Abstract
Moderate exercise in the form of treadmill training and brief electrical nerve stimulation both enhance axon regeneration after peripheral nerve injury. Different regimens of exercise are required to enhance axon regeneration in male and female mice (Wood et al., 2012), and androgens are suspected to be involved. We treated mice with the androgen receptor blocker, flutamide, during either exercise or electrical stimulation, to evaluate the role of androgen receptor signaling in these activity-based methods of enhancing axon regeneration. The common fibular (CF) and tibial (TIB) nerves of thy-1-YFP-H mice, in which axons in peripheral nerves are marked by yellow fluorescent protein (YFP), were transected and repaired using CF and TIB nerve grafts harvested from non-fluorescent donor mice. Silastic capsules filled with flutamide were implanted subcutaneously to release the drug continuously. Exercised mice were treadmill trained five days/week for two weeks, starting on the third day post transection. For electrical stimulation, the sciatic nerve was stimulated continuously for one hour prior to nerve transection. After two weeks, lengths of YFP+ profiles of regenerating axons were measured from harvested nerves. Both exercise and electrical stimulation enhanced axon regeneration, but this enhancement was blocked completely by flutamide treatments. Signaling through androgen receptors is necessary for the enhancing effects of treadmill exercise or electrical stimulation on axon regeneration in cut peripheral nerves.
Keywords: Androgen, Axon, Regeneration, Exercise, Stimulation
Introduction
Peripheral nerves have the capacity for regeneration, but functional recovery after peripheral nerve injury is rare (Frostick et al., 1998; Scholz et al., 2009). A primary cause of the poor outcomes following injury is slow axon regeneration (Gordon et al., 2009). To facilitate axon growth, several methods of enhancing axon regeneration after injury have been identified and investigated. Among these enhancing procedures are daily exercise in the form of treadmill training (Asensio-Pinilla et al., 2009; Ilha et al., 2008; Sabatier et al., 2008a; Seo et al., 2006; Udina et al., 2011) and brief electrical stimulation of the injured nerve at the time of transection (Al-Majed et al., 2000b).
A sex difference in the enhancement of mouse peripheral nerve regeneration due to treadmill training has been identified (Wood et al., 2012). Continuous training, an hour of daily slow walking, enhances axon regeneration in male mice, but not in female mice. Conversely, interval training, a series of interrupted short sprints, is effective in female mice, but not male mice. Androgens are implicated in this sex difference, as castration eliminates the enhancement effect of continuous training in male mice (Wood et al., 2012). When female mice are treated with a pharmacological dose of an inhibitor of cytochrome C P450 aromatase, an enzyme that catalyzes the conversion of testosterone or its precursors into estradiol, axon regeneration is enhanced significantly (Wood et al., 2012). Based on these results, we suggest that the availability of androgens is involved in the effects of training on nerve regeneration.
Brain-Derived Neurotrophic Factor (BDNF) is also important for axon regeneration after peripheral nerve injury. Both Schwann cell- and neuron-derived BDNF are facilitators of axon growth post-axotomy (Funakoshi et al., 1993), but BDNF of neuronal origin is required for the enhancement effect of exercise. Knocking out the gene for BDNF specifically in neurons completely blocks the enhancement of axon regeneration in peripheral nerves that is produced by exercise (Wilhelm et al., 2012). Expression of neuronal BDNF can be regulated by androgens. Prolonged treatments of castrated male rats with testosterone propionate resulted in a prolonged increase in BDNF mRNA levels in facial motoneurons (Sharma et al., 2010a). Based on these results, one might expect that androgens could play a role in the enhancement of axon regeneration due to exercise for both male and female mice. One goal of this study was to evaluate the androgen-dependence of the effect of exercise in enhancing axon regeneration in cut peripheral nerves in male and female mice.
Brief electrical stimulation, for as little as one hour at the time of surgical repair of a cut nerve, results in a marked enhancement of axon regeneration (Al-Majed et al., 2000b). It also causes a sharp but short-lived increase in motoneuron BDNF mRNA expression, both in gonadally intact female rats (Al-Majed et al., 2000b) and in castrated male rats (Sharma et al., 2010a). Despite these provocative findings, very little evidence exists to suggest that the effects of brief electrical stimulation might depend on androgens. A second goal of this study was to investigate whether the effectiveness of brief electrical stimulation in the enhancement of axon regeneration following peripheral nerve injury is dependent on androgen receptor signaling. A preliminary report of some of this work has been presented in abstract form (English et al., 2012).
Methods
Animals and surgical procedures
All experimental methods were approved by the Institutional Animal Care and Use Committee of Emory University. Axon regeneration was studied in the H strain of thy-1-YFP mice (Feng et al., 2000). In this strain, yellow fluorescent protein (YFP) completely fills a subset of axons in the peripheral nervous system, making regenerating axons visible with confocal microscopy.
All mice were anesthetized using 1% isoflurane. The common fibular and/or tibial nerves, two branches of the sciatic nerve, were transected and repaired bilaterally in each mouse using a 10–15 mm long segment of the same nerve harvested from a different transgenic donor mouse (B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J, Jackson Laboratories, stock 007676; Fig. 1A). In these animals, a red fluorescent protein, tomato, is expressed in all cells, facilitating the identification of grafts in our experiments. Both host and donor mice are of the same background strain (C57B6). We have described previously that no significant difference was found in the lengths of axons growing into grafts from wild type littermates or from these tomato mice and whether or not the common fibular or tibial nerves were studied (Wilhelm et al., 2012). Nerves and grafts were secured with fibrin glue (MacGillivray, 2003). The fibrin glue was created immediately preceding use from equal parts of thrombin and a 1:1 solution of fibrinogen and fibronectin, and applied to the nerves with a micropipette.
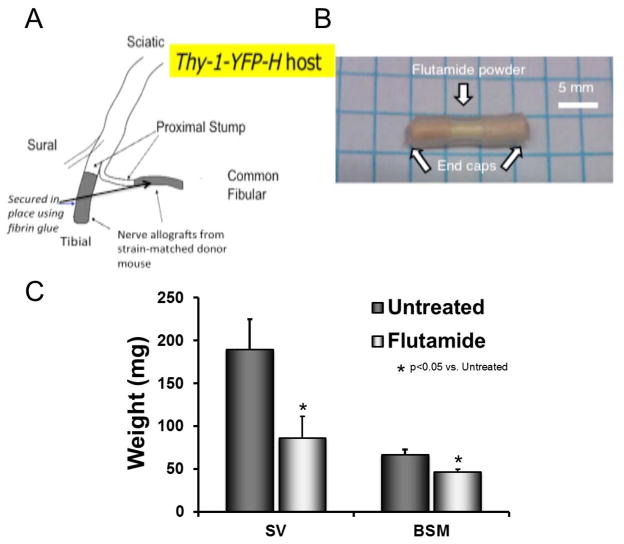
Figure 1.
Surgical Methods and Organ Weights. A: The common fibular and/or tibial nerves were transected in thy-1-YFP-H host mice. Nerve grafts from a strain-matched donor mouse, marked in grey, were attached to the proximal stumps of the transected nerves of the host mouse using fibrin glue. B: Silastic capsule filled with 5mm of flutamide. C: Mean (± 95% confidence intervals) weights of seminal vesicles (SV), bulbospongiosis muscle (BSM), and uterus (UT) are shown for animals treated for two weeks by capsules either containing flutamide (dark bars) or containing nothing (grey bars).
The YFP+ axons in the proximal segment of the host mouse were allowed to regenerate into the donor graft for two weeks, after which the mouse was euthanized with an overdose of pentobarbital and perfused with saline followed by periodate-lysate-paraformaldehyde fixative solution (McLean et al., 1974). Nerves and grafts were harvested, mounted onto slides, cover slipped with Vectashield®, and the edges sealed with nail polish. The nerves and grafts were then imaged at low magnification (10X) using confocal microscopy. Stacks of optical sections were obtained through contiguous and overlapping microscope fields, extending over the full extent of each nerve and graft, and these were stitched together using Adobe Photoshop. The result was a complete reconstruction of the repaired nerve and graft. Lengths of YFP+ axon profiles were measured from the resulting reconstructions using Image J software. For each nerve studied, a cumulative histogram of the distribution of axon profile lengths measured was constructed, with a bin size of 100 μm, ranging from 0–6300 μm (64 bins). Averages of these histograms were computed for each experimental group (see below).
Treatments
Treadmill exercise and brief electrical stimulation were employed in different groups of mice to enhance axon regeneration after injury. For treadmill exercise, males and females were trained using different protocols, as described elsewhere (Wood et al., 2012). Continuous training, i.e., slow walking at 10 m/min for one hour per day, was used for male mice. Interval training, i.e., four repetitions of short sprints at 20 m/min for 2 minutes followed by 5 min of rest, was used for female mice. All mice were exercised on a level treadmill five days/week for 2 weeks, beginning on the 3rd day post transection. For electrical stimulation, a bipolar cuff electrode (Stein et al., 1977) was placed around the sciatic nerve in the mid-thigh. Short (0.1 ms) pulses were delivered to the nerve via this cuff at a rate of 20 Hz, continuously for one hour immediately prior to nerve transection. Stimulus intensity was set at twice the minimum voltage needed to evoke a visible twitch in the gastrocnemius muscles.
To assess the importance of androgen receptor signaling in the enhancement of axon regeneration after injury, flutamide, an androgen receptor antagonist, was employed. The drug was applied systemically in a sustained release dosage form via Silastic capsules filled with flutamide powder. Capsules were prepared according to the methods of Smith et al. (1977). Capsules composed of 15mm long Silastic tubing (1.57 mm i.d.; 3.18 mm o.d.; Dow Corning Corp., Midland, MI) were packed with 5 mm of flutamide powder (2-methyl-N-[4-nitro-3-(trifluoromethyl) phenyl]-propanamide; Sigma Aldrich, Seelze, Germany) and flanked on each side by 5 mm wooden stint segments. The ends of the capsule were then covered externally by Medical Adhesive A (Fig. 1B). Capsules were soaked in normal saline solution at 37°C for 24 hours prior to implantation to prime the flutamide powder for release. The goal of this approach was to control the release of flutamide as it diffused through the thin-walled tubing; the rate of release is proportional to the effective capsule surface area (Smith et al., 1977). The primary purpose of the wooden stints at each end of the capsules was to provide a barrier to the diffusion of flutamide out the ends of the tubing. Two such flutamide capsules (a total of approximately 16.8 mg of flutamide) were implanted subcutaneously in each mouse three days prior to the onset of treadmill training or electrical stimulation to ensure disruption of androgen receptor signaling at the time of the enhancement procedure. Control mice received similar capsules that were not filled with flutamide (blank capsules). Flutamide treatment efficacy was measured using seminal vesicle and bulbospongiosis muscle weights in males.
