Abstract
Pancreatic cancer is one of most aggressive forms of cancer. After clinical detection it exhibits fast metastatic growth. Heat shock protein 27 (HSP27; HSPB1) has been characterized as a molecular chaperone which modifies the structures and functions of other proteins in cells when they are exposed to various stresses, such as chemotherapy. While the administration of gemcitabine, an anti-tumor drug, has been the standard treatment for patients with advanced pancreatic cancer, accumulating evidence shows that HSP27 plays a key role in the chemosensitivity to gemcitabine. In addition, phosphorylated HSP27 induced by gemcitabine has been associated with the inhibition of pancreatic cancer cell growth. In this review, we summarize the role of phosphorylated HSP27, as well as HSP27, in the regulation of chemosensitivity in pancreatic cancer.
Keywords: HSP27, pancreatic cancer, phosphorylated HSP27, chemosensitivity, prognosis
1. Introduction
Pancreatic cancer, which causes approximately 266,000 deaths per year, is the eighth leading cause of cancer-related deaths worldwide [1]. Surgery remains the best chance for achieving a cure, but at the time of diagnosis only approximately 10% of patients are eligible for surgery [2]. The majority of patients (80%–90%) present with incurable metastatic or locally-advanced disease. Therefore, the survival of patients with pancreatic cancer largely depends on chemotherapy.
Gemcitabine is a nucleoside analog of deoxycytidine which is enzymatically activated inside the cell where it subsequently inhibits DNA synthesis and induces apoptosis [3]. Thus far, gemcitabine has been shown to improve median overall survival from 4.4 to 5.6 months, in comparison to fluorouracil (5-FU), which is the chemotherapy that was classically used in the treatment of pancreatic cancer [4]. In 2011, Conroy et al., reported the efficacy of FOLFIRINOX, a combination therapy that is used in the treatment of metastatic pancreatic cancer which consists of a biweekly regimen of oxaliplatin, irinotecan, fluorouracil, and leucovorin [5]. They demonstrated that FOLFIRINOX was associated with a median overall survival improvement of more than 10 months in comparison to gemcitabine [5]. However, hematologic and non-hematologic events including neutropenia, febrile neutropenia, diarrhea, and sensory neuropathy are more frequently associated with the administration of FOLFIRINOX than with gemcitabine. In 2013, Von Hoff et al., reported that nab-paclitaxel with gemcitabine was more effective than gemcitabine alone, and that the combination resulted in an improvement in median overall survival that ranged from 6.7 to 8.5 months [6]. The combination of nab-paclitaxel with gemcitabine was associated with a lower risk of adverse events than FOLFIRINOX. Thus, in the treatment of pancreatic cancer, gemcitabine remains an efficient anticancer drug. Moreover, a large number of potential makers have been identified in pancreatic cancer, but their clinical utility as prognostic tools remains under investigation. With regard to gemcitabine, human equilibrative nucleoside transporter 1 (hENT1) [7,8], ribonucleotide reductase M1 (RRM1) [9], B7H4 [10], DJ-1 [10], and heat shock protein 27 (HSP27; HSPB1) are considered to be prognostic markers of resistance to chemotherapy in pancreatic cancer.
HSPs were first discovered as stress-inducible proteins [11]. HSPs have been characterized as molecular chaperones which prevent aggregation of proteins [12]. Based on their molecular masses, HSPs are currently classified into seven families, including HSPA (HSP70), HSPB (small HSPs), HSPC (HSP90), and HSPH (HSP110) [13]. The high molecular weight HSPs, such as HSPA (HSP70) and HSPC (HSP90), act as molecular chaperones in protein folding, oligomerization, and translocation. Although the functions of low molecular weight HSPs are not well characterized as those of high molecular weight HSPs, it is recognized that they may have chaperone activities. Among small HSPs with monomer molecular mass in the range of 12–43 kDa, HSP27, an ATP-independent molecular chaperone, is induced by the heat shock associated with physical and chemical stresses, including radiation, oxidative stress, and various chemotherapies [11,13]. HSP27 is able to bind to improperly folded proteins and further transfer them to the ATP-dependent chaperones such as HSPA (HSP70) or to the protein degradation machines including proteasomes or autophagosomes. HSP27 can also interact with various components including the regulated programmed cell death machinery, upstream and downstream of mitochondrial events [13,14]. The functions of HSP27 are regulated by post-translational modifications such as phosphorylation [15,16]. Human HSP27 is phosphorylated mainly at three sites (Ser-15, Ser-78, and Ser-82), and the phosphorylation is catalyzed by various protein kinases including mitogen-activated protein (MAP) kinase activated protein kinase 2 (MAPKAPK-2) [17]. Unphosphorylated HSP27 forms large aggregated large oligomers while its phosphorylation results in the conformational changes, leading to dissociated small oligomers [16].
