Abstract
α-Synucleinopathies (ASP) comprise adult-onset, progressive neurodegenerative disorders such as Parkinson’s disease (PD), dementia with Lewy bodies (DLB) and multiple system atrophy (MSA) that are characterized by α-synuclein (AS) aggregates in neurons or glia. PD and DLB feature neuronal AS-positive inclusions termed Lewy bodies (LB) whereas glial cytoplasmic inclusions (GCIs, Papp-Lantos bodies) are recognized as the defining hallmark of MSA. Furthermore, AS-positive cytoplasmic aggregates may also be seen in astroglial cells of PD/DLB and MSA brains. The glial AS-inclusions appear to trigger reduced trophic support resulting in neuronal loss. Moreover, microgliosis and astrogliosis can be found throughout the neurodegenerative brain and both are key players in the initiation and progression of ASP. In this review, we will highlight AS-dependent alterations of glial function and their impact on neuronal vulnerability thereby providing a detailed summary on the multifaceted role of glia in ASP.
Keywords: α-synuclein, oligodendroglia, microglia, astroglia, Parkinson’s disease, multiple system atrophy, dementia with Lewy bodies, glial cytoplasmic inclusions, Lewy bodies
Introduction
α-Synucleinopathies (ASP) are progressive, adult-onset neurodegenerative diseases that include Parkinson’s disease (PD), dementia with Lewy bodies (DLB) and multiple system atrophy (MSA) (Spillantini and Goedert 2000, Goedert 2001, Beyer and Ariza 2007). The main pathological hallmark of these diseases is the occurrence of hyperphosphorylated, misfolded and fibrillized α-synuclein (AS)-positive inclusions throughout the central nervous system (CNS) (Fujiwara et al., 2002, Uversky 2008, Vilar et al., 2008). In PD and DLB, neurons are the main cell type displaying cytoplasmic AS-positive aggregations which are called Lewy bodies (LB) and Lewy neurites (LN) (Baba et al., 1998, Beyer and Ariza 2007), whereas in MSA, these inclusions predominantly develop in oligodendroglia and are therefore named glial cytoplasmic inclusions (GCIs, Papp-Lantos bodies) (Spillantini et al., 1998, Dickson et al., 1999, Hasegawa et al., 2004, Song et al., 2009, Fellner et al., 2011, Fellner and Stefanova 2013). Furthermore, PD and DLB show AS depositions in astrocytes and oligodendrocytes (Wakabayashi et al., 2000, Braak et al., 2007, Song et al., 2009). Wenning and Jellinger described AS-positive deposits in astroglial cells in MSA (Wenning and Jellinger 2005), however they appear to be less prominent and sometimes absent (Song et al., 2009) compared to neuronal and oligodendroglial inclusion pathology. AS aggregation in astroglial cells and its relevance to disease initiation and progression require further attention in MSA.
The brain protein AS is predominantly located in presynaptic terminals of neurons in the hippocampus, striatum, thalamus, cerebellum and neocortex (Iwai et al., 1995, Norris et al., 2004). AS belongs to a family of three distinct genes, including SNCA, SNCB and SNCG (α-, β- and γ-synuclein) and is composed of 140 amino-acids (Dev et al., 2003, Eriksen et al., 2003). Although the precise function of the protein is not solved yet, the importance of AS in folding and refolding of synaptic proteins has been proven (Chandra et al., 2005). Moreover, AS directly interacts with phospholipid vesicle membranes suggesting an important regulatory role in both inhibitory or facilitatory transmitter release (Auluck et al., 2010) (Abeliovich et al., 2000, Cabin et al., 2002, Gitler and Shorter 2007).