Four experimental mouse groups were studied: flutamide treated and continuously trained males, flutamide treated and interval trained females, flutamide treated and electrically stimulated males, and flutamide treated and electrically stimulated females. Four additional control groups were used to assess the effect of flutamide treatment and capsule implantation: males flutamide treated with no enhancement procedure, females flutamide treated with no enhancement procedure, males continuously trained and implanted with blank (empty) capsules, and females interval trained and implanted with blank capsules. Additional data from previously published experimental results were used for comparison. Numbers of nerves studied in each group are shown in Table I.
Table I.
Treatment Groups Studied
| Treatment | Sex | Type of Enhancement | No. Nerves |
|---|---|---|---|
| This study | |||
| Blank Capsules | Female | Exercise – Interval Training | 3 |
| Flutamide | Female | None | 5 |
| Flutamide | Female | Exercise – Interval Training | 4 |
| Flutamide | Female | Electrical Stimulation | 4 |
| Blank Capsules | Male | Exercise – Continuous Training | 2 |
| Flutamide | Male | None | 6 |
| Flutamide | Male | Exercise – Continuous Training | 4 |
| Flutamide | Male | Electrical Stimulation | 3 |
|
From Previous studies
| |||
| None1 | Female | None | 4* |
| None3 | Female | None | 4 |
| None3 | Female | Exercise – Interval Training | 9 |
| None2 | Female | Electrical Stimulation | 4* |
| None1 | Male | None | 4* |
| None3 | Male | None | 4 |
| None3 | Male | Exercise – Continuous Training | 6 |
| Castration 3 | Male | Exercise – Continuous Training | 5 |
| None2 | Male | Electrical Stimulation | 4* |
from English et al, (2007) * used short (2 mm) nerve grafts. All others used longer grafts.
from Wood et al (2012)
Statistical methods
Significance of differences in the distributions of axon profile lengths measured in grafts used to repair cut nerves was evaluated using the Kolmogorov-Smirnov two-sample test (K-S test). This non-parametric test assumes nothing about the nature of these distributions. The null hypothesis of the K-S test is that the two frequency distributions studied are samples drawn from the same population distribution. A critical probability value of 0.05 was set for all K-S test results.
Median axon profile lengths were determined in each nerve and the significance of differences in average medians between groups was compared using a one-way analysis of variance (ANOVA) with post-hoc (Fisher’s least significant difference, LSD) paired testing, where appropriate. P values of <0.05 were considered significant.
The average weights of seminal vesicles and bulbospongiosis muscles in flutamide treated and untreated males was compared using t-tests for independent samples. P values of <0.05 were considered significant.
Results
Flutamide treatments disrupt androgen receptor signaling
The effectiveness of sustained release flutamide treatments was first evaluated by comparing the weights of seminal vesicles and bulbospongiosis muscles in treated and untreated males (Fig. 1C). Seminal vesicle weights from flutamide treated males (Mean=85.00 mg, SD=23.07) were significantly lower than those from untreated males (M=141.00 mg, SD=16.29; t(8)=−3.71, p<0.01). Similarly, bulbospongiosis muscle weights from flutamide treated males (M=52.40 mg, SD=7.27) were also significantly lower than those from untreated males (M=67.70 m., SD=7.24; t(6)= −2.89, p<0.05). Both of these structures are known to be dependent on androgen receptor signaling. Their atrophy in flutamide-treated mice is evidence that the drug treatments result in a systemic effect on androgen receptors. We assume that the flutamide had a similar effect on all tissues.
Flutamide treatments block the enhancing effects of exercise
Inhibition of androgen receptor signaling via flutamide treatment eliminated the enhancement of axon regeneration produced by continuous treadmill training in male mice. The distribution of axon profile lengths measured in flutamide treated male mice that were exposed to two weeks of daily continuous treadmill exercise is shown in Figure 2A. In flutamide treated, continuously trained males (Fig. 2A: grey symbols), this distribution is similar to that found in untreated, untrained males (Fig. 2A: solid line, data from Wood et al, 2012) (K-S test, D64,64=0.06, n.s.). It is also similar to the distribution of axon profile lengths obtained from castrated, continuously trained males, data that we have reported previously (Wood et al., 2012). All of these distributions are shifted significantly to the left of those from continuously trained male mice receiving blank capsules (Fig. 2A: black symbols) (K-S test, D64,64=0.41, p<0.01) or untreated continuously trained males that we reported previously (Wood et al., 2012).
