Abstract
Electrical stimulation of cut peripheral nerves at the time of their surgical repair results in an enhancement of axon regeneration. Regeneration of axons through nerve allografts was used to evaluate whether this effect is due to an augmentation of cell autonomous neurotrophin signaling in the axons or signaling from neurotrophins produced in the surrounding environment. In the thy-1-YFP-H mouse, a single one hour application of electrical stimulation at the time of surgical repair of the cut common fibular nerve results in a significant increase in the proportion of YFP+ dorsal root ganglion neurons that were also immunoreactive for BDNF or trkB as well as an increase in the length of regenerating axons through allografts from wild type litter mates, both one and two weeks later. Axon growth through allografts from neurotrophin-4/5 knockout mice or grafts made acellular by repeated cycles of freezing and thawing is normally very poor, but electrical stimulation results in a growth of axons through these grafts which is similar to that observed through grafts from wild type mice after electrical stimulation. When cut nerves in NT-4/5 knockout mice were electrically stimulated, no enhancement of axon regeneration was found. Electrical stimulation thus produces a potent enhancement of the regeneration of axons in cut peripheral nerves which is independent of neurotrophin production by cells in their surrounding environment but is dependent on stimulation of trkB and its ligands in the regenerating axons themselves.
INTRODUCTION
After injury to a peripheral nerve, axons proximal to the lesion are capable of regeneration. They form regenerative sprouts which, if they enter endoneurial tubes in the distal stump, can elongate and reinnervate their targets. Regenerating axons require the presence of growth promoting molecules to elongate in this pathway (Fu and Gordon, 1997). In addition to extracellular matrix molecules, such as laminin and fibronectin, which form a growth promoting substrate, regenerating axons require a continual supply of neurotrophins. If repair of a cut peripheral nerve is delayed, the regeneration of axons is impaired (Fu and Gordon, 1995b; Fu and Gordon, 1995a) and these effects can be reversed by application of moderate amounts of the trkB ligand, brain derived neurotrophic factor (BDNF) (Boyd and Gordon, 2001; Boyd and Gordon, 2002). The growth of regenerating axons is severely compromised if the environment through which they must grow is deficient in either BDNF (Zhang et al., 2000) or neurotrophin 4/5 (NT-4/5) (English et al., 2005a). Application of either of these molecules at the time of surgical repair of cut peripheral nerves results in an enhancement of the growth of these axons (English et al., 2005a). The traditional, target-derived neurotrophin signaling pathway could underlie this augmentation. The mRNA for both BDNF and NT-4/5 are known to be up regulated in Schwann cells in the distal stump, but not the proximal stump, one week following nerve transection (Funakoshi et al., 1993; Griesbeck et al., 1995; Zhang et al., 2000), and they could promote axon regeneration by binding to trkB receptors on regenerating neurites.
Gordon and colleagues (Al-Majed et al., 2000b; Brushart et al., 2002) suggested that an alternate source of these neurotrophins may be involved. They showed that if the proximal stump of a cut nerve is stimulated for as little as one hour at 20 impulses per second beginning at the time of its surgical repair, the speed of reinnervation of target muscles is improved. In an effort to identify how this stimulation produces enhanced growth of regenerating axons, they examined its effects on the expression of both BDNF and its receptor, trkB, in spinal motoneurons. They found that electrical stimulation produced a potent increase in the expression of BDNF mRNA in motoneurons and a lesser, but still marked, up regulation of the mRNA for trkB (Al-Majed et al., 2000a). A similar finding has been presented for the effects of electrical stimulation on dorsal root ganglion neurons (Geremia et al., 2005). Neurotrophin production by Schwann cells was not studied. They postulated that electrical stimulation promoted improved motor axon regeneration, not by an increase in neurotrophin signaling originating from Schwann cells, but by an augmented trkB signaling originating from the regenerating neurons themselves.
Using allografts from genetically altered tissues to repair cut peripheral nerves in mice, the ability of Schwann cells surrounding regenerating axons to produce or bind trkB ligands can be manipulated without altering directly the production of these molecules by neurons connected to those axons (English et al., 2005a). This approach was used in this study. Electrical stimulation of the proximal stump of the cut nerve at the time of repair is able to overcome a lack of NT-4/5 of Schwann cell origin in the environment of the regenerating axons and restore growth of regenerating axons. Using acellular allografts, which contain no intrinsic cellular source of neurotrophins or their receptors, it is shown that electrical stimulation is also sufficient to enhance axon regeneration in peripheral nerves. Growth of regenerating axons is not enhanced by electrical stimulation in cut nerves from NT-4/5 knockout mice. A preliminary report of these findings has been made (English et al., 2005b).
METHODS
Animals and Surgical Procedures
All experimental procedures conformed to the Guidelines for the Use of Animals in Research of the Society for Neuroscience and were approved by the Institutional Animal Care and Use Committee of Emory University. Experiments were performed using mice of the thy-1-YFP-H strain as experimental subjects. In these mice, a small subset of all of the neurons with axons in peripheral nerves contains yellow fluorescent protein (YFP) throughout their axonal domains (Feng et al., 2000). Both sensory and motor axons are labeled (Groves et al., 2005; Witzel et al., 2005), there being slightly more of the former marked. The regeneration of axons in the common fibular (CF) nerve of these mice was studied. The CF nerve supplies the anterior and lateral leg muscles and the skin over the dorsum of the foot. It is readily accessible in the popliteal fossa and in previous studies (Groves et al., 2005; Witzel et al., 2005), we showed that it contains more YFP+ axons (37± 2.5 (mean ± SD)) than observed in the other main branches of the sciatic nerve. All host mice were genotyped by observing fluorescence transcutaneously in the posterior auricular nerve.
To study regeneration of peripheral axons, the CF nerve was cut in pentobarbital anesthetized (90mg/kg, IP) thy-1-YFP-H mice and surgically repaired using a short (2-3 mm long) graft taken from the CF nerve of a donor mouse which did not contain the YFP transgene. This nerve allograft and the cut ends of the CF nerve in the host mouse were carefully arranged on a short length of Gore-Tex tubing which had been cut in half longitudinally. The cut ends of the nerves were aligned with those of the graft, placing the proximal stump of the host nerve with the proximal end of the graft from the donor, and similarly apposing the distal end of the graft with the original distal stump of the cut nerve. The stumps of the cut nerve were separated from each of the cut ends of the allograft by a very small gap (ca. 0.1 mm) at either end. The entire graft was then secured in place using a small volume (10 μl total) of fibrin glue which was added to the supporting tube using a pipette. The glue was prepared just prior to use by combining equal parts of fibrin and fibrinogen, and then mixing this with thrombin at equal volumes (de Vries et al., 2002; Suri et al., 2002). Once applied to the nerve, this solution cures rapidly to form a clot and a mechanically secure connection of the graft. Once the clot was formed, the Gore-Tex tubing was removed. The allograft was used initially to provide a dark background against which the regeneration of YFP+ axons could be studied. However, the allografts are also useful in manipulating the environment of the regenerating axons.
In these host mice, cut CF nerves were repaired with one of three different types of grafts which were used to define three different treatment groups, described below. In each group, nerves were studied at one (n=3 for each treatment group) and two week (n=4 for each treatment group) survival times. Two different host scenarios for each treatment group were also implemented. In a control scenario, the regeneration of axons in cut CF nerves was unaided. These nerves were considered unstimulated (US). In the second scenario, the repaired nerve was electrically stimulated (ES).
In one treatment group, the grafts used to repair cut CF nerves were obtained from litter mates of the host thy-1-YFP-H mice. The thy-1-YFP-H mice used as hosts are maintained as heterozygotes, so that half of the members of the F1 generation of a pairing of wild type C57BL/6J mice (the background strain) and thy-1-YFP-H mice will not contain the transgene. These mice were used as a source of control allografts.