With regard to cancer, HSP27 appears to be constitutively expressed at high levels in various tumors, including lung [18], gastric [19], prostate [20], and pancreatic cancers [21]. HSP27 expression has been reported to be associated with a superior treatment response [22], prognosis [23], and tumor progression [24]. Phosphorylated HSP27 expression also has been reported to play a suppressive role in the cell growth [25,26], chemosensitivity [27,28,29]. In pancreatic cancer, the expression of HSP27 and the phosphorylated HSP27 state are considered to play a critical role in gemcitabine resistance [10,28,29,30,31,32,33,34].
2. How Does HSP27 Predict Chemosensitivity?
HSP27 mainly acts as a molecular chaperone in cells exposed to different stresses, including heat shock and chemotherapy. It consecutively counteracts the formation of misfolded proteins and allows for correct protein folding [35,36]. In addition to these functions, HSP27 has been implicated in proteasome-mediated protein degradation as well as the regulation of the apoptotic pathway [13]. However, its precise role is still not fully understood.
Increasing evidence shows that HSP27 may predict the response of tumors in individual patients to radio- and chemotherapy. Studies have investigated the response in bladder [37], breast [38], esophageal [39], ovarian [40], and prostate cancers [41]. However, the role of HSP27 as a predictive marker for chemosensitivity in pancreatic cancer remains to be elucidated. There are several reports regarding indirect evidence on the influence of HSP27 on chemotherapy, including gemcitabine. Schafer et al., [32] reported that HSP27 was an independent prognostic marker and that the better survival demonstrated in patients with HSP27-positive tumors may be partly attributable to a better response to gemcitabine. Additionally, Guo et al., [33] reported that the overexpression of HSP27 increases the sensitivity to gemcitabine in the pancreatic cancer cell line, PL5, which is consistent with the in vivo results shown by Schafer et al. By contrast, Liu et al., [30] reported that the expression of HSP27 was decreased in a gemcitabine-resistant pancreatic cancer cell line, Capan-1. Taken together, a high expression of HSP27 in pancreatic cancer cells appears to lead to high sensitivity to gemcitabine.
On the contrary, it has been reported that HSP27 plays a role in resistance to gemcitabine treatment or that it causes gemcitabine treatment to become irrelevant. Mori-Iwamoto et al., [31] reported that the low expression of HSP27 is related to a better rate of survival in patients with pancreatic cancer in an in vivo study that used specimens obtained by endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA). This article also reported that knockdown of the HSP27 expression using siRNA targeting HSP27 increased gemcitabine sensitivity even in the gemcitabine-resistant pancreatic cell line, KLM1-R. Similarly, Taba et al., [42] reported that gemcitabine-resistant cell lines, KLM1-R and PK59, exhibited increased gemcitabine sensitivity after inhibition of the HSP27 expression by KNK43, a known HSP inhibitor that causes a decreased HSP27 expression in these cells. Zhang et al., [43] also reported that SW1900/GEM, a gemcitabine-resistant cell line, exhibited increased gemcitabine sensitivity when the HSP27 expression was inhibited by shRNA specific for HSP27. Similar results were also shown in MiaPaCa-2, HPAC, and BxPC3 cells [34]. Additionally, a high expression HSP27 pancreatic cell line, MiaPaCa-2-HSP27, was resistant to gemcitabine-induced apoptosis compared with a low expression HSP27 cell line, MiaPaCa-2-Mock [21]. These studies suggest that the HSP27 expression is related to gemcitabine resistance. In our previous study, the HSP27 expression level did not affect the cell growth or sensitivity to gemcitabine in HSP27-transfected Panc1 pancreatic cell lines [29]. Moreover, there was no relationship between the HSP27 expression and gemcitabine sensitivity in another in vivo study [10], which was inconsistent with the previous studies showing that a low expression of HSP27 results in a better survival [31] or that HSP27-positive tumors were an independent prognostic marker [32] (Table 1). Taken together, the relationship between the HSP27 expression and sensitivity to gemcitabine differs according to the cell line used; therefore, the effect of the HSP27 expression on gemcitabine sensitivity must be investigated using various pancreatic cancer cell lines under the same conditions.