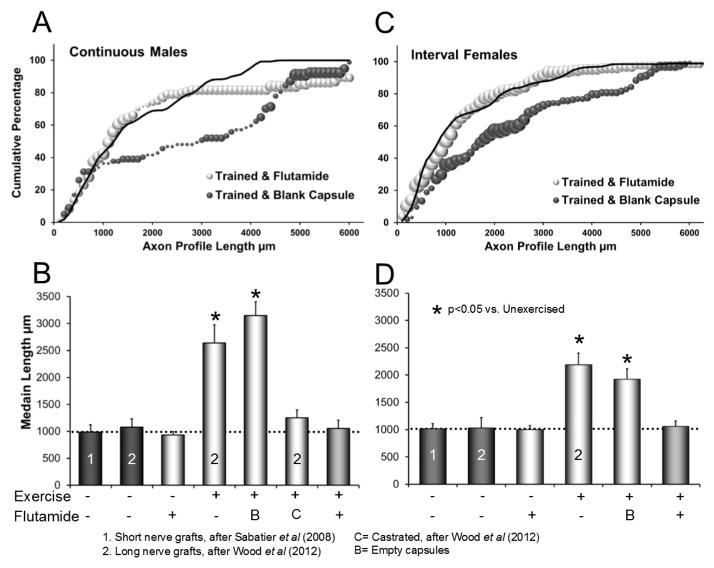
Figure 2.
Result of flutamide treatment on the enhancement effect of exercise. A: Cumulative histogram of measurements of regenerating axon profile lengths in grafts harvested from male mice. Three groups of male mice are represented: untreated and untrained (solid black line, data after Wood et al., 2012), continuously trained mice in which empty (blank) capsules had been implanted (black symbols), and flutamide treated and continuously trained male mice (grey symbols). Each data point represents an average of data from several mice (see Table I). The size of each symbol is proportional to the standard error of the mean. B: Average (± 95% confidence intervals) median axon profile lengths of male groups are shown. Treatment conditions for continuous treadmill exercise and flutamide treatments are shown below each bar. B= blank capsules, C=castrated. C: Cumulative histogram of measurements of regenerating axon profile lengths in grafts harvested from female mice. Three groups of female mice are represented: untreated and untrained (solid black line, data after Wood et al., 2012), interval trained mice in which blank capsules had been implanted (black symbols), and flutamide treated and interval trained mice (grey symbols). The formatting of this graph is similar to that in panel A. D. Average (± 95% confidence intervals) median axon profile lengths of female groups are shown. The formatting is similar to that in panel B.
The average median axon profile lengths of different groups of male mice are shown in Figure 2B. Data from three of the groups of male mice studied are original to this study: flutamide treated-unexercised; flutamide treated-exercised, and empty capsule treated-exercised. Data from unexercised and untreated mice whose cut nerves were repaired either with short (2 mm long) grafts (Sabatier et al., 2008a) or longer grafts such as used in the present study (Wood et al., 2012) also were compared. In addition, data from exercised and untreated mice, and exercised and castrated mice, which have been published earlier (Wood et al., 2012), were included in this set (Fig. 2B, see also Table I). The omnibus test of a one-way ANOVA conducted using these data was significant (F6,24=21.67, p<.01). Based on paired, post-hoc (Fisher’s least significant difference, LSD) testing, lengths of profiles of YFP+ regenerating axons in flutamide treated and continuously trained male mice were not significantly different from those found in untrained and untreated males, irrespective of the length of the grafts used to repair the nerves (LSD, n.s.). Profiles of YFP+ regenerating axons in flutamide treated and continuously trained male mice were significantly shorter than axon profile lengths measured in continuously trained male mice given either blank capsules (LSD, p<0. 01) or no capsules (Wood et al., 2012) (LSD, p<0.01) (Fig. 2B). Regenerating axon profile lengths in flutamide treated and untrained males were not significantly different from either group of untrained and untreated males (LSD, n.s.). Thus, signaling through the androgen receptor is necessary for the enhancement of axon regeneration due to continuous training in male mice, but is likely not a major factor in promoting axon regeneration after injury in untrained males.
In Figure 2C, the distributions of axon profile lengths measured in flutamide treated and untreated female mice that were each exposed to two weeks of daily interval treadmill exercise are presented. For comparison, analogous data are included from untreated, unexercised female controls (Wood et al., 2012). The distribution of flutamide treated, interval trained females (Fig. 2C: grey symbols) was similar to that of untreated, untrained controls (Fig. 2C: solid line) (K-S test, D64,64=0.15, n.s.), but to the left of the distribution from interval trained females implanted with blank capsules (Fig. 2C: black symbols) (K-S test, D64,64=0.37, p<0.01).
Average median axon profile lengths for the different groups of female mice are shown in Figure 2D. Data analyzed included previously reported measurements from unexercised and untreated females whose nerves were repaired with short grafts (Sabatier et al., 2008a) or grafts of the length used in the present study from unexercised and untreated mice or from interval trained mice not treated with flutamide (Wood et al., 2012). Flutamide treatment eliminated the enhancement effect of interval training in female mice (ANOVA, F5,21,=12.01, p<0.01). Lengths of profiles of YFP+ regenerating axons from female mice that were interval trained and given blank capsules (LSD, p<0.01) or interval trained without treatment (LSD, p<0.01) (Wood et al., 2012) were significantly longer than found in untrained females, regardless of graft length, but this enhancement effect was eliminated in interval trained female mice treated with flutamide (LSD, n.s.). Regenerating axon profile lengths in flutamide treated and untrained females were not significantly different from those found in both groups of untrained and untreated females (LSD, n.s.).