In the second group, NT-4/5 knockout mice (Liu et al., 1995) were used as graft donors. Founders for these mice were obtained from The Jackson Laboratory. All mice were genotyped from tail DNA prior to use according to the protocol provided by the supplier. The thy-1-YFP-H mice were developed on a C57BL/6J background. The NT-4/5−/− mice used were developed on a strain 129 background. These two strains share the H2b major histocompatibility locus, but differ in other loci (Snell, 1958; Fischer Lindahl, 1997). On the basis of reports in the literature (reviewed by (Evans et al., 1994), one might expect little immediate rejection of nerve allografts involving such minor histocompatibility disparities, although effects at times later than those we studied might be anticipated.
In the third group, segments of CF nerves were used from wild type C57BL/6J mice which were rendered acellular (Ide et al., 1998; Krekoski et al., 2001). Segments of nerves were removed from the euthanized wild type donor mouse and frozen in liquid nitrogen for two minutes. The segment was then removed and placed in normal saline at 37° C for two minutes before repeating this freeze-thaw cycle two more times. The acellular nerve grafts were then frozen in liquid nitrogen until used to repair cut nerves. For use as grafts, a 2-3 mm long segment of nerve was removed, using frozen razor blades, and allowed to come to room temperature. It was then secured in place using fibrin glue, as described above.
In an additional group of 12 mice, the effect of eliminating NT-4/5 from the host was studied. The thy-1-YFP-H mice were bred to NT-4/5 knockout mice, and then back crossed until mice containing the thy-1-YFP-H transgene that were also homozygous for the NT-4/5 knockout were created. The CF nerves of these mice were then cut and repaired with nerve allografts, as described above. Four treatment groups were studied over a two week survival period. In six mice, the cut nerve was repaired with a graft taken from a wild type littermate. In three of these mice, the repaired nerve was electrically stimulated at the time of surgical repair. In six mice, the cut CF nerve of the NT-4/5−/−/YFP+ host mouse was repaired with a graft taken from a mouse homozygous for the NT-4/5 knockout. In three of these mice, the repaired nerve was electrically stimulated.
Electrical stimulation
In experiments involving electrical stimulation, after the CF nerve had been repaired, the sciatic nerve was exposed just distal to its emergence from the pelvis, and a small bipolar cuff electrode was placed around the nerve. These electrodes were constructed from an incised piece of silastic tubing in which two sets of fine stainless steel wires had been sewn. The holes in the tubing created by passing the wires were sealed using Medical Adhesive A (Dow Corning). The inter electrode spacing for the wires was 1mm. Similar cuff electrodes have been described in more detail by others (Loeb and Peck, 1996). Nerves were stimulated with short duration (0.1msec) constant voltage pulses which were delivered through a stimulus isolation unit at 20Hz for one hour. Stimulus intensity was adjusted to a level twice that required for just evoking a visible twitch in the gastrocnemius muscle. Typically stimulus intensities in the range of 0.5-5.0 volts were used. The cut and repaired CF nerves in the unstimulated mice were fitted with cuff electrodes, but were not subject to stimulation. After one hour of continuous stimulation, the cuffs were removed, all wounds were sutured closed, and animals were allowed to recover from anesthesia before returning them to their cages.
Tissue preparation and data collection
After a one- or two-week survival period, mice were euthanized with pentobarbital (150mg/kg, IP) and perfused, transcardially, with normal saline followed by fixation in periodate-lysate-paraformaldehyde (PLP) fixative solution (McLean and Nakane, 1974). On both sides of each animal the entire sciatic nerve was exposed, removed, and placed on a microscope slide. The branches of the nerve were carefully arranged such that the grafted CF nerve was oriented as in the body and then the entire nerve was cover slipped using Vectashield mounting media. Edges of the cover slip were sealed using nail polish.
These whole mounts were viewed using a laser scanning confocal microscope (Zeiss LSM-510). Stacks of optical sections through the nerve were obtained at relatively low power (10x) at 10μm intervals. Stacks from adjacent microscope fields were stitched together using Adobe Photoshop such that the entire CF nerve from just proximal to the surgical repair site to its entrance into the peroneal muscles was visualized as one large stack of aligned optical sections. Profiles of regenerating axons could be followed through this stack from their distal tips to the proximal surgical repair site. The lengths of these profiles were then measured using Image Pro Plus software.
The YFP+ axon profiles measured are often branches of individual axons. Since the branching often occurs distal to the injury site, the lengths of these profiles, from their distal tip to the surgical repair sites, contains both the lengths of the branch and the length of its parent axon below the surgical repair site. If several branches emanate from the same parent axon, the length of the parent axon from the injury site to the branch point is included in the length measurements made of each branch. We understand that by measuring axon profiles in this manner, we may not be able to differentiate between the effect of electrical stimulation on an increase in branching of a subset of axons (especially if the branching occurs far from the repair site) and an effect due to a more uniform axon elongation.
Data analysis
As has been shown elsewhere (English et al., 2005a; Groves et al., 2005), the distribution of axon profile lengths measured in nerve allografts is not statistically normal. Some axons grow very little, even during a two week survival period, while others grow considerably, giving rise to a bimodal distribution of lengths. Thus the significance of differences between treatment groups in these experiments could not be evaluated using parametric statistical methods, such as t-test or analysis of variance (ANOVA), because they assume that the data are derived from normally distributed samples. Instead, two different approaches were taken. First, a non-parametric measure, the Kolmogorov-Smirnov (KS) two-sample test, was used to examine the probability that the distributions of axon profile lengths measured in different treatment groups are samples drawn from the same population. This method makes no assumption about the nature of these distributions. Second, the median axon profile length was determined in each nerve studied in each mouse. Because these individual values were found to be distributed normally, the significance of differences in average median axon profile lengths between the different treatment groups was evaluated using ANOVA and post-hoc (Fisher Least Significant Differences (LSD)) testing.
Histology
To investigate the structure of the endoneurial tubes of acellular grafts, we prepared acellular grafts by repeated freezing and thawing and either used them to repair cut nerves, as described above, or fixed them immediately in PLP. The grafts used to repair cut nerves were harvested one or two weeks later and fixed similarly. Harvested grafts were sectioned on a cryostat at 20μm thickness. The sections were mounted on slides, reacted with an antibody to laminin-2 (Sigma, Clone 4H8-2) at a dilution of 4μg/ml for 18 hours at 4° C, washed, and then reacted with a species appropriate secondary antibody conjugated to Alexa Fluor 594 (Molecular Probes). Slides were cover slipped with Entellan and viewed using fluorescence microscopy.
To study the effects of electrical stimulation on the expression of BDNF and the full length trkB receptor (trkBFL) in regenerating neurons, we compared immunoreactivity to these two molecules in sections through dorsal root ganglion (DRG) neurons. In 16 thy-1-YFP-H mice, the CF nerve was cut and surgically repaired, bilaterally, using an acellular allograft from a non-fluorescent donor, as described above. In half of these animals, the sciatic nerve proximal to the transection was electrically stimulated at the time of nerve repair, as described above. In an additional eight mice, the intact sciatic nerve was stimulated without nerve transection. Seven or 14 days later, all of these animals were euthanized and perfused with PLP fixative and the L4 DRGs were harvested for histology. The ganglia were serially sectioned in a horizontal plane on a cryostat and alternate 20 μm thick sections were reacted with antibodies either to the full length trkB receptor (SC-12, Santa Cruz Biotechnology) or to BDNF (SC-546, Santa Cruz Biotechnology). The primary antibodies were diluted (1:150) in a solution consisting of 0.1 M phosphate buffer solution (PBS), pH 7.4, 10% normal goat serum, and 0.3% Triton-X 100 for 18 hours at 4° C, washed in buffer, and then reacted with biotinylated goat antirabbit IgG (Jackson ImmunoResearch Laboratories) diluted 1:200 in buffer for two hours at 23° C. Following a wash in buffer solution, the sections were then reacted with Cy5 conjugated streptavidin (Jackson ImmunoResearch Laboratories) diluted to a concentration of 2 μg/ml in PBS for one hour at 23° C. Washed and dried sections were cover slipped with Entellan. The YFP fluorescence is very bright in these sections. The very long wavelength fluorophore, Cy5, was chosen for immunofluorescence to insure that any fluorescence noted in analysis was not due to YFP. In histological sections containing profiles of YFP+ DRG neurons, 5 μm thick optical sections were obtained at a plane through the nucleus using confocal microscopy. Each such YFP+ DRG neuron was scored as positive of negative for immunoreactivity to BDNF or the full length trkB receptor. The mean percentage of YFP+ cells scored as positive was compared between the three groups of mice using ANOVA and appropriate post-hoc paired (LSD) testing. Significance levels were set at p<0.05.