The development of the AS-positive GCI, LB and LN has not been completely elucidated yet. However, different studies demonstrated that AS overexpression impairs macroautophagy suggesting that reduced AS clearance is involved in the generation of AS inclusions in DLB and PD (Winslow et al., 2010, Xilouri and Stefanis 2011). Furthermore, alterations in the autophagosomal proteins in MSA brains and the participation of macroautophagy in the MSA pathogenesis have been suggested (Tanji et al., 2011, Schwarz et al., 2012). Post-translational modifications of AS, such as ubiquitination, nitration and phosphorylation may promote pathological inclusion formation and enhance disease progression (Giasson et al., 2000, Tofaris et al., 2003, Xilouri and Stefanis 2011). Moreover, Ozawa et al. showed a connection between neuronal cell loss, aggregation of AS and disease severity in MSA (Ozawa et al., 2004). Prion-like cell-to-cell propagation of AS has been suggested a crucial contributor to neurodegeneration and therefore to the progression of ASP (Desplats et al., 2009, Lee et al., 2010, Hansen et al., 2011, Reyes et al., 2014).
Glial cells are important in supporting neuronal survival, synaptic functions and local immunity (Webster and Astrom 2009, Hauser and Cookson 2011). However, glial cells might be crucial for the initiation and progression of different neurodegenerative diseases, including ASP (Gerhard et al., 2003, Gerhard et al., 2006, Fellner et al., 2011, Halliday and Stevens 2011). Due to various stimuli, e.g. infection or injury, astroglial and microglial cells get activated (Nimmerjahn et al., 2005, Wilhelmsson et al., 2006). Neurons may benefit from activated microglia and astroglia due to the release of trophic factors or the clearance of damaged cells by microglia (Liberto et al., 2004, van Rossum and Hanisch 2004, Nimmerjahn et al., 2005, Wilhelmsson et al., 2006).
Especially in neurodegenerative diseases microglia and astroglia can get over-activated resulting in reactive microgliosis and astrogliosis. It was described that astroglial cells can activate microglial cells (Gu et al., 2010, Halliday and Stevens 2011, Schmidt et al., 2011), or vice versa microglial activation can induce astrogliosis (Balasingam et al., 1996, Hanisch 2002, Rohl et al., 2007). Reactive gliosis might induce neurotoxicity, perturbation of the neuronal network, maladaptive plasticity and further lead to tissue damage (Papa et al., 2014). Moreover, it was demonstrated that neuronal cells have the ability to release excessive AS leading to the activation of an inflammatory response in microglia (Lee et al., 2010, Kim et al., 2013). Furthermore, the before mentioned prion-like spreading of pathological AS (Luk et al., 2009, Hansen et al., 2011, Masuda-Suzukake et al., 2013, Watts et al., 2013) could be a possible mechanism of AS aggregation in ASP and further cause activation of microglia and astroglia. Aggregated AS was shown to induce reactive microgliosis resulting in dopaminergic cell death (Zhang et al., 2005). Glial overactivation results in the release of (pro)-inflammatory cytokines, nitric oxide (NO) and reactive oxygen species (ROS) (Neumann et al., 2002, Deshpande et al., 2005, Mizuno et al., 2005, Zhang et al., 2005, Qian and Flood 2008, Dean et al., 2010, Lee et al., 2010, Qian et al., 2010).
Besides, oligodendroglial cells that are exposed to oxidative stress and cytokines present with cellular dysfunction, demyelination and cell death, as well as reduced trophic support which consequently affects neuronal survival (Thorburne and Juurlink 1996, Jurewicz et al., 2005).
This review summarizes the main features of ASP and the involvement of glial cells regarding the initiation and progression of these neurodegenerative diseases. We will discuss the main changes of glial cells during disease initiation and progression.