Table 1.
The relationship between the HSP27 expression and the response to gemcitabine.
| Author | Year | in Vitro/Vivo | HSP27 Expression | Response to Gemcitabine | Pancreatic Cancer Cell Lines (Response to Gemcitabine) |
|---|---|---|---|---|---|
| Mori-Iwamoto et al. [31] | 2007 | in vivo/vitro | ↑ | ↓ | KLM1-R (resistant) |
| Taba et al. [42] | 2011 | in vitro | ↓ | ↑ | KLM1-R, PK59 (resistant) |
| Baylot et al. [21] | 2011 | in vitro | ↑ | ↓ | MiaPaCa-2 (resistant) |
| Nakashima et al. [29] | 2011 | in vitro | not related | not related | Panc1 |
| Liu et al. [30] | 2012 | in vitro | ↓ | ↓ | Capan-1 |
| Schafer et al. [32] | 2012 | in vivo/vitro | ↑ | ↑ | PL5 |
| Tsiaousidou et al. [10] | 2013 | in vivo | not related | not related | – |
| Guo et al. [33] | 2014 | in vitro | ↑ | ↑ | PL5 |
| Kang et al. [34] | 2015 | in vitro | ↓ | ↑ | MiaPaCa-2, HPAC, BxPC3 |
| Zhang et al. [43] | 2015 | in vitro | ↓ | ↑ | SW1900, SW1900/GEM (resistant) |
As for paclitaxel, the role of HSP27 on the response to paclitaxel in cervical [44], ovarian [45,46], prostate [47], and bladder cancer [48] were reported recently. The cervical cancer tissues which were sensitive to paclitaxel or cisplatin showed the decreased expression of HSP27 after chemotherapy [44]. In addition, down-regulation of HSP27 expression increased the chemosensitivity to paclitaxel and paclitaxel-induced apoptosis in ovarian cancer [45]. Moreover, the expression of HSP27 helps progression by inhibiting apoptotic cell death in the bladder cancer patients treated with paclitaxel [48]. Taken together, it is more likely that higher HSP27 expression plays a protective role in cell proliferation of various cancers with paclitaxel-based chemotherapy. Future studies using a molecular approach, as well as large cohort study of patients treated with gemcitabine or paclitaxel, will be required to elucidate the mechanisms underlying the role of HSP27 as a prognostic marker.
3. How Does Phosphorylated HSP27 Affect Chemosensitivity?
Phosphorylated HSP27 reportedly plays a suppressive role in the cell growth of hepatocellular carcinomas [25,26]. In contrast, Matsunaga et al., showed that the inhibition of HSP27 phosphorylation results in the increased sensitivity of colorectal cancer cells to chemotherapy (5-FU) [27]. However, the exact role of phosphorylated HSP27 in the various types of cancer is not fully understood.
With regard to pancreatic cancer, several reports discuss chemosensitivity to gemcitabine. Habiro et al., reported that gemcitabine-induced apoptosis is mediated by p38 MAP kinase activation [49]. Taba et al. showed that the phosphorylation levels of HSP27 at Ser-78 and Ser-82 are elevated in gemcitabine-resistant pancreatic cancer cells, KLM1-R, in comparison to gemcitabine-sensitive pancreatic cancer cells, KLM1 [28].