RESULTS
Effects of electrical stimulation on peripheral axon regeneration
In thy-1-YFP-H mice, CF nerves were cut and repaired with a 2-3 mm long allograft harvested from a wild type litter mate, and then the proximal stump of the sciatic nerve was electrically stimulated continuously for one hour at 20Hz. One or two weeks later these repaired nerves were harvested and YFP+ axons which had regenerated into the graft from the proximal stump were studied using confocal microscopy. From reconstructed optical sections through the nerve, the lengths of axon profiles were measured from their distal tips to the surgical repair site between the proximal stump and the graft. Examples of representative optical sections through nerves that had been surgically repaired two weeks earlier using grafts from wild type mice and were either unstimulated (US) or electrically stimulated (ES) are shown in figure 1.
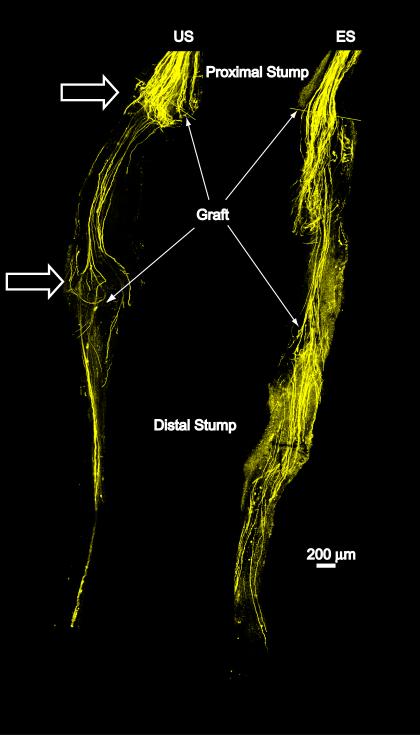
Figure 1.
Low magnification images of two common fibular nerves are used to show the effects of electrical stimulation on the regeneration of cut axons. Each nerve had been cut and surgically repaired two weeks earlier using a ca. 2 mm long graft from a wild type donor mouse. The boundaries of each graft are indicated by arrows. The overall image of each nerve is a montage, constructed from images of several microscope fields, each taken at the same confocal plane through whole mounts of the nerve. They thus represent a single optical section through the nerve from proximal to the lesion site to its muscle entry point. By two weeks after nerve repair, any residual fluorescence in the original distal stump (distal to the graft) due to the degeneration of the host axons has disappeared. Fluorescence found in this region thus represents profiles of regenerating axons that have grown entirely through the grafts. The nerve on the right (ES) was stimulated for one hour at the time of the nerve repair. The nerve on the left (US) was from a mouse that was unstimulated. Note that in the US nerve, build-up and recurrent loopings of regenerating axons are found near the proximal and distal attachments of the host nerve to the graft (open arrows). No such effects were noted in the electrically stimulated nerve and larger numbers of regenerating axon profiles are present.
Grafts were used to repair cut nerves partly because they form a dark background against which regenerating YFP+ axons could be visualized in the absence of any fluorescence associated with degenerating host axons distal to the injury site. Such fluorescence usually disappears from the original distal stump approximately 10 days after nerve repair (English et al., 2005a). In the animals surviving two weeks, growth of some regenerating axons is sufficient to have passed completely through the 2-3 mm long graft and into the original distal stump of the cut nerve. Confusion of fluorescence in the original distal stump between the products of anterograde degeneration and profiles of regenerating axons was never a problem in the nerves studied. Similarly, the presence of fluorescence in the original distal stumps in animals surviving one week was never a confounding issue, since regenerating axons rarely grow this far during the first post-injury week (Groves et al., 2005) (see below).
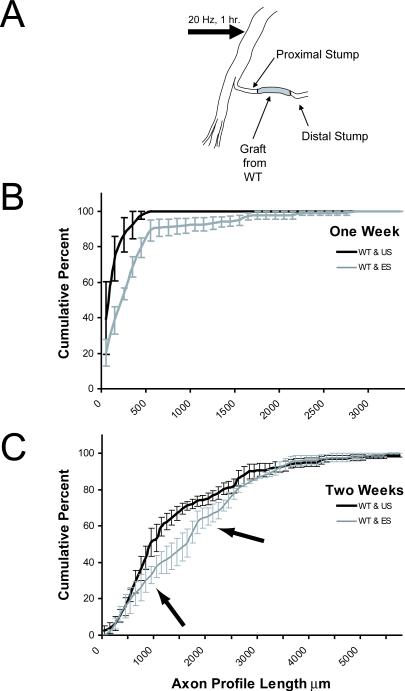
Frequency distributions of axon profile lengths were constructed for each graft using a bin size of 100 μm. The distributions for each of the grafts studied in each of the treatment groups were then averaged (n = 3 or 4, depending on the survival time) and the resulting average distributions were plotted as cumulative frequency distributions (±SEM). The distributions of axon profile lengths measured in these grafts for mice in which the proximal stumps were electrically stimulated (ES) and mice in which no stimulation was applied (US) are shown in figure 2. Data are presented for one week (B) and two week (C) survival periods. A statistically significant (KS, p<0.05) shift in the cumulative distribution of axon profile lengths to the right in the electrical stimulation group one week after nerve repair reflects an enhancement of axon regeneration produced in response to electrical stimulation (Fig. 2B). This shift means that, at any axon profile length, significantly more axon profiles are found at that length or longer in the electrically stimulated group than the unstimulated group. A more subtle but significant (KS, p<0.05) effect is noted two weeks after nerve repair (Fig. 2C). Arrows point to the region near the center of the cumulative frequency distribution which is shifted significantly to the right of that found in controls.
Figure 2.
A. Diagram of experimental paradigm used for electrical stimulation. The common fibular branch of the sciatic nerve in thy-1-YFP-H mice was cut and repaired with a graft from a wild type litter mate. The entire sciatic nerve was then electrically stimulated for one hour at 20 impulses per second. In a separate set of mice, the stimulating electrode was placed around the sciatic nerve for one hour, but no electrical stimuli were delivered. B and C. Cumulative frequency distributions of YFP+ axon profile lengths measured in grafts one (B) or two weeks (C) after the nerve repair. Each 100 μm bin in these distributions represents the mean (±SEM) of three (B) or four (C) mice. Dark lines represent controls (grafts from wild type, unstimulated); grey lines represent values from electrically stimulated (ES) mice. Arrows in C mark the portion of the frequency distributions in which all values between groups are significantly different.