Glia in PD and DLB
PD and DLB are common neurodegenerative diseases in the population over the age of 65. About 3% of the general population develops PD after the age of 65, whereas about 20% of all diagnosed dementia patients have DLB (McKeith 2004, Dorsey et al., 2007). In both disorders movement and cognition, as well as mood and autonomic function are severely affected. Diagnosis to distinguish PD and DLB is very difficult, because of the overlap of symptoms and signs (Henchcliffe et al., 2011). In search for new biomarkers different factors were examined in the cerebrospinal fluid (CSF) of PD and DLB patients in comparison with Alzheimer disease (AD) patients and controls. Nagatsu and colleagues described elevated levels of pro-inflammatory cytokines such as Interleukine (IL)-1β, tumor necrosis factor (TNF)-α and IL-6, as well as decreased levels of neurotrophins such as brain-derived neurotrophic factor (BDNF) in the ventricular or lumbar cerebrospinal fluid (CSF) of PD patients (Nagatsu and Sawada 2005). Moreover, elevated levels of the astroglial protein glial fibrillary acidic protein (GFAP), as well as the neurofilament light protein (NFL), which is used as a marker of neuronal damage, and AS were found in the CSF of PD patients (Constantinescu et al., 2010, Gao et al., 2014). Different studies could show that CSF AS levels are lower in PD and DLB compared to AD patients and controls (Mollenhauer et al., 2008, Wennstrom et al., 2013). Additionally, Wennström and colleagues described a decrease of neurosin, an AS degrading protease, in the CSF of patients with PD and DLB (Wennstrom et al., 2013). Furthermore, it was suggested that an altered ratio of phosphorylated AS CSF levels might serve as a biomarker to distinguish PD from controls (Foulds et al., 2011).
Both diseases feature LB consisting of aggregated AS as a hallmark lesion of degenerating neurons. PD patients show enhanced neuronal loss in the substantia nigra (SN) compared to DLB patients (Tsuboi and Dickson 2005). Immunohistochemical studies showed a significantly higher amount of amyloid plaques in the putamen and caudate nucleus and more severe tau pathology in DLB compared to PD brains. Additionally, Jellinger and colleagues suggested an elevated level of AS-lesions in DLB compared to PD (Jellinger and Attems 2006). The accumulation of AS is increased with the occurrence of point mutations or duplications as well as triplications of the SNCA gene (Polymeropoulos et al., 1997, Singleton et al., 2003, Zarranz et al., 2004, Nishioka et al., 2006). Recent studies confirmed the association between PD and both SNCA single nucleotide polymorphisms (SNPs) and the H1 haplotype of microtubule-associated protein tau (MAPT) (Edwards et al., 2010, Elbaz et al., 2011, Trotta et al., 2012). Other genetic risk factors in the development of PD include leucine-rich repeat kinase 2 (LRRK2), the human leukocyte antigen (HLA) region and DJ-1 (Bonifati et al., 2003, Zimprich et al., 2004, Simon-Sanchez et al., 2009, Hamza et al., 2010). Genetic observations show also overlaps between PD and DLB. Mutations in the genes encoding AS (El-Agnaf et al., 1998, Ibanez et al., 2004), leucine-rich repeat kinase (Zimprich et al., 2004) and glucocerebrosidase (Goker-Alpan et al., 2006) were found in some DLB patients. However, also sporadic PD and DLB cases occur suggesting that genetic predisposition and environmental factors might play together in the initiation of the disease.
Due to PD progression and the development of LB and LN, dopaminergic terminals in the striatum and dopaminergic neurons in SN get affected and finally degenerate (Fearnley and Lees 1991, Jellinger 2003, Savitt et al., 2006). An attempt to classify the stages of PD was undertaken in 2003 by Braak and colleagues: (1) The stages 1-2 affect the lower raphe nuclei, lower brainstem nuclei, including the dorsal motor nucleus of the vagus, the locus coeruleus as well as the olfactory system. (2) Thereafter, LB pathology affects the SN pars compacta (SNpc), intralaminar thalamic nuclei, hippocampal CA2 and amygdala (stage 3-4). (3) Finally, in stage 5-6 of PD LB pathology expands to the neocortex (Braak et al., 2002, Braak et al., 2003b). Yet, the suggested Braak stages were challenged for different reasons, one being the lack of a definite correlation between clinical course and neuronal loss (Calne et al., 1992, Parkkinen et al., 2005, Burke et al., 2008, Jellinger 2009). Given that neuronal loss is not only dependent on the occurrence of AS aggregates, different other factors must have a major impact on disease progression in PD and DLB. Additionally to the AS positive aggregations in neurons and glia, it is suggested that reactive astrogliosis and microgliosis and therefore chronic inflammation play a crucial role in the initiation and progression of PD and DLB (Fellner et al., 2011, Halliday and Stevens 2011). However, as microglia and astroglia might display beneficial and detrimental effects on neuronal cells, the complete involvement of glial activation in PD and DLB is contradictory and has not been elucidated yet (Knott et al., 2002, Hashioka et al., 2009).
AS-positive inclusions in oligodendroglial cells were also confirmed in PD brains (Wakabayashi et al., 2000). However, oligodendroglial involvement in neuronal ASP seems not so profound for disease initiation, but in late disease progression nonmyelinating oligodendroglial cells may play a more crucial role (Halliday and Stevens 2011).
Microglia
[(11)C]-PK11195 Positron Emission Tomography (PET) imaging revealed profound microglial activation especially in pons, basal ganglia, frontal and temporal cortical regions of PD patients (Gerhard et al., 2006). Iannaccone et al described microglial activation in SN and putamen in PD as revealed by PET (Iannaccone et al., 2013). Moreover, early-stage drug-naïve PD patients displayed enhanced microglial activation only in midbrain which correlated with the loss of dopaminergic terminals in the striatum using [(11)C]-PK11195 PET and [(11)C]CFT binding the dopamine transporter (Ouchi et al., 2005). In a follow-up study, microgliosis also affected extra-striatal regions of the brain in these PD patients (Ouchi et al., 2009). In post-mortem PD brains, microglial activation has been identified in different brain regions, including SN, putamen, hippocampus, transentorhinal, cingulate and temporal cortex, as well as the limbic system (Imamura et al., 2003). However, profound activation of microglia in the SN, but no inflammatory changes such as microgliosis was reported in the putamen (Mirza et al., 2000). The inconsistent reports regarding microglial activation in different regions of PD brains might reflect the various stages of the disease and the individual differences of the disease pattern. In DLB patients, microglial activation in the SN and putamen was found. Further, comparisons of PD with DLB patients using the [(11)C]-PK11195 PET revealed additional microglial activation in several associative cortices in early DLB patients (Iannaccone et al., 2013). Moreover, reactive microglial cells were found to be more frequent around AS-positive LBs in PD and also DLB (Mackenzie 2000, Gerhard et al., 2003), and they were described in close proximity of dying neurons (Imamura et al., 2003).
In a recent study, post-mortem analyses of PD brains revealed region-specific variations of different microglial phenotypes in the SN and the hippocampus (Doorn et al., 2014). Furthermore, an enhanced expression of Toll-like receptor 2 (TLR2) on microglia in SN and hippocampus of incidental Lewy Body disease cases, which is thought to be a prodromal state of PD, and PD patients was described indicating a role for TLR2 and also microglia in the early stages of PD pathology (Doorn et al., 2014).
The hypothesis that microglial cells get activated by extracellular AS or astroglia even before neuronal loss occurs in SN pars compacta (SNpc) has been proposed previously (Su et al., 2009, Halliday and Stevens 2011). These data and the observations in PD patients support the presumption that microglial activation is involved in the initiation and progression of PD and DLB including the secretion of pro-inflammatory cytokines and ROS.
Especially in many cell culture studies a correlation between AS and microglial activation was described. The treatment of murine wild type (wt) microglia with aggregated AS in vitro led to the activation of antigen processing and presentation of antigen, inducing e.g. cytokine release (Harms et al., 2013). The PD-associated mutant forms of AS (A30P, E46K and A53T) extracellular applied, induced microglial activation in vitro and thus the release of pro-inflammatory cytokines including IL-6, IL-1β and TNF-α and the anti-inflammatory cytokine IL-10 as well as chemokines such as RANTES, monocyte chemotactic protein 1 (MCP-1), (C-X-C motif) ligand 10 (CXCL-10) and the macrophage inflammatory protein 1 α (MIP-1α) respectively (Roodveldt et al., 2010). Moreover, it was also shown that AS treatment of human primary microglial cells causes a dose-dependent release of pro-inflammatory molecules (Klegeris et al., 2008, Su et al., 2008). Experiments with the microglial cell line BV2 revealed that neuron-derived wt and mutant AS increased the pro-inflammatory response, and primarily mutant AS induced an enhanced release of nitric oxide and inflammatory cytokines, such as IL-6 and TNF-α (Alvarez-Erviti et al., 2011, Rojanathammanee et al., 2011). Furthermore, it was demonstrated that especially recombinant C-terminally truncated AS induced an enhanced release of pro-inflammatory cytokines (e.g. IL-6, TNF-α) or chemokines (e.g. CXCL-1) and production of ROS (Fellner et al., 2013a). In another study it was also found that extracellular aggregated AS induced NADPH oxidase activation and ROS production in rat primary mesencephalic microglia which led to dopaminergic neuronal loss (Zhang et al., 2005). In addition, nitrated and aggregated AS increased oxidative stress, inflammation and neuronal cell death in mesencephalic neuron microglia co-cultures (Zhang et al., 2005, Reynolds et al., 2008). These studies highlight the impact of AS on microglial cells and suggest the importance of microglial overactivation on neuronal survival and therefore, in the progression of PD and DLB.
Another important feature of microglial cells is the clearance of debris, including dead cells and AS (del Rio-Hortega 1932, Zhang et al., 2005, Park et al., 2008), thereby supporting neuronal survival. Different studies could show that microglial cells are capable of internalizing and degrading different forms of extracellular and cell-derived AS in vitro (Lee et al., 2008, Park et al., 2008, Stefanova et al., 2011, Fellner et al., 2013a). Recently, it was described that Toll-like receptors (TLRs) might play an important role in the recognition, internalization and activation of microglial cells. Particularly, the pattern-recognition receptors TLR2 and TLR4 were found to play a crucial role regarding AS phagocytosis and AS-dependent activation (Stefanova et al., 2011, Fellner et al., 2013a, Kim et al., 2013). Kim and colleagues suggested TLR2 as a fundamental link between recognition of neuron-released oligomeric AS, microglial activation and inflammatory responses (Kim et al., 2013). In vivo and in vitro studies showed that TLR4 ablation led to a disturbed clearance of overexpressed or recombinant AS by mouse microglia linked to aggravated nigral neurodegeneration (Stefanova et al., 2011, Fellner et al., 2013a). On the other hand TLR4 deficiency in microglia induced a decreased release of pro-inflammatory cytokines and ROS in response to AS exposure suggesting an involvement of TLR4 in inflammation and oxidative stress in ASP (Fellner et al., 2013a).
Studies performed in different animal models overexpressing AS highlight the link between AS or modified forms of AS and microglial activation. The following studies were able to demonstrate the importance of microglial activation in PD and DLB and the impact of microglial activation on dopaminergic neuronal survival which indicates a leading role for microglial cells in disease initiation and progression. In a mouse model overexpressing wt AS under the rat tyrosine hydroxylase promoter premature microglial activation was detected (Su et al., 2008). Moreover, enhanced microglial activation was described in mice overexpressing mutant human AS (A53T and A30P homozygous double-mutants) under a neuronal promoter (Su et al., 2009). Interestingly, the intramuscular injections of fibrillized AS in mice expressing the mutant human A53T AS led to a widespread CNS AS-inclusion pathology with elevated levels of microgliosis in brain areas presenting AS pathology compared to control animals (Sacino et al., 2014). A correlation between the level of AS expression and cell numbers of microglia was found in a rat PD model with rAAV-based overexpression of AS in midbrain (Sanchez-Guajardo et al., 2010). Furthermore, in this rat model delayed but long lasting microglial activation upon dopaminergic degeneration was described. An early and transient activation of microglial cells was induced although no dopaminergic cell death occurred. In the rat model with rAAV-based overexpression of AS the progression of the neurodegeneration was associated with four different types of microglial activation (Sanchez-Guajardo et al., 2010). Moreover, it has been suggested that modified forms of AS, particularly nitrated species, may be released as a consequence of dopaminergic neurodegeneration and that these trigger subsequent immune responses (Theodore et al., 2008). Evidence has been provided that a PD mouse model with rAAV-based human AS overexpression triggered microglial activation and further stimulated the adaptive immune system (Theodore et al., 2008). Microglial cells lacking the Fc gamma receptor, which participates in the regulation of the immune response by binding antibodies, can be activated by AS, further leading to stress and therefore to NF-κB/p65 expression, the release of pro-inflammatory cytokines as well as neurodegeneration respectively. These results suggest an involvement of the humoral adaptive immune system in AS-mediated microglial activation and neuronal cell death (Cao et al., 2010). It was described that glucocorticoid receptors are decreased in the SN of PD patients and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-intoxicated mice. Therefore, in a recent study the knock-out of glucocorticoid receptors, which are involved in the immune response and inflammation by binding cortisol and glucocorticoids that can be released by stress, on microglia in an MPTP mouse model has been characterized and revealed an increased dopaminergic neurodegeneration in a model for parkinsonism (Ros-Bernal et al., 2011). In a different approach it was found that rats with induced inflammation in the midbrain and exposed to stress showed an increased microglial activation resulting in a higher rate of dopaminergic neuronal cell death, suggesting that stress might increase the progression of PD (de Pablos et al., 2014).
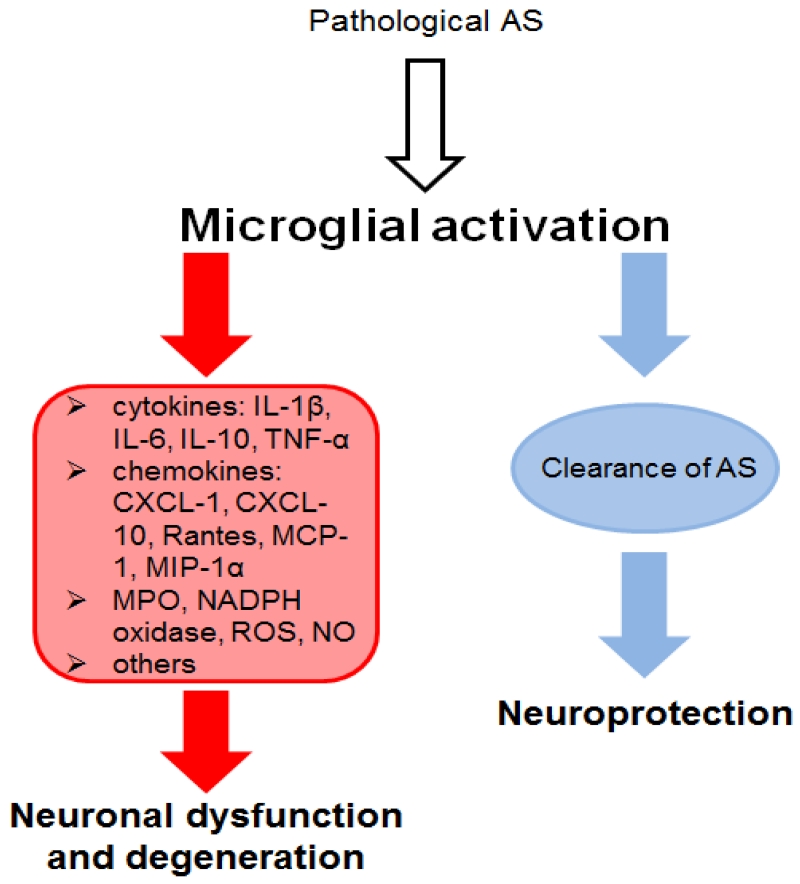
In conclusion, microglial activation is a very important mechanism in PD and DLB and seems to occur in correlation with the AS pathology in the CNS as seen in experimental models. However, the exact role of microglial cells has not been elucidated completely in these neurodegenerative diseases. On the one hand, microglial cells contribute to the clearance of debris, dead cells and AS thereby supporting neuronal survival. But on the other hand, microglial cells can get over-activated in the course of the disease and might contribute to disease initiation and progression by enhancing neurodegeneration through elevated oxidative stress and inflammatory processes (Figure1).
Figure 1. Microglial involvement in α-synucleinopathies (ASP).
Microglial cells can get activated by pathological α-synuclein (AS) (Su et al., 2009, Halliday and Stevens 2011, Fellner et al., 2013a). Different sources of these pathological AS species were proposed including release by neurons to the extracellular space or cell-to-cell propagation (Braak et al., 2007, Lee et al., 2010). Activation of microglial cells induces an oxidative stress response including the release of reactive oxygen species (ROS) and nitric oxide (NO) as well as the production of NADPH oxidase. Furthermore, pro-inflammatory cytokines, such as Interleukine-1β (IL-1β), IL-6, and tumor necrosis factor α (TNF-α), and the anti-inflammatory cytokine IL-10 as well as pro-inflammatory chemokines including (C-X-C motif) ligand 1 (CXCL-1), CXCL-10, Rantes, monocyte chemotactic protein 1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α) are released by activated microglial cells (Zhang et al., 2005, Su et al., 2008, Roodveldt et al., 2010, Alvarez-Erviti et al., 2011, Rojanathammanee et al., 2011, Fellner et al., 2013a). An involvement of Toll-like receptor 4 (TLR4), TLR2 and myeloperoxidase (MPO, key enzyme related to oxidative stress during inflammation) in inflammation and oxidative stress has been suggested (Stefanova et al., 2012a, Fellner et al., 2013a, Kim et al., 2013). Inflammation and oxidative stress mediated through microglial cells can further lead to neuronal dysfunction and cell death (Zhang et al., 2005, Reynolds et al., 2008). Thereby, dying neurons might release accumulated AS that stays in the extracellular space and again leads to the activation of microglial cells. This feedback loop might increase microglial activation leading to microgliosis. However, microglial cells are also able to phagocytose different forms of extracellular AS via TLR4 (Stefanova et al., 2011, Fellner et al., 2013a). This clearance mechanism might be even beneficial for neuronal survival. The different features displayed by microglial cells make it hard to categorize the role of microglial cells in ASP. Yet, the detrimental and beneficial functions of microglial cells suggest an involvement of microglial activation in the initiation and progression of ASP (Halliday and Stevens 2011). However, further studies have to be conducted to understand the complete participation of microglial activation in ASP.
Astroglia
Astroglial cells may play an important role in PD and DLB, as they display AS-positive accumulations in the cytoplasm and an activated phenotype in these diseases. In DLB brains, processes of astroglia that were TNF-α and inducible nitric oxide synthase (iNOS)-positive were characterized around AS-positive irregular LB (Katsuse et al., 2003). Different reports exist on astroglial activation in PD. Astroglial activation in PD post-mortem brains has been described as not existing, mild or marked. Different studies proposed that no reactive astroglial cells occurred in the SN, putamen and pons of PD brains (Mirza et al., 2000, Song et al., 2009). