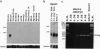Abstract
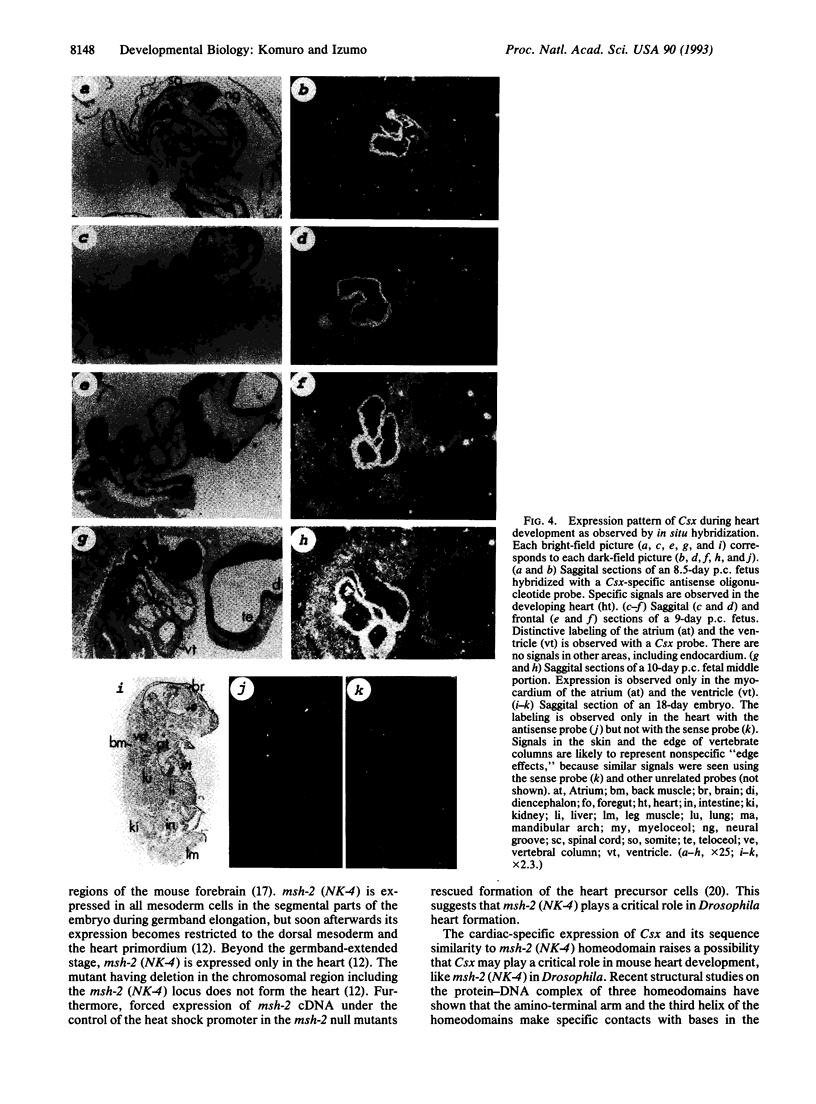
The molecular control of the differentiation process depends in part on lineage-restricted transcription factors that regulate expression of tissue-specific genes. Although significant progress has been made in molecular understanding of skeletal muscle differentiation, no information is available concerning the genes involved in development of the heart, the first organ to form in vertebrate embryos. Many vertebrate homeobox-containing genes have been shown to be expressed in broad regions of the mouse embryo, but no expression of a homeobox gene has been found in the most anterior region of the early embryo, the heart primordium. We report here on the cloning of a murine homeobox cDNA, Csx (cardiac-specific homeobox). The Csx homeodomain sequence is divergent from those of the Hox class genes but is related to that of Drosophila msh-2 (NK-4), which plays a key role in Drosophila heart formation. Csx is conserved in evolution and Csx homologs exist in all vertebrates examined. Transcripts of Csx are detected from the presomite stage (7.5 days postcoitum), when mesoderm differentiates into promyocardium. Csx expression is restricted in the myocardial cells from 8.5 days postcoitum through adult. Csx is not expressed in skeletal or smooth muscle or any other tissues examined. Expression of Csx precedes that of cardiac-specific genes in embryonic stem cells differentiating into beating myocardial cells in vitro. Although physiological function of Csx is yet to be determined, the temporal and spacial pattern of Csx expression raises a possibility that Csx may play a critical role in the differentiation of cardiac cells.
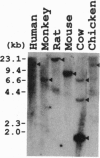
Full text
PDF


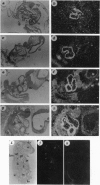
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akam M. The molecular basis for metameric pattern in the Drosophila embryo. Development. 1987 Sep;101(1):1–22. [PubMed] [Google Scholar]
- Bodmer R., Jan L. Y., Jan Y. N. A new homeobox-containing gene, msh-2, is transiently expressed early during mesoderm formation of Drosophila. Development. 1990 Nov;110(3):661–669. doi: 10.1242/dev.110.3.661. [DOI] [PubMed] [Google Scholar]
- Bodmer R. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development. 1993 Jul;118(3):719–729. doi: 10.1242/dev.118.3.719. [DOI] [PubMed] [Google Scholar]
- Castrillo J. L., Theill L. E., Karin M. Function of the homeodomain protein GHF1 in pituitary cell proliferation. Science. 1991 Jul 12;253(5016):197–199. doi: 10.1126/science.1677216. [DOI] [PubMed] [Google Scholar]
- Chisaka O., Capecchi M. R. Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature. 1991 Apr 11;350(6318):473–479. doi: 10.1038/350473a0. [DOI] [PubMed] [Google Scholar]
- DeRuiter M. C., Poelmann R. E., VanderPlas-de Vries I., Mentink M. M., Gittenberger-de Groot A. C. The development of the myocardium and endocardium in mouse embryos. Fusion of two heart tubes? Anat Embryol (Berl) 1992;185(5):461–473. doi: 10.1007/BF00174084. [DOI] [PubMed] [Google Scholar]
- Dohrmann C., Azpiazu N., Frasch M. A new Drosophila homeo box gene is expressed in mesodermal precursor cells of distinct muscles during embryogenesis. Genes Dev. 1990 Dec;4(12A):2098–2111. doi: 10.1101/gad.4.12a.2098. [DOI] [PubMed] [Google Scholar]
- Fahrner K., Hogan B. L., Flavell R. A. Transcription of H-2 and Qa genes in embryonic and adult mice. EMBO J. 1987 May;6(5):1265–1271. doi: 10.1002/j.1460-2075.1987.tb02363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A., Papalopulu N., Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989 May 5;57(3):367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Scott M. P. What determines the specificity of action of Drosophila homeodomain proteins? Cell. 1990 Nov 30;63(5):883–894. doi: 10.1016/0092-8674(90)90492-w. [DOI] [PubMed] [Google Scholar]
- He X., Treacy M. N., Simmons D. M., Ingraham H. A., Swanson L. W., Rosenfeld M. G. Expression of a large family of POU-domain regulatory genes in mammalian brain development. Nature. 1989 Jul 6;340(6228):35–41. doi: 10.1038/340035a0. [DOI] [PubMed] [Google Scholar]
- Holland P. W., Hogan B. L. Expression of homeo box genes during mouse development: a review. Genes Dev. 1988 Jul;2(7):773–782. doi: 10.1101/gad.2.7.773. [DOI] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y. Genes required for specifying cell fates in Drosophila embryonic sensory nervous system. Trends Neurosci. 1990 Dec;13(12):493–498. doi: 10.1016/0166-2236(90)90083-m. [DOI] [PubMed] [Google Scholar]
- Kessel M., Balling R., Gruss P. Variations of cervical vertebrae after expression of a Hox-1.1 transgene in mice. Cell. 1990 Apr 20;61(2):301–308. doi: 10.1016/0092-8674(90)90810-2. [DOI] [PubMed] [Google Scholar]