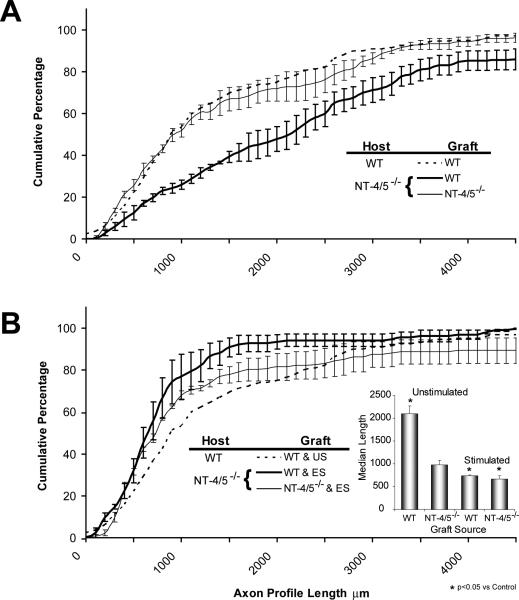
Effects of electrical stimulation on regeneration of axons through grafts from NT-4/5−/− mice
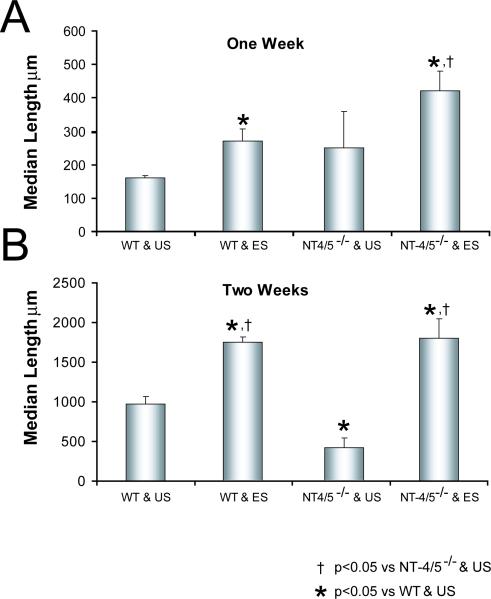
The use of grafts from NT-4/5 knockout mice to repair cut CF nerves was designed to manipulate the environment surrounding the regenerating axons by using cells in the graft that could not make NT-4/5. As has been shown previously (English et al., 2005a), the distribution of axon profile lengths measured in grafts from NT-4/5−/− mice surviving two weeks is shifted significantly (KS, p<0.05) to the left of that from measurements made in grafts from wild type mice (data not shown). This difference reflects the poor growth of regenerating axons in an environment in which Schwann cell-derived NT-4/5 is absent (English et al., 2005a). Median axon profile length measured in grafts from NT-4/5 knockout mice one week after nerve repair is not significantly different from that measured at the same time in grafts from wild type mice (LSD, p=0.55) (Fig. 3A). This was not surprising. The mRNA for NT-4/5 is not elevated in the distal stump of cut and repaired nerves until nearly a week after injury (Funakoshi et al., 1993; Griesbeck et al., 1995; Zhang et al., 2000). After two weeks, the growth of axons through an environment devoid of Schwann cell-derived NT-4/5 is slowed markedly (LSD, p<0.001) (Fig. 3B), as has been noted elsewhere (English et al., 2005a). If nerves repaired using grafts from NT-4/5 knockout mice are electrically stimulated, average median lengths of axon profiles measured in grafts from NT-4/5−/− mice are significantly longer, after both one (LSD, p< 0.001) and two weeks (LSD, p<0.001) (Fig. 3A, B).
Figure 3.
Effects of electrical stimulation on the regeneration of axons through grafts from NT-4/5 knockout mice. The protocol is similar to that described in figure 2, except that the graft used to repair the nerve was taken either from a wild type (WT) mouse or from a neurotrophin 4/5 knockout (NT-4/5−/−) knockout mouse. Median lengths (+SEM) of YFP+ axon profiles measured in grafts from these different donors which were used to repair electrically stimulated (ES) nerves or unstimulated (US) nerves are shown one (A) or two weeks (B) after the nerve repair.
Effects of electrical stimulation on axon regeneration through acellular grafts
The effect of electrical stimulation in overcoming the lack of NT-4/5 in the pathway through which they regenerate could be the result of an enhancement of trkB signaling known to be produced by electrical stimulation (Al-Majed et al., 2000a). Presumably normal amounts of BDNF in these grafts could signal more strongly to regenerating neurons through the increased amounts of neuronal trkB produced by electrical stimulation. However, it is also possible that electrical stimulation is able to reverse the effects of deleting Schwann cell-derived NT-4/5 in the environment of regenerating axons by enhancing a cell autonomous stimulation of axon growth, as suggested previously (Al-Majed et al., 2000a). Regenerating axons could regulate their own growth by signals they produce in response to electrical stimulation. To investigate this latter possibility, grafts of CF nerves from wild type mice, which were made acellular by repeated freezing and thawing, were used to repair cut CF nerves in thy-1-YFP-H mice. It was assumed that this process kills all of the cells in the grafts, leaving only the endoneurial tubes and extracellular matrix intact (Ide et al., 1998; Krekoski et al., 2001). It was also assumed that, over the time periods studied, migration of host cells into the graft was minimal (Hall and Berry, 1989; Anderson et al., 1991; Dubovy et al., 2001; Kimura et al., 2005). Data from stimulated and unstimulated acellular grafts at one and two weeks after nerve repair were compared to data from unstimulated cellular (control) grafts obtained from wild type mice.
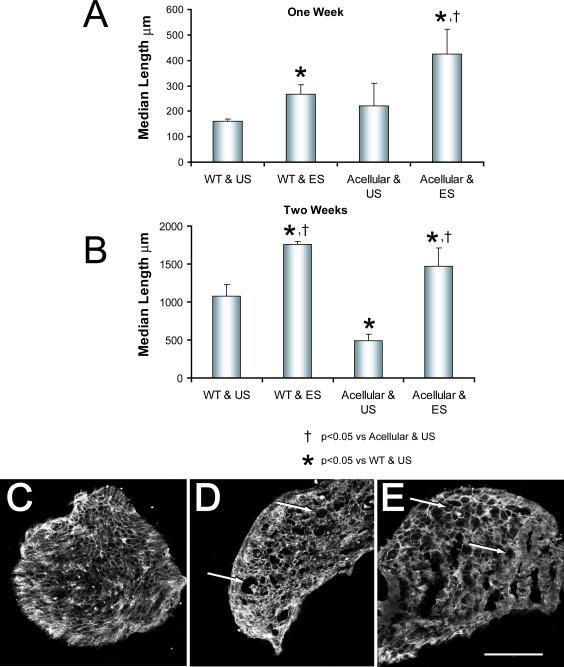
Median axon profile lengths measured in grafts in these four groups are shown in figures 4A and 4B. One week after repair of the cut CF nerve with an acellular graft, the average median axon profile length was not significantly different (LSD, p=0.52) from that measured in grafts from wild type mice (Fig. 4A). By two weeks after nerve repair, the median axon profile lengths measured in acellular grafts are significantly (LSD, p<0.0001) shorter than those found in cellular grafts (Fig. 4B). Electrical stimulation of the proximal stumps of the cut CF nerves results in a marked change in this pattern. The median length of axon profiles measured in electrically stimulated mice whose nerves were repaired using acellular grafts is significantly greater than that found in controls (LSD, p<0.001) at both times studied (Fig. 4A, B).
Figure 4.
Effects of electrical stimulation on the regeneration of axons through acellular grafts. The protocol is similar to that described in figure 2, except that the grafts from wild type (WT) mice used to repair the nerve either were untreated or were made acellular by repeated freeze-thawing before use. Median lengths (+SEM) of YFP+ axon profiles measured in grafts from these different donors which were used to repair electrically stimulated (ES) nerves or unstimulated (US) nerves are shown one (A) or two weeks (B) after the nerve repair. Panels C-E are images of transverse sections through acellular grafts before (C), one week (D) and two weeks (E) after being used to repair cut nerves. Each has been reacted with an antibody to laminin-2. Arrows indicate examples of enlarged spaces found in implanted grafts. Scale bar = 200 μm.
As noted above, it is assumed that making these grafts acellular will remove any source of neurotrophins from them but that the Schwann cell basal lamina within the endoneurial tubes in the grafts will be retained. Using immunohistofluorescence analysis of tissue sections made through regions of acellular grafts used to repair cut nerves, we have validated this assumption. Transverse sections through such grafts were reacted for the demonstration of immunoreactivity to laminin-2. Grafts were studied at the time of implantation (Fig. 4C) and one (Fig. 4D) and two weeks (Fig. 4E) after being used to repair cut nerves in unstimulated mice. Distinct laminin-2-lined endoneurial tubes are present in all three images, even those from grafts that had been used to repair cut nerves and in which little axon regeneration had progressed. Enlarged laminin-2-lined spaces, distinct from blood vessels, are visible among smaller endoneurial tubes (Fig. 4 D, E: arrows) in sections from grafts that had been implanted. As has been reported by others (Ide et al., 1998; Krekoski et al., 2001), such seemingly enlarged endoneurial tubes also were found in control (cellular) grafts (data not shown).
Effects of electrical stimulation on axon regeneration in NT-4/5 knockout mice
When axons in the CF nerve of NT-4/5 knockout mice are constrained to grow through an environment in which NT-4/5 is absent, the distribution of axon profile lengths (Fig. 5A, thin solid line) is not significantly different from that observed in controls (KS, p=0.39) – that is axons from wild type mice regenerating into grafts from wild type mice (Fig. 5A, dashed line). If regenerating axons from NT-4/5 knockout mice grow through a graft from a wild type mouse, the distribution of axon profile lengths (Fig. 5A, thick solid line) is shifted significantly (KS, p<0.01) to the right of that observed either in controls or when both graft and host contained no NT-4/5. This shift means that for any given axon profile length, significantly more axon profiles are found that length or longer in grafts from wild type mice. Similarly, median axon profile length is significantly (LSD, p<0.001) larger than controls when measured in grafts from wild type mice used to repair cut CF nerves in NT-4/5 knockout mice, but not when the axon profiles are measured in grafts from NT-4/5 knockout mice (LSD, p=0.93) (Fig.5B, inset).
Figure 5.
Regeneration in NT-4/5 knockout mice. Mice homozygous null for the NT-4/5 gene were mated with thy-1-YFP-H mice and then back crossed until offspring that were both NT-4/5−/− and YFP+ were identified. The common fibular nerves of these mice were cut and repaired using grafts obtained either from NT-4/5−/− mice that were YFP− or wild type (NT-4/5+/+ and YFP−) mice. Cumulative frequency distributions of axon profile lengths measured in these unstimulated mice are shown in panel A. The format is the same as that of figure 2. In panel B, data are shown in a similar format from measurements of axon profile lengths in a set of these animals in which the repaired nerve was electrically stimulated. Inset is a graph of average median axon profile lengths (+SEM) for these four treatment groups.
If the cut and repaired CF nerves of NT-4/5 knockout mice were electrically stimulated at the time of nerve repair, a different situation was found (Fig. 5B). Irrespective of the type of graft used to repair the cut CF nerve of NT -4/5−/− mice, electrical stimulation produced no significant enhancement of the growth of regenerating axons. The distributions of axon profile lengths were not significantly different from those of unstimulated mice repaired with the same type of graft (Fig. 5B). Whether the type of graft used to repair the cut nerves was obtained from a wild type mouse or from an NT-4/5 knockout mouse, median axon profile lengths were actually significantly (LSD, p<0.0001) smaller than in unstimulated NT-4/5 knockout mice (Fig. 5B, inset). When compared to median axon profile lengths measured in grafts from wild type mice used to repair cut nerves in unstimulated wild type mice, no significant (LSD, p=0.29) differences were found.
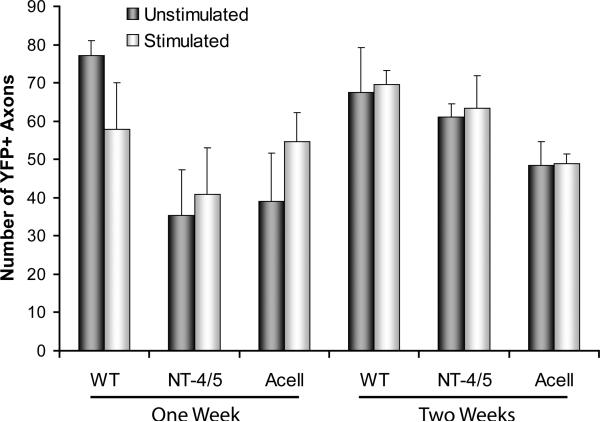
Number of Axon Profiles
It is possible that the effects of electrical stimulation could be due to an increase in the growth of regenerating axons, as we have assumed above. It is also possible that the effects observed could be accounted for by a change in the extent of branching of a subset of axons, especially if that branching occurred far distal to the surgical repair site. Because of our subjective impression that such branching was not a hallmark of the measurements made, we tend to favor the former interpretation. However, to assay for the extent of branching of regenerating axons, we counted the number of YFP+ axon profiles in each case studied, and evaluated the significance of differences between the different treatment groups using ANOVA. These data are shown in figure 6. No significant differences were found between electrically stimulated and unstimulated mice.
Figure 6.
The mean (+SEM) number of YFP+ axon profiles measured in grafts of different types, in stimulated (ES) and unstimulated (US) mice are shown after one (left) and two (right) week survival periods. No significant differences were found between stimulated and unstimulated mice.
BDNF and trkBFL content of regenerating axons
In previous experiments, it has been shown using in situ hybridization that the mRNA for both BDNF and trkB is elevated in rats in which cut and repaired nerves were electrically stimulated. This up regulation of these persisted for several days (Al-Majed et al., 2000a). It was assumed that a similar increase would be found in the mouse. We studied the effects of ES on the expression of BDNF and trkB in YFP+ neurons in sections through the L4 DRG that had been reacted with well characterized antibodies. To evaluate this expression among neurons whose axons had been studied in regenerating peripheral nerves, we scored each YFP+ DRG neuron for its immunoreactivity to one or the other of these antibodies. We have shown that in thy-1-YFP-H mice, DRG neurons containing the YFP marker are approximately equally divided between large and small sized cells (English et al., 2005a). In comparison to the overall cell size distribution in these ganglia (e.g. Liebl et al., 1999), this means that the sample of DRG neurons defined by the presence of YFP is slightly biased toward larger cells.
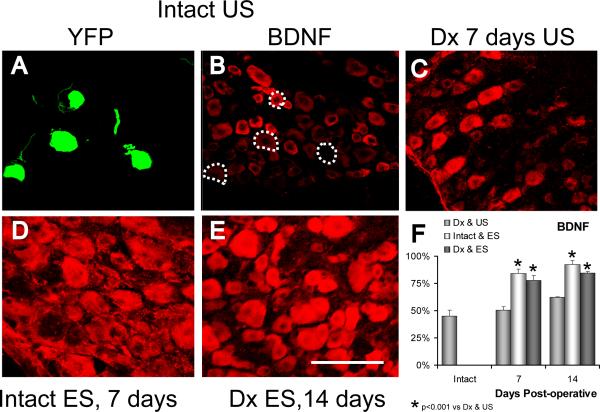
In intact mice, immunoreactivity to BDNF is strongest in small to medium sized neurons and weak or absent in larger cells (Fig. 7A, B). Slightly fewer than half of the YFP+ DRG neurons studied in ganglia from intact mice were scored as immunoreactive for BDNF (Fig. 7F). If the sciatic nerve is transected and surgically repaired, the pattern of immunoreactivity found in the ganglia changes (Michael et al., 1999; Zhou et al., 1999; Karchewski et al., 2002). Immunoreactivity to BDNF decreases in small diameter neurons and large cells become much more strongly immunoreactive, both seven and 14 days later (Fig. 7C). However, among YFP+ DRG neurons, no significant (LSD, p=0.87 at one week, p=0.372 at two weeks) change in the proportion of neurons scored as positive for BDNF was noted (Fig. 7F). If the sciatic nerve was electrically stimulated for one hour in intact mice, a striking increase in the amount of BDNF immunoreactivity was noted in sections through the L4 DRG (Fig. 7D). Both one and two weeks later, most neurons were immunoreactive and among YFP+ DRG neurons, the proportion scored as immunoreactive increased significantly relative to unstimulated mice (LSD, p<0.001 at both times) (Fig. 7F). If the proximal stumps of cut sciatic nerves were electrically stimulated at the time of their surgical repair a similar increase in immunoreactivity was observed both one and two weeks later (Fig. 7E). Among YFP+ neurons, a significant (LSD, p<0.001 at both times) increase in the proportion of neurons scored as immunoreactive to BDNF, relative to unstimulated mice was found. This increase was not significantly different from that noted in intact electrically stimulated neurons (LSD, p=0.25 at one week, p=0.20 at two weeks) (Fig. 7F).
Figure 7.
Immunoreactivity to BDNF is shown in representative sections through the L4 dorsal root ganglion of thy-1-YFP-H mice. In panel A, four YFP+ DRG neurons are shown in a section through the L4 ganglion of an intact mouse. In panel B, positive immunoreactivity to BDNF is shown in the same microscope field when viewed with filters appropriate for demonstration of CY5. The YFP+ cells are shown by the dashed outlines. Immunoreactivity to BDNF is shown in sections from an unstimulated mouse seven days after transection and surgical repair of the sciatic nerve (C), seven days after application of one hour of electrical stimulation to the intact sciatic nerve (D) or 14 days after sciatic nerve transection in mice that had been electrically stimulated for one hour at the time of nerve repair (E). Scale bar = 100 μm. In panel F, the proportions of YFP+ DRG neurons that were scored positive for immunoreactivity for BDNF are shown for denervated and unstimulated (Dx & US), intact and stimulated (Intact & ES), and denervated and stimulated (Dx & ES) mice at one and two weeks following nerve transection and/or stimulation. Each column represents the mean (+SEM) of four ganglia.
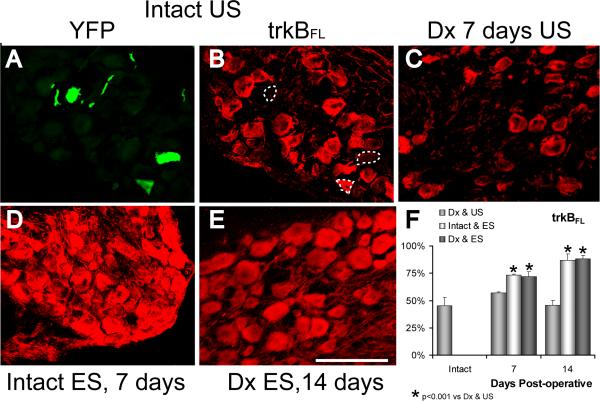
In intact mice, immunoreactivity to the full length trkB is strongest in medium to large sized neurons and weak or absent in smaller cells (Fig. 8A, B). As we have shown previously (English et al., 2005a), slightly fewer than half of the YFP+ DRG neurons studied in the L4 ganglia from intact mice were scored as immunoreactive for trkBFL (Fig. 8F). If the sciatic nerve is transected and surgically repaired, the intensity of immunoreactivity is decreased somewhat (Karchewski et al., 2002), but no obvious change in this general pattern is observed (Fig. 8C). Among YFP+ DRG neurons, no significant change in the proportion of neurons scored as positive for trkBFL was noted, either one (LSD, p=0.44) or two (LSD, p=0.68) weeks later (Fig. 8F). If the sciatic nerve was electrically stimulated for one hour in intact mice, a striking increase in the amount of trkBFL immunoreactivity was noted in sections through the L4 DRG (Fig. 8D). Both one and two weeks later, many more neurons were immunoreactive and, among YFP+ DRG neurons, the proportion scored as immunoreactive increased significantly (LSD, p<0.001 at both times) (Fig. 8F). If the proximal stumps of cut sciatic nerves were electrically stimulated at the time of their surgical repair, a similar increase was observed both one and two weeks later (Fig. 8E). Among YFP+ neurons, a significant (LSD, p<0.001 at both times) increase in the proportion of neurons scored as immunoreactive to trkBFL was found relative to unstimulated mice. This increase was not significantly different (LSD, p=0.23 at one week, p=0.84 at two weeks) from that noted in intact electrically stimulated neurons (Fig. 8F).
Figure 8.
Immunoreactivity to the full length trkB receptor is shown in representative sections through the L4 dorsal root ganglion of thy-1-YFP-H mice. In panel A, three YFP+ DRG neurons are shown in a section through the L4 ganglion of an intact mouse. In panel B, positive immunoreactivity to trkBFL is shown in the same microscope field when viewed with filters appropriate for demonstration of CY5. The YFP+ cells are shown by the dashed outlines. Immunoreactivity to trkBFL is shown in sections from an unstimulated mouse seven days after transection and surgical repair of the sciatic nerve (C), seven days after application of one hour of electrical stimulation to the intact sciatic nerve (D) or 14 days after sciatic nerve transection in mice that had been electrically stimulated for one hour at the time of nerve repair (E). Scale bar = 100 μm. In panel F, the proportions of YFP+ DRG neurons that were scored as positive for immunoreactivity to trkBFL are shown for denervated and unstimulated (Dx & US), intact and stimulated (Intact & ES), and denervated and stimulated (Dx & ES) mice at one and two weeks following nerve transection and/or stimulation. Each column represents the mean (+SEM) of four ganglia.
DISCUSSION
In pioneering work, Gordon and colleagues showed that both the speed of reinnervation of target muscles and the sensorimotor precision with which they were reinnervated was enhanced if the proximal stump of the cut nerve was stimulated electrically at the time of surgical repair (Al-Majed et al., 2000b). Since such stimulation resulted in the up regulation of the mRNA for both BDNF and its receptor, trkB, in motoneurons and dorsal root ganglion cells (Geremia et al., 2005), they postulated that the enhancement of axon regeneration produced by electrical stimulation was the result of an enhancement of a cell autonomous regulation of axon growth. In particular, they speculated that electrical stimulation promoted an increase in trkB signaling originating from BDNF of neuronal origin (Al-Majed et al., 2000a).
All of the results presented above are consistent with the interpretation that electrical stimulation exerts its effects through such a cellular mechanism. As shown previously (English et al., 2005a), if the environment surrounding regenerating axons in peripheral nerves is made deficient in the trkB ligand NT-4/5 by repairing cut nerves with grafts from NT-4/5−/− mice, the growth of regenerating axons is markedly reduced. If the proximal stumps of cut CF nerves of wild type mice, which were repaired with grafts from NT-4/5−/− mice, are electrically stimulated at the time of surgical repair, the anticipated defect in regenerative axon growth is completely reversed. It is notable that this effect of electrical stimulation is similar to the effect observed when grafts from NT-4/5−/− mice were used to repair cut CF nerves in wild type mice and they were treated at the time of surgical repair either with recombinant human BDNF or NT-4/5 (English et al., 2005a). The simplest explanation of these findings is that the increase in neuronal BDNF and trkB which is thought to accompany electrical stimulation (Al-Majed et al., 2000a) forms the basis for a cell autonomous regulation of axon growth, but evidence to support such a mechanism of action has been lacking.
Even though electrical stimulation might result in an increase in trkB signaling in regenerating axons, our interpretation is complicated by the fact that more than one cellular source of the ligands for that receptor are known. In addition to the production of trkB ligands by most motoneurons (Buck et al., 2000; Copray and Kernell, 2000) and some dorsal root ganglion cells (Mendell, 1996), Schwann cells in the distal stump synthesize both BDNF and NT-4/5, beginning as early as four days after injury (Funakoshi et al., 1993; Griesbeck et al., 1995; Zhang et al., 2000). Thus, the rapid up regulation of the mRNA for trkB in motoneurons described following electrical stimulation could result in an increase in trkB signaling in regenerating axons produced by BDNF of neuronal origin (which acts on the regenerating axons themselves and/or the surrounding axons) or BDNF (or/and NT-4/5) produced by Schwann cells in the surrounding environment (a form of target derived neurotrophin signaling). The finding that electrical stimulation of the proximal stump at the time of surgical repair of cut nerves is sufficient to overcome the lack of NT-4/5 in the regeneration pathway supports the hypothesis that the efficacy of electrical stimulation is related to an up regulation of signaling through neuronal trkB. The source of the ligands for these receptors is not addressed by these experiments.