Flutamide treatment blocks the effect of brief electrical stimulation
As little as one hour of electrical stimulation (ES) applied to peripheral nerves at the time of their surgical repair has been shown to produce a marked enhancement of axon regeneration (Al-Majed et al., 2000b). We reported a similar enhancement of axon regeneration with brief electrical stimulation in mice in which cut nerves in thy-1-YFP mice were repaired with 2 mm long grafts from non-fluorescent littermates (English et al., 2007). When we re-analyzed these data, we found no significant sex differences, either in unstimulated controls or mice in which nerves had been stimulated. Data from males and females in these samples were pooled and form the control groups (no stimulation-no flutamide and stimulation-no flutamide) shown in Figure 3A.
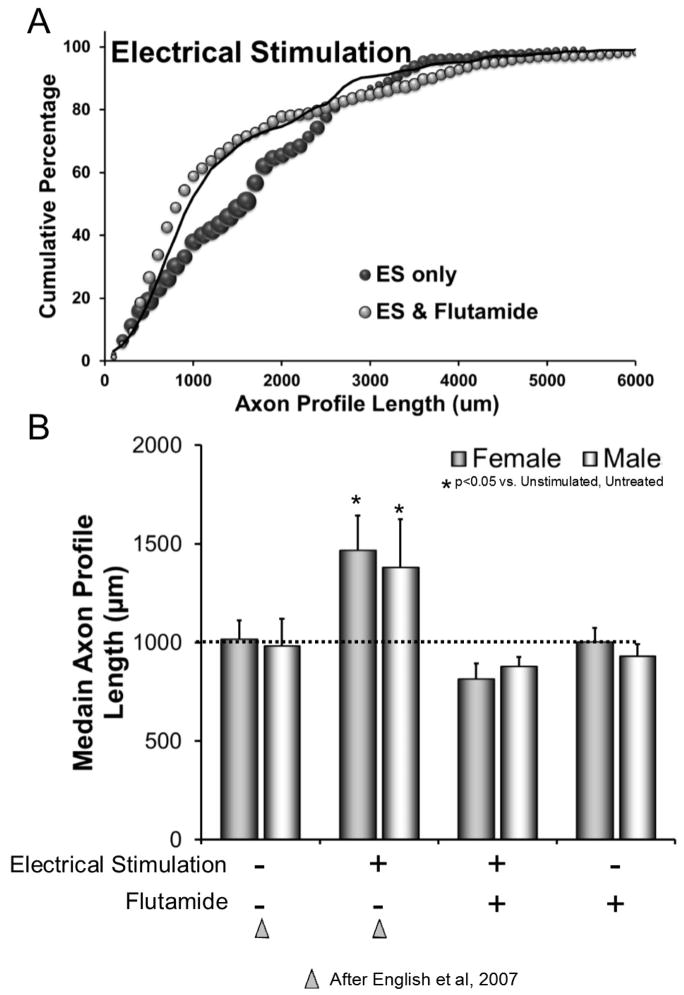
Figure 3.
Result of flutamide treatment on the enhancement effect of electrical stimulation. A: Cumulative histogram of measurements of regenerating axon profile lengths in grafts harvested from mice whose nerves had been electrically stimulated. Three groups of mice are represented: untreated and non-stimulated (solid black line, after (English et al., 2007), untreated and electrically stimulated (ES) (dark symbols), and flutamide treated and electrically stimulated (light symbols). Each data point represents an average of data from several mice (see Table I). The size of each symbol is proportional to the standard error of the mean. B: Average median axon profile lengths (± SEM) of all groups in both sexes are compared. Treatment conditions for ES and flutamide treatments are shown below each bar. Data for bars published previously (English et al., 2007) are indicated by triangles.
The distribution of axon profile lengths measured in flutamide treated mice whose nerves were exposed to one hour of electrical stimulation is shown in Figure 3A. These new data are from experiments using the longer grafts employed in the present study. For comparison, previously published distributions were included (English et al., 2007), as described above. The distribution found in flutamide treated mice whose nerves were electrically stimulated (Fig. 3A: grey symbols) was similar to that of untreated, untrained controls (Fig. 3A: solid line) (K-S test, D64,64=0.07, n.s.), but shifted to the left of the distribution from untreated mice whose nerves had been electrically stimulated (Fig. 3A: black symbols) (K-S test, D64,64=0.23, p<0.05).
Differences in average median axon profile lengths in nerves from these groups of mice, as well from flutamide treated mice whose nerves had not been stimulated (controls), were significant (F7,26=5.99, p<0.01) (Fig. 3B). In flutamide treated mice whose nerves were electrically stimulated, average median lengths of regenerating axon profiles were not significantly different from those of untreated male (LSD, n.s.) or female (LSD, n.s.) mice whose nerves were not stimulated, nor those of flutamide treated male (LSD, n.s.) or female (LSD, n.s.) mice whose nerves were not stimulated. However, the median axon profile lengths measured in electrically stimulated nerves of flutamide treated mice were significantly shorter than the median lengths measured in electrically stimulated nerves of untreated mice for both males (LSD, p< 0.01) and females (LSD, p<0.01). Thus, the enhancement of axon regeneration after peripheral nerve injury produced by brief electrical stimulation is dependent on androgen receptor signaling in both males and females.
Discussion
Even though axons in cut peripheral nerves are capable of considerable regeneration, functional recovery following peripheral nerve injury is poor (Frostick et al., 1998; Scholz et al., 2009). One reason for this poor clinical outcome is that axons in injured nerves regenerate slowly and very asynchronously (Gordon et al., 2009). Both exercise, in the form of treadmill training (Sabatier et al., 2008a), and brief electrical stimulation, either alone (Al-Majed et al., 2000b) or in conjunction with treadmill exercise (Asensio-Pinilla et al., 2009), have been shown to increase regenerating axon elongation significantly in the weeks following axotomy. Androgens are suspected to be involved in the enhancement of axon regeneration due to treadmill training because of a sex difference we previously identified (Wood et al., 2012). The sex dependence of electrical stimulation had not been investigated. In this study, we explored the involvement of androgen receptor signaling in the enhancement of axon regeneration produced by these activity-based treatments using flutamide, an androgen receptor antagonist. The main finding of this study is that, in both sexes, the effects of exercise and electrical stimulation were eliminated completely upon flutamide treatment, indicating a requirement for androgen receptor signaling in their cellular mechanisms.
The androgen receptor dependence of the enhancement of axon regeneration due to exercise is in keeping with previous findings from this laboratory. Castration of male mice eliminated the enhancement effect of continuous training (Wood et al., 2012). The flutamide treatments resulted in a similar effect in male mice. For female mice, the involvement of androgens may be different. Interval training has been shown to increase skeletal muscle androgen receptor expression (Aizawa et al., 2010) and decrease muscle cytochrome C P450 aromatase mRNA levels in female rats (Aizawa et al., 2008). Because aromatase converts testosterone or its precursors into estradiol, any exercise-induced decrease in aromatase activity would be expected to result in greater availability of testosterone. Indeed, pharmacologic inhibition of aromatase in unexercised female mice led to increased axon regeneration (Wood et al., 2012), causing us to speculate that local inhibition of aromatase might underlie the effect of interval training in females. The finding presented above, that flutamide treatment eliminates the effect of interval training on the enhancement of axon regeneration in female mice is consistent with this hypothesized increase in local androgen availability.
We have shown elsewhere (Wilhelm et al., 2012) that increased signaling via neuron-derived BDNF is required for the enhancement of axon regeneration in peripheral nerves produced by exercise. A similar increase in neuronal BDNF expression has been suggested to underlie the effects of electrical stimulation in enhancing axon regeneration (Al-Majed et al., 2000a; Sharma et al., 2010a). We propose three different testable models to hypothesize how both neuronal BDNF and androgen receptor signaling are necessary for an enhancement of axon regeneration.
The simplest hypothetical model is an in-series mechanism. Increased neuronal activity, induced by exercise or electrical stimulation, could increase the availability of androgens to neurons whose axons are regenerating. Such increased availability could be the result of stimulation of androgen synthesis, by aromatase inhibition, or both. The expected increase in neuronal androgen receptor signaling would then stimulate BDNF production and result in enhanced axon regeneration.
Production of BDNF is known to be downstream from motoneuron androgen receptor signaling. In castrated rats treated with testosterone, an increased expression of BDNF by facial motoneurons was found (Sharma et al., 2010a). The increase was not noted until at least two days after the start of treatment, but it was maintained for at least two weeks. Electrical stimulation has been shown to increase mRNA expression for both BDNF and its receptor, trkB, in motoneurons in both gonadally intact female rats (Al-Majed et al., 2000a) and castrated male rats (Sharma et al., 2010a), but whether this increased neuronal activity leads to a local increase in androgen availability is not yet known. Our hypothetical model could be tested by evaluating markers of androgen biosynthesis and aromatase activity in different cell types in response to increases in neuronal activity that are effective in promoting axon regeneration.
Alternatively, androgen receptor signaling could converge with increased neuronal activity to produce an increase in neuronal BDNF expression that is greater than that found with increased activity or androgen receptor signaling alone. Increased synthesis of BDNF in response to neuronal activity is well known (Hong et al., 2008). As noted above, androgen receptor signaling leads to BDNF expression. In this hypothetical model, the increased BDNF expression produced separately by either androgen receptor signaling or neuronal activity would not be adequate to enhance axon regeneration. Parallel activations of the BDNF gene could combine to overcome a BDNF threshold, which then leads to enhanced axon regeneration. Unlike the effects of androgens, the increase in neuronal BDNF expression in response to electrical stimulation has a rapid onset, but a relatively short duration, but when used together, electrical stimulation and androgens produce an increase in neuronal BDNF expression that is both rapid and prolonged (Sharma et al., 2010a). This hypothetical model could be tested by comparing BDNF expression levels in neurons whose axons are regenerating, in response to increased activity, in the cell-type specific presence or absence of androgen receptors or where activity during exercise is blocked.
