. [DOI] [PubMed] [Google Scholar]
- 16.Goldsmith S.R., Francis G.S., Cowley A.W., Jr., Levine T.B., Cohn J.N. Increased plasma arginine vasopressin levels in patients with congestive heart failure. J. Am. Coll. Cardiol. 1983;1:1385–1390. doi: 10.1016/S0735-1097(83)80040-0. [DOI] [PubMed] [Google Scholar]
- 17.Szatalowicz V.L., Arnold P.E., Chaimovitz C., Bichet D., Berl T., Schrier R.W. Radioimmunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure. N. Engl. J. Med. 1981;305:263–266. doi: 10.1056/NEJM198107303050506. [DOI] [PubMed] [Google Scholar]
- 18.Pruszczynski W., Vahanian A., Ardaillou R., Acar J. Role of antidiuretic hormone in impaired water excretion of patients with congestive heart failure. J. Clin. Endocrinol. Metab. 1984;58:599–605. doi: 10.1210/jcem-58-4-599. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T., Funayama H., Yoshimura A., Tsuruya Y., Saito M., Kawakami M., Ishikawa S.E. Possible vascular role of increased plasma arginine vasopressin in congestive heart failure. Int. J. Cardiol. 2006;106:191–195. doi: 10.1016/j.ijcard.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 20.Bettari L., Fiuzat M., Shaw L.K., Wojdyla D.M., Metra M., Felker G.M., O'Connor C.M. Hyponatremia and long-term outcomes in chronic heart failure—An observational study from the duke databank for cardiovascular diseases. J. Card. Fail. 2012;18:74–81. doi: 10.1016/j.cardfail.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Konishi M., Haraguchi G., Ohigashi H., Sasaoka T., Yoshikawa S., Inagaki H., Ashikaga T., Isobe M. Progression of hyponatremia is associated with increased cardiac mortality in patients hospitalized for acute decompensated heart failure. J. Card. Fail. 2012;18:620–625. doi: 10.1016/j.cardfail.2012.06.415. [DOI] [PubMed] [Google Scholar]
- 22.Sato N., Gheorghiade M., Kajimoto K., Munakata R., Minami Y., Mizuno M., Aokage T., Asai K., Sakata Y., Yumino D., et al. Hyponatremia and in-hospital mortality in patients admitted for heart failure (from the attend registry) Am. J. Cardiol. 2013;111:1019–1025. doi: 10.1016/j.amjcard.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Gheorghiade M., Abraham W.T., Albert N.M., Gattis Stough W., Greenberg B.H., O’Connor C.M., She L., Yancy C.W., Young J., Fonarow G.C., et al. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: An analysis from the OPTIMIZE-HF registry. Eur. Heart J. 2007;28:980–988. doi: 10.1093/eurheartj/ehl542. [DOI] [PubMed] [Google Scholar]
- 24.Izumi Y., Miura K., Iwao H. Therapeutic potential of vasopressin-receptor antagonists in heart failure. J. Pharmacol. Sci. 2014;124:1–6. doi: 10.1254/jphs.13R13CP. [DOI] [PubMed] [Google Scholar]
- 25.Imamura T., Kinugawa K., Minatsuki S., Muraoka H., Kato N., Inaba T., Maki H., Hatano M., Yao A., Komuro I. Tolvaptan can improve clinical course in responders. Int. Heart J. 2013;54:377–381. doi: 10.1536/ihj.54.377. [DOI] [PubMed] [Google Scholar]
- 26.Imamura T., Kinugawa K., Shiga T., Kato N., Endo M., Inaba T., Maki H., Hatano M., Yao A., Hirata Y., et al. Correction of hyponatremia by tolvaptan before left ventricular assist device implantation. Int. Heart J. 2012;53:391–393. doi: 10.1536/ihj.53.391. [DOI] [PubMed] [Google Scholar]
- 27.Imamura T., Kinugawa K., Kato N., Minatsuki S., Muraoka H., Inaba T., Maki H., Shiga T., Hatano M., Yao A., et al. Successful conversion from thiazide to tolvaptan in a patient with stage D heart failure and chronic kidney disease before heart transplantation. Int. Heart J. 2013;54:48–50. doi: 10.1536/ihj.54.48. [DOI] [PubMed] [Google Scholar]
- 28.Kinugawa K., Imamura T., Komuro I. Experience of a vasopressin receptor antagonist, tolvaptan, under the unique indication in Japanese heart failure patients. Clin. Pharmacol. Ther. 2013;94:449–451. doi: 10.1038/clpt.2013.147. [DOI] [PubMed] [Google Scholar]
- 29.Costello-Boerrigter L.C., Smith W.B., Boerrigter G., Ouyang J., Zimmer C.A., Orlandi C., Burnett J.C., Jr. Vasopressin-2-receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am. J. Physiol. Ren. Physiol. 2006;290:F273–F278. doi: 10.1152/ajprenal.00195.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schrier R.W., Gross P., Gheorghiade M., Berl T., Verbalis J.G., Czerwiec F.S., Orlandi C. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N. Engl. J. Med. 2006;355:2099–2112. doi: 10.1056/NEJMoa065181. [DOI] [PubMed] [Google Scholar]
- 31.Udelson J.E., Orlandi C., Ouyang J., Krasa H., Zimmer C.A., Frivold G., Haught W.H., Meymandi S., Macarie C., Raef D., et al. Acute hemodynamic effects of tolvaptan, a vasopressin V2 receptor blocker, in patients with symptomatic heart failure and systolic dysfunction: An international, multicenter, randomized, placebo-controlled trial. J. Am. Coll. Cardiol. 2008;52:1540–1545. doi: 10.1016/j.jacc.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 32.Berl T., Quittnat-Pelletier F., Verbalis J.G., Schrier R.W., Bichet D.G., Ouyang J., Czerwiec F.S., Investigators S. Oral tolvaptan is safe and effective in chronic hyponatremia. J. Am. Soc. Nephrol. 2010;21:705–712. doi: 10.1681/ASN.2009080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinugawa K., Sato N., Inomata T., Shimakawa T., Iwatake N., Mizuguchi K. Efficacy and safety of tolvaptan in heart failure patients with volume overload. Circ. J. 2014;78:844–852. doi: 10.1253/circj.CJ-66-0093. [DOI] [PubMed] [Google Scholar]
- 34.Matsuzaki M., Hori M., Izumi T., Fukunami M., Tolvaptan I. Efficacy and safety of tolvaptan in heart failure patients with volume overload despite the standard treatment with conventional diuretics: A phase III, randomized, double-blind, placebo-controlled study (quest study) Cardiovasc. Drugs Ther. 2011;25:S33–S45. doi: 10.1007/s10557-011-6304-x. [DOI] [PubMed] [Google Scholar]
- 35.Imamura T., Kinugawa K. Mid-term administration of tolvaptan improves renal function accompanied by dose-reduction in furosemide in aquaporin-defined responders. Int. Heart J. 2015;56:686–687. doi: 10.1536/ihj.15-298. [DOI] [PubMed] [Google Scholar]
- 36.Imamura T., Kinugawa K., Kato N., Minatsuki S., Muraoka H., Inaba T., Maki H., Shiga T., Hatano M., Hosoya Y., et al. A case with recovery of response to tolvaptan associated with remission of acute kidney injury and increased urine osmolality. Int. Heart J. 2013;54:115–118. doi: 10.1536/ihj.54.115. [DOI] [PubMed] [Google Scholar]
- 37.Imamura T., Kinugawa K., Fujino T., Inaba T., Maki H., Hatano M., Yao A., Komuro I. Increased urine aquaporin-2 relative to plasma arginine vasopressin is a novel marker of response to tolvaptan in patients with decompensated heart failure. Circ. J. 2014;78:2240–2249. doi: 10.1253/circj.CJ-14-0244. [DOI] [PubMed] [Google Scholar]
- 38.Konstam M.A., Gheorghiade M., Burnett J.C., Jr., Grinfeld L., Maggioni A.P., Swedberg K., Udelson J.E., Zannad F., Cook T., Ouyang J., et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The everest outcome trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 39.Hauptman P.J., Burnett J., Gheorghiade M., Grinfeld L., Konstam M.A., Kostic D., Krasa H.B., Maggioni A., Ouyang J., Swedberg K., et al. Clinical course of patients with hyponatremia and decompensated systolic heart failure and the effect of vasopressin receptor antagonism with tolvaptan. J. Card. Fail. 2013;19:390–397. doi: 10.1016/j.cardfail.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Imamura T., Kinugawa K., Shiga T., Kato N., Muraoka H., Minatsuki S., Inaba T., Maki H., Hatano M., Yao A., et al. Novel criteria of urine osmolality effectively predict response to tolvaptan in decompensated heart failure patients—Association between non-responders and chronic kidney disease. Circ. J. 2013;77:397–404. doi: 10.1253/circj.CJ-12-0971. [DOI] [PubMed] [Google Scholar]