Acellular grafts were used as a means of evaluating the source of the ligand. In these grafts, Schwann cells have been destroyed, leaving behind endoneurial tubes and their associated extracellular matrix (Ide et al., 1998; Krekoski et al., 2001) (Fig. 4C-E). These grafts contain neither neurotrophins nor the cellular machinery with which to synthesize them. Even though some migration of cells into these grafts from the cut stumps of the CF host nerve can occur (Hall and Berry, 1989; Anderson et al., 1991; Dubovy et al., 2001; Kimura et al., 2005), it was assumed that, since an up regulation of the RNA for BDNF is not found in the proximal stumps of cut nerves (Zhang et al., 2000), their ability to synthesize and secrete neurotrophins into the environment of the regenerating axons was much less than the Schwann cells destroyed in the repeated freeze-thaw process used to prepare them. In two weeks, Schwann cells migrate only 100-200 μm into acellular grafts (Dubovy et al., 2001). Therefore, neurotrophins of Schwann cell origin would not be expected to contribute significantly to axon regeneration during the first week following nerve repair. Consistent with our assumption and these data, the growth of regenerating axons through unstimulated acellular grafts is not significantly different than through intact grafts after one week, but it is markedly slower at the two week survival time studied.
An underlying assumption of our interpretations is that electrical stimulation of the proximal stump of a cut peripheral nerve will result in an increase in both trkB and its ligands in the regenerating axons. This assumption is based on the finding of Gordon and colleagues that the mRNA for both BDNF and trkB is increased significantly and persistently in rat femoral motoneurons after electrical stimulation (Al-Majed et al., 2000a). To provide some validity to our assumption in the mouse, we investigated the expression of these proteins in the L4 DRG in stimulated and unstimulated mice. In contrast to highly immunoreactive motoneurons (Buck et al., 2000; Copray and Kernell, 2000), only about half of the YFP+ DRG neurons in intact mice are immunoreactive for the full length trkB molecule (English et al., 2005a). As others have shown (Michael et al., 1999; Zhou et al., 1999; Karchewski et al., 2002), axotomy results in a change in BDNF expression in DRG neurons from mainly small, presumably trkA containing cells to medium to large size neurons. In addition, the proportion of DRG neurons containing both BDNF and trkB is increased (Karchewski et al., 2002). We found that the proportions of YFP+ DRG neurons that express either BDNF or trkBFL increase dramatically following electrical stimulation, whether or not peripheral nerves had been cut. We interpret these findings as in support of our assumption that electrical stimulation produces an increase in both BDNF and trkBFL protein in regenerating axons. Coupled with the finding that electrical stimulation enhances the regeneration of axons through acellular grafts, which have no known source of Schwann cell neurotrophins, at both survival times, we feel that these observations are strong evidence in support of the cellular mechanism proposed by others (Al-Majed et al., 2000a), that electrical stimulation promotes axon regeneration by enhancing trkB signaling originating from the regenerating axons.
Electrical stimulation failed to enhance the growth of regenerating axons in NT-4/5 knockout mice. In fact, when compared to appropriate controls, electrical stimulation actually resulted in a significant inhibition of growth of regenerating axons in these mice. Unfortunately, it was not possible to conduct analogous experiments with BDNF knockout mice, since they do not survive. (Jones et al., 1994; Ernfors et al., 1995; Conover and Yancopoulos, 1997) Given that BDNF and NT-4/5 seem to be interchangeable in promoting the growth of regenerating axons, (English et al., 2005a) we would speculate that if we could perform such an experiment, its outcome would be the same as has been observed in the present study using NT-4/5 knockout mice. These findings are interpreted to mean that the growth enhancing effect of electrical stimulation requires trkB signaling, and they are consistent with the hypothesized role of neuronal neurotrophins regulating the growth of their own or surrounding regenerating axons (Al-Majed et al., 2000a).
However, we draw this conclusion with a slight reservation. It is important to consider that the population of neurons studied in the NT-4/5 knockout might not be exactly the same as those studied in wild type mice. In NT-4/5 knockout mice, it has been reported that some lumbar dorsal root ganglion neurons die (Conover and Yancopoulos, 1997) and others do not differentiate normally (Kirstein and Farinas, 2002). It is not yet known if the same is true for motoneurons. Because of this potential difference, it is possible that the growth of regenerating axons in NT-4/5 knockout mice might be less dependent on NT-4/5 from any source than the growth of regenerating axons in wild type mice. The relatively normal growth of axons in NT-4/5 knockout mice through grafts from NT-4/5 knockout mice might reflect that independence. The enhanced growth noted when these axons grow through grafts from wild type mice might be interpreted as a growth promoting effect of NT-4/5 produced by Schwann cells in the graft. Even though such an interpretation does not necessarily degrade the conclusions of the present study, it does complicate them. Study of axon regeneration in a conditional NT-4/5 (or BDNF) knockout mouse would resolve this concern.
One might postulate that two parallel trkB signaling pathways exist in regenerating peripheral axons. A target derived pathway utilizing BDNF and/or NT-4/5 becomes active as Schwann cells in the distal stump begin to produce larger quantities of these ligands and secrete them into the environment of the regenerating axons. The timing of activation of this pathway corresponds well with the growth of regenerating axons which have formed regenerative sprouts and crossed the injury site to invade the distal stump, or in the case of the experiments described above, a nerve allograft. A second trkB signaling pathway is driven by NT-4/5 (and/or BDNF) secretion from the regenerating axons themselves. Under normal circumstances signaling via this route is not sufficient to promote axon regeneration by itself, since the growth of regenerating axons is profoundly slowed in the absence of NT-4/5 in their environment. Neurotrophin signaling originating from regenerating axons is found early in the regeneration process and, based on its selective recruitment by electrical stimulation; it can be enhanced sufficiently to support the regeneration of axons in peripheral nerves irrespective of the neurotrophin content in their immediate environment.
ACKNOWLEDGEMENTS
Thanks are due to Dr. Dario Carrasco, who read and commented on earlier versions of the manuscript. Thanks also to W. L. Gore & Associates, Inc., for the gift of the Gore-Tex tubing. This work was completed with funding from grant HD43596 from the USPHS.