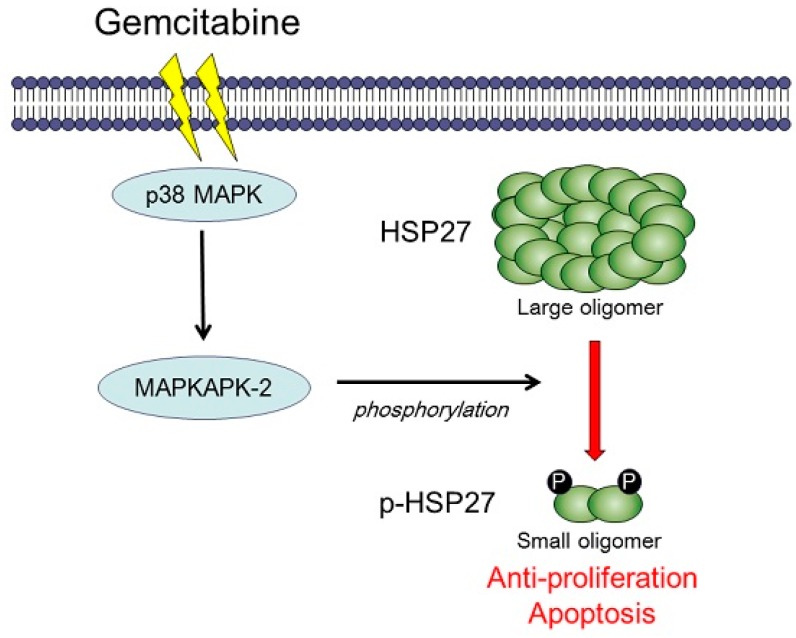
On the contrary, Kang et al., reported that the ratio of phosphorylated HSP27 to non-phosphorylated HSP27 was significantly increased after gemcitabine treatment using pancreatic cancer cell lines MiaPaCa-2, HPAC, and BxPC3, and that an increase in the ratio of phosphorylated HSP27 to non-phosphorylated HSP27 was related to cell death [34]. In our recent study [29], we investigated the relationship between the effect of gemcitabine and the phosphorylated HSP27 state using pancreatic cancer cell lines, KP3 and Panc1. We found that gemcitabine induces the phosphorylation of p38 MAPK, and subsequently MAPKAPK-2, which leads to the phosphorylation of HSP27 at Ser-15, Ser-78, and Ser-82 without affecting the total level of HSP27 [29]. Moreover, the phosphorylation of HSP27 induced by gemcitabine has a suppressive role in the pancreatic cancer cell growth and induces apoptosis [29]. In order to elucidate the precise role of phosphorylated HSP27 in the sensitivity to gemcitabine, we established two types of mutant HSP27-transfected Panc1 pancreatic cell lines, overexpressed non-phosphorylatable HSP27 cells and phosphorylated HSP27 cells [29], and found that the cell growth of phosphorylated HSP27 cells was markedly retarded compared with that of non-phosphorylatable HSP27 cells [29], indicating that gemcitabine-induced HSP27 phosphorylation via the p38 MAPK-MAPKAP-2 pathway leads to the growth suppression of pancreatic cancer cells. We summarized the hypothetical role of phosphorylated HSP27 in the gemcitabine-induced growth suppression of pancreatic cancer (Figure 1) and reviewed these studies in Table 2.
Figure 1.
A schematic representation of the role of HSP27 in the sensitivity to gemcitabine in human pancreatic cancer. Gemcitabine induces the phosphorylation of HSP27 via the MAPK-MAPKAPK-2 pathway and phosphorylated HSP27 leads cells to growth suppression in pancreatic cancer.
Table 2.
The relationship between the phosphorylated HSP27 (p-HSP27) expression and the response to gemcitabine
| Author | Year | in Vitro/Vivo | p-HSP27 Expression | Response to Gemcitabine | Pancreatic Cancer Cell Lines (Response to Gemcitabine) |
|---|---|---|---|---|---|
| Taba et al. [28] | 2010 | in vitvo | ↑ | ↓ | KLM1/KLM1-R (resistant) |
| Nakashima et al. [29] | 2011 | in vitvo | ↑ | ↑ | Panc1 |
| Kang et al. [34] | 2015 | in vitvo | ↑ (p-HSP27/HSP27) | ↑ | MiaPaCa-2, HPAC, BxPC3 |
4. Conclusions and Future Directions
Phosphorylated HSP27 could, therefore, be a potentially-useful biomarker that predicts the sensitivity of pancreatic cancer to gemcitabine-based chemotherapy. Further investigation might provide a more effective combination chemotherapy that uses gemcitabine in the treatment of human pancreatic cancer. Compounds which collaborate with HSP27 might, therefore, be useful in this regard.