- 41.Imamura T., Kinugawa K., Minatsuki S., Muraoka H., Kato N., Inaba T., Maki H., Shiga T., Hatano M., Yao A., et al. Urine osmolality estimated using urine urea nitrogen, sodium and creatinine can effectively predict response to tolvaptan in decompensated heart failure patients. Circ. J. 2013;77:1208–1213. doi: 10.1253/circj.CJ-12-1328. [DOI] [PubMed] [Google Scholar]
- 42.Toda H., Nakamura K., Nakahama M., Wada T., Watanabe A., Hashimoto K., Terasaka R., Tokioka K., Nishii N., Miyoshi T., et al. Clinical characteristics of responders to treatment with tolvaptan in patients with acute decompensated heart failure: Importance of preserved kidney size. J. Cardiol. 2015;67:177–183. doi: 10.1016/j.jjcc.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka A., Katsuno T., Ozaki T., Sakata F., Kato N., Suzuki Y., Kosugi T., Kato S., Tsuboi N., Sato W., et al. The efficacy of tolvaptan as a diuretic for chronic kidney disease patients. Acta Cardiol. 2015;70:217–223. doi: 10.1080/ac.70.2.3073514. [DOI] [PubMed] [Google Scholar]
- 44.Tominaga N., Kida K., Matsumoto N., Akashi Y.J., Miyake F., Kimura K., Shibagaki Y. Safety of add-on tolvaptan in patients with furosemide-resistant congestive heart failure complicated by advanced chronic kidney disease: A sub-analysis of a pharmacokinetics/pharmacodynamics study. Clin. Nephrol. 2015;84:29–38. doi: 10.5414/CN108457. [DOI] [PubMed] [Google Scholar]
- 45.Iwatani H., Kawabata H., Sakaguchi Y., Yamamoto R., Hamano T., Rakugi H., Isaka Y. Urine osmolarity predicts the body weight-reduction response to tolvaptan in chronic kidney disease patients: A retrospective, observational study. Nephron. 2015;130:8–12. doi: 10.1159/000381859. [DOI] [PubMed] [Google Scholar]
- 46.Imamura T. Aquaporin-2-guided tolvaptan therapy in patients with congestive heart failure accompanied by chronic kidney disease. Int. Heart J. 2014;55:482–483. doi: 10.1536/ihj.14-217. [DOI] [PubMed] [Google Scholar]
- 47.Jensen J.M., Mose F.H., Kulik A.E., Bech J.N., Fenton R.A., Pedersen E.B. Abnormal urinary excretion of NKCC2 and AQP2 in response to hypertonic saline in chronic kidney disease: An intervention study in patients with chronic kidney disease and healthy controls. BMC Nephrol. 2014;15:101. doi: 10.1186/1471-2369-15-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato E., Nakamura T., Amaha M., Nomura M., Matsumura D., Yamagishi H., Ono Y., Ueda Y. Effect of tolvaptan in patients with chronic kidney disease due to diabetic nephropathy with heart failure. Int. Heart J. 2014;55:533–538. doi: 10.1536/ihj.14-190. [DOI] [PubMed] [Google Scholar]
- 49.Hirano D., Kakegawa D., Yamada A., Ito A., Miwa S., Ida H. Tolvaptan in a pediatric patient with diuretic-resistant heart and kidney failure. Pediatr. Int. 2015;57:183–185. doi: 10.1111/ped.12590. [DOI] [PubMed] [Google Scholar]
- 50.Otsuka T., Sakai Y., Ohno D., Murasawa T., Sato N., Tsuruoka S. The effects of tolvaptan on patients with severe chronic kidney disease complicated by congestive heart failure. Clin. Exp. Nephrol. 2013;17:834–838. doi: 10.1007/s10157-013-0788-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Imamura T., Kinugawa K., Komuro I. Tolvaptan prolongs blockage of the vasopressin type II receptor over 24 hours in responders with stage D heart failure. Int. Heart J. 2016 doi: 10.1536/ihj.15-297. in press. [DOI] [PubMed] [Google Scholar]