LITERATURE CITED
- Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci. 2000a;12:4381–4390. [PubMed] [Google Scholar]
- Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000b;20:2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson PN, Nadim W, Turmaine M. Schwann cell migration through freeze-killed peripheral nerve grafts without accompanying axons. Acta Neuropathol (Berl) 1991;82:193–199. doi: 10.1007/BF00294445. [DOI] [PubMed] [Google Scholar]
- Boyd JG, Gordon T. The neurotrophin receptors, trkB and p75, differentially regulate motor axonal regeneration. J. Neurobiol. 2001;49:314–325. doi: 10.1002/neu.10013. [DOI] [PubMed] [Google Scholar]
- Boyd JG, Gordon T. A dose-dependent facilitation and inhibition of peripheral nerve regeneration by brain-derived neurotrophic factor. Eur J Neurosci. 2002;15:613–626. doi: 10.1046/j.1460-9568.2002.01891.x. [DOI] [PubMed] [Google Scholar]
- Brushart TM, Hoffman PN, Royall RM, Murinson BB, Witzel C, Gordon T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002;22:6631–6638. doi: 10.1523/JNEUROSCI.22-15-06631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CR, Seburn KL, Cope TC. Neurotrophin expression by spinal motoneurons in adult and developing rats. J. Comp. Neurol. 2000;416:309–318. doi: 10.1002/(sici)1096-9861(20000117)416:3<309::aid-cne3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Conover JC, Yancopoulos GD. Neurotrophin regulation of the developing nervous system: analyses of knockout mice. Rev. Neurosci. 1997;8:13–27. doi: 10.1515/revneuro.1997.8.1.13. [DOI] [PubMed] [Google Scholar]
- Copray S, Kernell D. Neurotrophins and trk-receptors in adult rat spinal motoneurons: differences related to cell size but not to 'slow/fast' specialization. Neurosci. Let. 2000;289:217–220. doi: 10.1016/s0304-3940(00)01305-7. [DOI] [PubMed] [Google Scholar]
- de Vries J, Menovsky T, van Gulik S, Wesseling P. Histological effects of fibrin glue on nervous tissue: a safety study in rats. Surg Neurol. 2002;57:415–422. doi: 10.1016/s0090-3019(02)00736-x. [DOI] [PubMed] [Google Scholar]
- Dubovy P, Svizenska I, Klusakova I, Zitkova A, Houst'Ava L, Haninec P. Laminin molecules in freeze-treated nerve segments are associated with migrating Schwann cells that display the corresponding alpha6beta1 integrin receptor. Glia. 2001;33:36–44. doi: 10.1002/1098-1136(20010101)33:1<36::aid-glia1004>3.3.co;2-2. [DOI] [PubMed] [Google Scholar]
- English AW, Meador W, Carrasco DI. Neurotrophin 4/5 is required for the early growth of regenerating axons in peripheral nerves. Eur J Neurosci. 2005a;21:2624–2634. doi: 10.1111/j.1460-9568.2005.04124.x. [DOI] [PubMed] [Google Scholar]
- English AW, Mulligan A, Schwartz G. Electrical stimulation promotes peripheral axon regeneration by enhancing autocrine/paracrine neurotrophin signaling. Abstr. Soc. Neurosci. 2005b doi: 10.1002/dneu.20339. Program No. 29.11. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Kucera J, Lee KF, Loring J, Jaenisch R. Studies on the physiological role of brain-derived neurotrophic factor and neurotrophin-3 in knockout mice. Int. J. Dev. Biol. 1995;39:799–807. [PubMed] [Google Scholar]
- Evans PJ, Midha R, Mackinnon SE. The peripheral nerve allograft: a comprehensive review of regeneration and neuroimmunology. Progr. Neurobiol. 1994;43:187–233. doi: 10.1016/0301-0082(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Fischer Lindahl K. On naming H2 haplotypes: functional significance of MHC class Ib alleles. Immunogenetics. 1997;46:53–62. doi: 10.1007/s002510050242. [DOI] [PubMed] [Google Scholar]
- Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged axotomy. J. Neurosci. 1995a;15:3876–3885. doi: 10.1523/JNEUROSCI.15-05-03876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J. Neurosci. 1995b;15:3886–3895. doi: 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Frisen J, Barbany G, Timmusk T, Zachrisson O, Verge VM, Persson H. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J. Cell Biol. 1993;123:455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geremia NM, Gordon T, Al-Majed AA, Brushart TM, Verge VMK. Brief electrical stimulation promotes sensory neuron regeneration and intrinsic growth-associated gene expression. Society for Neuroscience. 2005 doi: 10.1016/j.expneurol.2007.01.040. Program No. 28.26. [DOI] [PubMed] [Google Scholar]
- Griesbeck O, Parsadanian AS, Sendtner M, Thoenen H. Expression of neurotrophins in skeletal muscle: quantitative comparison and significance for motoneuron survival and maintenance of function. J. Neurosci. Res. 1995;42:21–33. doi: 10.1002/jnr.490420104. [DOI] [PubMed] [Google Scholar]
- Groves ML, McKeon R, Werner E, Nagarsheth M, Meador W, English AW. Axon regeneration in peripheral nerves is enhanced by proteoglycan degradation. Exp. Neurol. 2005;195:278–292. doi: 10.1016/j.expneurol.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Hall S, Berry M. Electron microscopic study of the interaction of axons and glia at the site of anastomosis between the optic nerve and cellular or acellular sciatic nerve grafts. J Neurocytol. 1989;18:171–184. doi: 10.1007/BF01206660. [DOI] [PubMed] [Google Scholar]
- Ide C, Tohyama K, Tajima K, Endoh K, Sano K, Tamura M, Mizoguchi A, Kitada M, Morihara T, Shirasu M. Long acellular nerve transplants for allogeneic grafting and the effects of basic fibroblast growth factor on the growth of regenerating axons in dogs: a preliminary report. Exp Neurol. 1998;154:99–112. doi: 10.1006/exnr.1998.6921. [DOI] [PubMed] [Google Scholar]
- Jones FR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karchewski LA, Gratto KA, Wetmore C, Verge VM. Dynamic patterns of BDNF expression in injured sensory neurons: differential modulation by NGF and NT-3. Eur J Neurosci. 2002;16:1449–1462. doi: 10.1046/j.1460-9568.2002.02205.x. [DOI] [PubMed] [Google Scholar]
- Kimura A, Ajiki T, Takeuchi K, Hakamata Y, Murakami T, Hoshino Y, Kobayashi E. Transmigration of donor cells involved in the sciatic nerve graft. Transplant Proc. 2005;37:205–207. doi: 10.1016/j.transproceed.2004.12.052. [DOI] [PubMed] [Google Scholar]
- Kirstein M, Farinas I. Sensing life: regulation of sensory neuron survival by neurotrophins. Cell Mol Life Sci. 2002;59:1787–1802. doi: 10.1007/PL00012506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krekoski CA, Neubauer D, Zuo J, Muir D. Axonal regeneration into acellular nerve grafts is enhanced by degradation of chondroitin sulfate proteoglycan. J Neurosci. 2001;21:6206–6213. doi: 10.1523/JNEUROSCI.21-16-06206.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl DJ, Mbiene JP, Parada LF. NT4/5 mutant mice have deficiency in gustatory papillae and taste bud formation. Dev Biol. 1999;213:378–389. doi: 10.1006/dbio.1999.9385. [DOI] [PubMed] [Google Scholar]
- Liu X, Enfors P, Wu H, Jaenisch R. Sensory but not motor neuron deficits in mice lacking NT4 and BDNF. Nature. 1995;375:238–241. doi: 10.1038/375238a0. [DOI] [PubMed] [Google Scholar]
- Loeb GE, Peck RA. Cuff electrodes for chronic stimulation and recording of peripheral nerve activity. J. Neurosci. Meth. 1996;64:95–103. doi: 10.1016/0165-0270(95)00123-9. [DOI] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysate-paraformaldehyde fixative. A new fixative for immunoelectron microscopy. J. Histochem. Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Mendell LM. Neurotrophins and sensory neurons: role in development, maintenance and injury. A thematic summary. Philosophical Transactions of the Royal Society of London - Series B: Biological Sciences. 1996;351:463–467. doi: 10.1098/rstb.1996.0043. [DOI] [PubMed] [Google Scholar]
- Michael GJ, Averill S, Shortland PJ, Yan Q, Priestley JV. Axotomy results in major changes in BDNF expression by dorsal root ganglion cells: BDNF expression in large trkB and trkC cells, in pericellular baskets, and in projections to deep dorsal horn and dorsal column nuclei. Eur J Neurosci. 1999;11:3539–3551. doi: 10.1046/j.1460-9568.1999.00767.x. [DOI] [PubMed] [Google Scholar]
- Snell GD. Histocompatibility genes in the mouse. II. Production and analysis of isogenic resistant lines. J. Natl. Cancer Inst. 1958;21:843–877. [PubMed] [Google Scholar]
- Suri A, Mehta VS, Sarkar C. Microneural anastomosis with fibrin glue: an experimental study. Neurol. India. 2002;50:23–26. [PubMed] [Google Scholar]
- Witzel C, Rohde C, Brushart TM. Pathway sampling by regenerating peripheral axons. J. Comp. Neurol. 2005;485:183–190. doi: 10.1002/cne.20436. [DOI] [PubMed] [Google Scholar]
- Zhang J-Y, Luo X-G, Xian CJ, Liu Z-H, Zhou X-F. Endogenous BDNF is required for myelination and regeneration of inured sciatic nerve in rodents. Eur J Neurosci. 2000;12:4171–4180. [PubMed] [Google Scholar]
- Zhou XF, Chie ET, Deng YS, Zhong JH, Xue Q, Rush RA, Xian CJ. Injured primary sensory neurons switch phenotype for brain-derived neurotrophic factor in the rat. Neuroscience. 1999;92:841–853. doi: 10.1016/s0306-4522(99)00027-5. [DOI] [PubMed] [Google Scholar]