Acknowledgments
We gratefully appreciate the efforts of everyone who collaborated with us in our research.
Author Contributions
Conception and design: Mitsuru Okuno, Seiji Adachi, Ichiro Yasuda; Development of methodology: Mitsuru Okuno, Seiji Adachi, Osamu Kozawa, Masahito Shimizu, Ichiro Yasuda
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2012. CA Cancer J. Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Plunkett W., Huang P., Searcy C.E., Gandhi V. Gemcitabine: Preclinical pharmacology and mechanisms of action. Semin. Oncol. 1996;23:3–15. [PubMed] [Google Scholar]
- 4.Burris H.A., 3rd, Moore M.J., Andersen J., Green M.R., Rothenberg M.L., Modiano M.R., Cripps M.C., Portenoy R.K., Storniolo A.M., Tarassoff P., et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 5.Conroy T., Desseigne F., Ychou M., Bouche O., Guimbaud R., Becouarn Y., Adenis A., Raoul J.L., Gourgou-Bourgade S., de la Fouchardiere C., et al. Folfirinox versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 6.Von Hoff D.D., Ervin T., Arena F.P., Chiorean E.G., Infante J., Moore M., Seay T., Tjulandin S.A., Ma W.W., Saleh M.N., et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spratlin J., Sangha R., Glubrecht D., Dabbagh L., Young J.D., Dumontet C., Cass C., Lai R., Mackey J.R. The absence of human equilibrative nucleoside transporter 1 is associated with reduced survival in patients with gemcitabine-treated pancreas adenocarcinoma. Clin. Cancer Res. 2004;10:6956–6961. doi: 10.1158/1078-0432.CCR-04-0224. [DOI] [PubMed] [Google Scholar]
- 8.Nordh S., Ansari D., Andersson R. hENT1 expression is predictive of gemcitabine outcome in pancreatic cancer: A systematic review. World J. Gastroenterol. 2014;20:8482–8490. doi: 10.3748/wjg.v20.i26.8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakahira S., Nakamori S., Tsujie M., Takahashi Y., Okami J., Yoshioka S., Yamasaki M., Marubashi S., Takemasa I., Miyamoto A., et al. Involvement of ribonucleotide reductase M1 subunit overexpression in gemcitabine resistance of human pancreatic cancer. Int. J. Cancer. 2007;120:1355–1363. doi: 10.1002/ijc.22390. [DOI] [PubMed] [Google Scholar]
- 10.Tsiaousidou A., Lambropoulou M., Chatzitheoklitos E., Tripsianis G., Tsompanidou C., Simopoulos C., Tsaroucha A.K. B7H4, HSP27 and DJ-1 molecular markers as prognostic factors in pancreatic cancer. Pancreatology. 2013;13:564–569. doi: 10.1016/j.pan.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Lindquist S., Craig E.A. The heat-shock proteins. Annu. Rev. Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 12.Freeman B.C., Yamamoto K.R. Disassembly of transcriptional regulatory complexes by molecular chaperones. Science. 2002;296:2232–2235. doi: 10.1126/science.1073051. [DOI] [PubMed] [Google Scholar]
- 13.Garrido C., Brunet M., Didelot C., Zermati Y., Schmitt E., Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- 14.Melle C., Ernst G., Escher N., Hartmann D., Schimmel B., Bleul A., Thieme H., Kaufmann R., Felix K., Friess H.M., et al. Protein profiling of microdissected pancreas carcinoma and identification of HSP27 as a potential serum marker. Clin. Chem. 2007;53:629–635. doi: 10.1373/clinchem.2006.079194. [DOI] [PubMed] [Google Scholar]
- 15.Benjamin I.J., McMillan D.R. Stress (heat shock) proteins: Molecular chaperones in cardiovascular biology and disease. Circ. Res. 1998;83:117–132. doi: 10.1161/01.RES.83.2.117. [DOI] [PubMed] [Google Scholar]