- Kim Y., Nirenberg M. Drosophila NK-homeobox genes. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7716–7720. doi: 10.1073/pnas.86.20.7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissinger C. R., Liu B. S., Martin-Blanco E., Kornberg T. B., Pabo C. O. Crystal structure of an engrailed homeodomain-DNA complex at 2.8 A resolution: a framework for understanding homeodomain-DNA interactions. Cell. 1990 Nov 2;63(3):579–590. doi: 10.1016/0092-8674(90)90453-l. [DOI] [PubMed] [Google Scholar]
- Komuro I., Schalling M., Jahn L., Bodmer R., Jenkins N. A., Copeland N. G., Izumo S. Gtx: a novel murine homeobox-containing gene, expressed specifically in glial cells of the brain and germ cells of testis, has a transcriptional repressor activity in vitro for a serum-inducible promoter. EMBO J. 1993 Apr;12(4):1387–1401. doi: 10.1002/j.1460-2075.1993.tb05783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaro D., Price M., de Felice M., Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991 Dec;113(4):1093–1104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- Lufkin T., Dierich A., LeMeur M., Mark M., Chambon P. Disruption of the Hox-1.6 homeobox gene results in defects in a region corresponding to its rostral domain of expression. Cell. 1991 Sep 20;66(6):1105–1119. doi: 10.1016/0092-8674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- Lyons G. E., Schiaffino S., Sassoon D., Barton P., Buckingham M. Developmental regulation of myosin gene expression in mouse cardiac muscle. J Cell Biol. 1990 Dec;111(6 Pt 1):2427–2436. doi: 10.1083/jcb.111.6.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis W., Krumlauf R. Homeobox genes and axial patterning. Cell. 1992 Jan 24;68(2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Olson E. N. Regulation of muscle transcription by the MyoD family. The heart of the matter. Circ Res. 1993 Jan;72(1):1–6. doi: 10.1161/01.res.72.1.1. [DOI] [PubMed] [Google Scholar]
- Otting G., Qian Y. Q., Billeter M., Müller M., Affolter M., Gehring W. J., Wüthrich K. Protein--DNA contacts in the structure of a homeodomain--DNA complex determined by nuclear magnetic resonance spectroscopy in solution. EMBO J. 1990 Oct;9(10):3085–3092. doi: 10.1002/j.1460-2075.1990.tb07505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M., Lazzaro D., Pohl T., Mattei M. G., Rüther U., Olivo J. C., Duboule D., Di Lauro R. Regional expression of the homeobox gene Nkx-2.2 in the developing mammalian forebrain. Neuron. 1992 Feb;8(2):241–255. doi: 10.1016/0896-6273(92)90291-k. [DOI] [PubMed] [Google Scholar]
- Robert B., Sassoon D., Jacq B., Gehring W., Buckingham M. Hox-7, a mouse homeobox gene with a novel pattern of expression during embryogenesis. EMBO J. 1989 Jan;8(1):91–100. doi: 10.1002/j.1460-2075.1989.tb03352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D., Nüsslein-Volhard C. The origin of pattern and polarity in the Drosophila embryo. Cell. 1992 Jan 24;68(2):201–219. doi: 10.1016/0092-8674(92)90466-p. [DOI] [PubMed] [Google Scholar]
- Sánchez A., Jones W. K., Gulick J., Doetschman T., Robbins J. Myosin heavy chain gene expression in mouse embryoid bodies. An in vitro developmental study. J Biol Chem. 1991 Nov 25;266(33):22419–22426. [PubMed] [Google Scholar]
- Wolberger C., Vershon A. K., Liu B., Johnson A. D., Pabo C. O. Crystal structure of a MAT alpha 2 homeodomain-operator complex suggests a general model for homeodomain-DNA interactions. Cell. 1991 Nov 1;67(3):517–528. doi: 10.1016/0092-8674(91)90526-5. [DOI] [PubMed] [Google Scholar]