Both of these hypothetical models assume that the site of the androgen receptor signaling that is required for enhancing axon regeneration using activity-based treatments is the neurons whose axons are regenerating. However, androgens also might act on other cell types, as androgen receptor immunoreactivity has been described on astrocytes and microglia, especially after injury (DonCarlos et al., 2006; García-Ovejero et al., 2002). Thus a third model is proposed in which non-neuronal androgen receptor signaling could initiate an intercellular signaling mechanism that results in increased expression of BDNF in neurons whose axons are regenerating. We believe that it is important to recognize that such a more complex mechanism might be responsible for the results presented above.
The different time courses of the effects of increased activity and androgens on neuronal BDNF expression are notable. Jones and colleagues have argued that the effect of increased activity, such as electrical stimulation, may have effects on different aspects of the regenerative process than the effects of androgens (Sharma et al., 2010a). For example, increased activity, because of the rapid but transient way that it increases neuronal BDNF expression, might be more influential on the early outgrowth of regenerative sprouts and navigation past the injury site than the longer-latency but longer duration effects of administered androgens (Sharma et al., 2010b). The effect of androgen treatments might be greater on the rate of elongation of these sprouts once they have entered a regeneration pathway (Kujawa et al., 1991; Kujawa et al., 1993). Although we find these ideas compelling, we have not addressed them specifically in the present study. We would only comment that our effects of exercise, where neuronal activity is increased daily for two weeks, results in an immediate enhancement of axon regeneration that is quantitatively greater than the short–term effects of either a single application of electrical stimulation or androgen treatments. For both electrical stimulation and exercise, the effects on axon regeneration and functional recovery are notable well after the end of the treatments (Boeltz et al., 2013; English et al., 2011a; English et al., 2011b; Sabatier et al., 2008b). Whether this subtle difference in acute effects and similarity in long-lasting effects is the result of the combinatorial effect of the increased neuronal activity and androgens on different aspects of the regenerative process will have to await the results of future studies.
Based on the results presented above and those we have presented previously (Wilhelm et al., 2012; Wood et al., 2012), we conclude that the effectiveness of activity-based therapies for promoting axon regeneration in injured peripheral nerves requires both an increase in BDNF expression in the neurons whose axons are regenerating and signaling through androgen receptors. The interaction of BDNF and its trkB receptor and androgens/androgen receptors in the mechanism underlying the effectivenss of these treatments is likely complex, as they show at least some capacity for regulating each other (Verhovshek et al., 2013). However, we feel that by identifying these requirements for activity-based therapies and posing testable hypotheses, we have moved them closer to their translational potential.
Acknowledgments
This material is based upon work supported by grant NS057190 from the USPHS.
References
- Aizawa K, Iemitsu M, Otsuki T, Maeda S, Miyauchi T, Mesaki N. Sex differences in steroidogenesis in skeletal muscle following a single bout of exercise in rats. J Appl Physiol. 2008;104:67–74. doi: 10.1152/japplphysiol.00558.2007. [DOI] [PubMed] [Google Scholar]
- Aizawa K, Iemitsu M, Maeda S, Otsuki T, Sato K, Ushida T, Mesaki N, Akimoto T. Acute exercise activates local bioactive androgen metabolism in skeletal muscle. Steroids. 2010;75:219–223. doi: 10.1016/j.steroids.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci. 2000a;12:4381–4390. [PubMed] [Google Scholar]
- Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000b;20:2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio-Pinilla E, Udina E, Jaramillo J, Navarro X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp Neurol. 2009;219:258–265. doi: 10.1016/j.expneurol.2009.05.034. [DOI] [PubMed] [Google Scholar]
- Boeltz T, Ireland M, Mathis K, Nicolini J, Poplavski K, Rose SJ, Wilson E, English AW. Effects of treadmill training on functional recovery following peripheral nerve injury in rats. J Neurophysiol. 2013;109:2645–2657. doi: 10.1152/jn.00946.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DonCarlos L, Sarkey S, Lorenz B, Azcoitia I, Garcia-Ovejero D, Huppenbauer C, Garcia-Segura L. Novel cellular phenotypes and subcellular sites for androgen action in the forebrain. Neuroscience. 2006;138:801–807. doi: 10.1016/j.neuroscience.2005.06.020. [DOI] [PubMed] [Google Scholar]
- English AW, Mulligan A, Meador W, Sabatier MJ, Schwartz G. Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling. Dev Neurobiol. 2007;67:158–172. doi: 10.1002/dneu.20339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Cucoranu D, Mulligan A, Rodriguez JA, Sabatier MJ. Neurotrophin-4/5 is implicated in the enhancement of axon regeneration produced by treadmill training following peripheral nerve injury. Eur J Neurosci. 2011a;33:2265–2271. doi: 10.1111/j.1460-9568.2011.07724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Wilhelm JC, Sabatier MJ. Enhancing recovery from peripheral nerve injury using treadmill training. Ann Anat. 2011b;193:354–361. doi: 10.1016/j.aanat.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Thompson N, Sengelaub D. Enhancement of peripheral axon regeneration by exercise requires androgen receptor signaling in both male and female mice. Abstr Soc Neurosci. 2012;352:312. [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18:397–405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Frisen J, Barbany G, Timmusk T, Zachrisson O, Verge VM, Persson H. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol. 1993;123:455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Ovejero D, Veiga S, García-Segura L, Doncarlos L. Glial expression of estrogen and androgen receptors after rat brain injury. J Comp Neurol. 2002;450:256–271. doi: 10.1002/cne.10325. [DOI] [PubMed] [Google Scholar]
- Gordon T, Chan KM, Sulaiman OA, Udina E, Amirjani N, Brushart TM. Accelerating axon growth to overcome limitations in functional recovery after peripheral nerve injury. Neurosurgery. 2009;65:A132–144. doi: 10.1227/01.NEU.0000335650.09473.D3. [DOI] [PubMed] [Google Scholar]
- Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008;60:610–624. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilha J, Araujo RT, Malysz T, Hermel EE, Rigon P, Xavier LL, Achaval M. Endurance and resistance exercise training programs elicit specific effects on sciatic nerve regeneration after experimental traumatic lesion in rats. Neurorehabil Neural Repair. 2008;22:355–366. doi: 10.1177/1545968307313502. [DOI] [PubMed] [Google Scholar]
- Kujawa KA, Emeric E, Jones KJ. Testosterone differentially regulates the regenerative properties of injured hamster facial motoneurons. J Neurosci. 1991;11:3898–3906. doi: 10.1523/JNEUROSCI.11-12-03898.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa KA, Jacob JM, Jones KJ. Testosterone regulation of the regenerative properties of injured rat sciatic motor neurons. J Neurosci Res. 1993;35:268–273. doi: 10.1002/jnr.490350306. [DOI] [PubMed] [Google Scholar]
- MacGillivray TE. Fibrin sealants and glues. J Card Surg. 2003;18:480–485. doi: 10.1046/j.0886-0440.2003.02073.x. [DOI] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Sabatier M, Redmon N, Schwartz G, English A. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp Neurol. 2008a;211:489–493. doi: 10.1016/j.expneurol.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier MJ, Redmon N, Schwartz G, English AW. Treadmill training promotes axon regeneration in injured peripheral nerves. Experimental Neurology. 2008b;211:489–493. doi: 10.1016/j.expneurol.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz T, Krichevsky A, Sumarto A, Jaffurs D, Wirth GA, Paydar K, Evans GR. Peripheral nerve injuries: an international survey of current treatments and future perspectives. J Reconstr Microsurg. 2009;25:339–344. doi: 10.1055/s-0029-1215529. [DOI] [PubMed] [Google Scholar]
- Seo TB, Han IS, Yoon JH, Hong KE, Yoon SJ, Namgung U. Involvement of Cdc2 in axonal regeneration enhanced by exercise training in rats. Med Sci Sports Exerc. 2006;38:1267–1276. doi: 10.1249/01.mss.0000227311.00976.68. [DOI] [PubMed] [Google Scholar]
- Sharma N, Marzo SJ, Jones KJ, Foecking EM. Electrical stimulation and testosterone differentially enhance expression of regeneration-associated genes. Exp Neurol. 2010a;223:183–191. doi: 10.1016/j.expneurol.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Sharma N, Moeller CW, Marzo SJ, Jones KJ, Foecking EM. Combinatorial treatments enhance recovery following facial nerve crush. Laryngoscope. 2010b;120:1523–1530. doi: 10.1002/lary.20997. [DOI] [PubMed] [Google Scholar]
- Smith E, Damassa D, Davidson J. Methods Psychobiol. New York: Academic Press; 1977. Hormone Administration: Peripheral and Intracranial Implants; pp. 259–279. [Google Scholar]
- Stein RB, Nichols TR, Jhamandas J, Davis L, Charles D. Stable long-term recordings from cat peripheral nerves. Brain Res. 1977;128:21–38. doi: 10.1016/0006-8993(77)90233-5. [DOI] [PubMed] [Google Scholar]
- Udina E, Puigdemasa A, Navarro X. Passive and active exercise improve regeneration and muscle reinnervation after peripheral nerve injury in the rat. Muscle & Nerve. 2011;43:500–509. doi: 10.1002/mus.21912. [DOI] [PubMed] [Google Scholar]
- Verhovshek T, Rudolph LM, Sengelaub DR. Brain-derived neurotrophic factor and androgen interactions in spinal neuromuscular systems. Neuroscience. 2013;239:103–114. doi: 10.1016/j.neuroscience.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm JC, Cucoranu D, Xu M, Chmielewski S, Holmes T, Lau KS, Bassell GJ, English AW. Cooperative roles of BDNF expression in neurons and Schwann cells are modulated by exercise to facilitate nerve regeneration. J Neurosci. 2012;32:5002–5009. doi: 10.1523/JNEUROSCI.1411-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K, Wilhelm JC, Sabatier MJ, English AW. Sex differences in the effects of treadmill training on axon regeneration in cut peripheral nerves. Dev Neurobiol. 2012;72:688–698. doi: 10.1002/dneu.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]