- 16.Lambert H., Charette S.J., Bernier A.F., Guimond A., Landry J. HSP27 multimerization mediated by phosphorylation-sensitive intermolecular interactions at the amino terminus. J. Biol. Chem. 1999;274:9378–9385. doi: 10.1074/jbc.274.14.9378. [DOI] [PubMed] [Google Scholar]
- 17.Rouse J., Cohen P., Trigon S., Morange M., Alonso-Llamazares A., Zamanillo D., Hunt T., Nebreda A.R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 18.Malusecka E., Zborek A., Krzyzowska-Gruca S., Krawczyk Z. Expression of heat shock proteins HSP70 and HSP27 in primary non-small cell lung carcinomas. An immunohistochemical study. Anticancer Res. 2001;21:1015–1021. [PubMed] [Google Scholar]
- 19.Huang Q., Ye J., Huang Q., Chen W., Wang L., Lin W., Lin J., Lin X. Heat shock protein 27 is over-expressed in tumor tissues and increased in sera of patients with gastric adenocarcinoma. Clin. Chem. Lab. Med. 2010;48:263–269. doi: 10.1515/CCLM.2010.043. [DOI] [PubMed] [Google Scholar]
- 20.Rocchi P., So A., Kojima S., Signaevsky M., Beraldi E., Fazli L., Hurtado-Coll A., Yamanaka K., Gleave M. Heat shock protein 27 increases after androgen ablation and plays a cytoprotective role in hormone-refractory prostate cancer. Cancer Res. 2004;64:6595–6602. doi: 10.1158/0008-5472.CAN-03-3998. [DOI] [PubMed] [Google Scholar]
- 21.Baylot V., Andrieu C., Katsogiannou M., Taieb D., Garcia S., Giusiano S., Acunzo J., Iovanna J., Gleave M., Garrido C., et al. OGX-427 inhibits tumor progression and enhances gemcitabine chemotherapy in pancreatic cancer. Cell Death Dis. 2011;2:137. doi: 10.1038/cddis.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciocca D.R., Calderwood S.K. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zoubeidi A., Gleave M. Small heat shock proteins in cancer therapy and prognosis. Int. J. Biochem. Cell Biol. 2012;44:1646–1656. doi: 10.1016/j.biocel.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Sherman M., Multhoff G. Heat shock proteins in cancer. Ann. N. Y. Acad. Sci. 2007;1113:192–201. doi: 10.1196/annals.1391.030. [DOI] [PubMed] [Google Scholar]
- 25.Yasuda E., Kumada T., Takai S., Ishisaki A., Noda T., Matsushima-Nishiwaki R., Yoshimi N., Kato K., Toyoda H., Kaneoka Y., et al. Attenuated phosphorylation of heat shock protein 27 correlates with tumor progression in patients with hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2005;337:337–342. doi: 10.1016/j.bbrc.2005.08.273. [DOI] [PubMed] [Google Scholar]
- 26.Matsushima-Nishiwaki R., Takai S., Adachi S., Minamitani C., Yasuda E., Noda T., Kato K., Toyoda H., Kaneoka Y., Yamaguchi A., et al. Phosphorylated heat shock protein 27 represses growth of hepatocellular carcinoma via inhibition of extracellular signal-regulated kinase. J. Biol. Chem. 2008;283:18852–18860. doi: 10.1074/jbc.M801301200. [DOI] [PubMed] [Google Scholar]
- 27.Matsunaga A., Ishii Y., Tsuruta M., Okabayashi K., Hasegawa H., Kitagawa Y. Inhibition of heat shock protein 27 phosphorylation promotes sensitivity to 5-fluorouracil in colorectal cancer cells. Oncol. Lett. 2014;8:2496–2500. doi: 10.3892/ol.2014.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taba K., Kuramitsu Y., Ryozawa S., Yoshida K., Tanaka T., Maehara S., Maehara Y., Sakaida I., Nakamura K. Heat-shock protein 27 is phosphorylated in gemcitabine-resistant pancreatic cancer cells. Anticancer Res. 2010;30:2539–2543. [PubMed] [Google Scholar]
- 29.Nakashima M., Adachi S., Yasuda I., Yamauchi T., Kawaguchi J., Itani M., Yoshioka T., Matsushima-Nishiwaki R., Hirose Y., Kozawa O., et al. Phosphorylation status of heat shock protein 27 plays a key role in gemcitabine-induced apoptosis of pancreatic cancer cells. Cancer Lett. 2011;313:218–225. doi: 10.1016/j.canlet.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Liu Q.H., Zhao C.Y., Zhang J., Chen Y., Gao L., Ni C.Y., Zhu M.H. Role of heat shock protein 27 in gemcitabine-resistant human pancreatic cancer: Comparative proteomic analyses. Mol. Med. Rep. 2012;6:767–773. doi: 10.3892/mmr.2012.1013. [DOI] [PubMed] [Google Scholar]
- 31.Mori-Iwamoto S., Kuramitsu Y., Ryozawa S., Mikuria K., Fujimoto M., Maehara S., Maehara Y., Okita K., Nakamura K., Sakaida I. Proteomics finding heat shock protein 27 as a biomarker for resistance of pancreatic cancer cells to gemcitabine. Int. J. Oncol. 2007;31:1345–1350. doi: 10.3892/ijo.31.6.1345. [DOI] [PubMed] [Google Scholar]
- 32.Schafer C., Seeliger H., Bader D.C., Assmann G., Buchner D., Guo Y., Ziesch A., Palagyi A., Ochs S., Laubender R.P., et al. Heat shock protein 27 as a prognostic and predictive biomarker in pancreatic ductal adenocarcinoma. J. Cell. Mol. Med. 2012;16:1776–1791. doi: 10.1111/j.1582-4934.2011.01473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo Y., Ziesch A., Hocke S., Kampmann E., Ochs S., de Toni E.N., Goke B., Gallmeier E. Overexpression of heat shock protein 27 (HSP27) increases gemcitabine sensitivity in pancreatic cancer cells through S-phase arrest and apoptosis. J. Cell. Mol. Med. 2014;19:340–350. doi: 10.1111/jcmm.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang D., Choi H.J., Kang S., Kim S.Y., Hwang Y.S., Je S., Han Z., Kim J.H., Song J.J. Ratio of phosphorylated HSP27 to nonphosphorylated HSP27 biphasically acts as a determinant of cellular fate in gemcitabine-resistant pancreatic cancer cells. Cell Signal. 2015;27:807–817. doi: 10.1016/j.cellsig.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Georgopoulos C., Welch W.J. Role of the major heat shock proteins as molecular chaperones. Annu. Rev. Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- 36.Arrigo A.P., Simon S., Gibert B., Kretz-Remy C., Nivon M., Czekalla A., Guillet D., Moulin M., Diaz-Latoud C., Vicart P. HSP27 (HSPB1) and αB-crystallin (HSPB5) as therapeutic targets. FEBS Lett. 2007;581:3665–3674. doi: 10.1016/j.febslet.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 37.Kassem H., Sangar V., Cowan R., Clarke N., Margison G.P. A potential role of heat shock proteins and nicotinamide N-methyl transferase in predicting response to radiation in bladder cancer. Int. J. Cancer. 2002;101:454–460. doi: 10.1002/ijc.10631. [DOI] [PubMed] [Google Scholar]
- 38.Vargas-Roig L.M., Gago F.E., Tello O., Aznar J.C., Ciocca D.R. Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int. J. Cancer. 1998;79:468–475. doi: 10.1002/(SICI)1097-0215(19981023)79:5<468::AID-IJC4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 39.Langer R., Ott K., Specht K., Becker K., Lordick F., Burian M., Herrmann K., Schrattenholz A., Cahill M.A., Schwaiger M., et al. Protein expression profiling in esophageal adenocarcinoma patients indicates association of heat-shock protein 27 expression and chemotherapy response. Clin. Cancer Res. 2008;14:8279–8287. doi: 10.1158/1078-0432.CCR-08-0679. [DOI] [PubMed] [Google Scholar]
- 40.Arts H.J., Hollema H., Lemstra W., Willemse P.H., de Vries E.G., Kampinga H.H., van der Zee A.G. Heat-shock-protein-27 (HSP27) expression in ovarian carcinoma: Relation in response to chemotherapy and prognosis. Int. J. Cancer. 1999;84:234–238. doi: 10.1002/(SICI)1097-0215(19990621)84:3<234::AID-IJC6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 41.Bubendorf L., Kolmer M., Kononen J., Koivisto P., Mousses S., Chen Y., Mahlamaki E., Schraml P., Moch H., Willi N., et al. Hormone therapy failure in human prostate cancer: Analysis by complementary DNA and tissue microarrays. J. Natl. Cancer Inst. 1999;91:1758–1764. doi: 10.1093/jnci/91.20.1758. [DOI] [PubMed] [Google Scholar]
- 42.Taba K., Kuramitsu Y., Ryozawa S., Yoshida K., Tanaka T., Mori-Iwamoto S., Maehara S., Maehara Y., Sakaida I., Nakamura K. KNK437 downregulates heat shock protein 27 of pancreatic cancer cells and enhances the cytotoxic effect of gemcitabine. Chemotherapy. 2011;57:12–16. doi: 10.1159/000321019. [DOI] [PubMed] [Google Scholar]
- 43.Zhang S., Zhang X.Q., Huang S.L., Chen M., Shen S.S., Ding X.W., Lv Y., Zou X.P. The effects of HSP27 on gemcitabine-resistant pancreatic cancer cell line through snail. Pancreas. 2015;44:1121–1129. doi: 10.1097/MPA.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 44.Liu H., Han Y., Mi R., Zhang Y., Su G., Wang H., Zhou X., Liu X., Zhu B. Identification of cervical cancer proteins associated with treatment with paclitaxel and cisplatin in patients. Int. J. Gynecol. Cancer. 2011;21:1452–1457. doi: 10.1097/IGC.0b013e31822491d0. [DOI] [PubMed] [Google Scholar]
- 45.Song T.F., Zhang Z.F., Liu L., Yang T., Jiang J., Li P. Small interfering RNA-mediated silencing of heat shock protein 27 (HSP27) increases chemosensitivity to paclitaxel by increasing production of reactive oxygen species in human ovarian cancer cells (HO8910) J. Int. Med. Res. 2009;37:1375–1388. doi: 10.1177/147323000903700512. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka Y., Fujiwara K., Tanaka H., Maehata K., Kohno I. Paclitaxel inhibits expression of heat shock protein 27 in ovarian and uterine cancer cells. Int. J. Cancer. 2004;14:616–620. doi: 10.1111/j.1048-891X.2004.14409.x. [DOI] [PubMed] [Google Scholar]
- 47.Stope M.B., Weiss M., Preuss M., Streitborger A., Ritter C.A., Zimmermann U., Walther R., Burchardt M. Immediate and transient phosphorylation of the heat shock protein 27 initiates chemoresistance in prostate cancer cells. Oncol. Rep. 2014;32:2380–2386. doi: 10.3892/or.2014.3492. [DOI] [PubMed] [Google Scholar]
- 48.Kamada M., So A., Muramaki M., Rocchi P., Beraldi E., Gleave M. Hsp27 knockdown using nucleotide-based therapies inhibit tumor growth and enhance chemotherapy in human bladder cancer cells. Mol. Cancer Ther. 2007;6:299–308. doi: 10.1158/1535-7163.MCT-06-0417. [DOI] [PubMed] [Google Scholar]
- 49.Habiro A., Tanno S., Koizumi K., Izawa T., Nakano Y., Osanai M., Mizukami Y., Okumura T., Kohgo Y. Involvement of p38 mitogen-activated protein kinase in gemcitabine-induced apoptosis in human pancreatic cancer cells. Biochem. Biophys. Res. Commun. 2004;316:71–77. doi: 10.1016/j.bbrc.2004.02.017. [DOI] [PubMed] [Google Scholar]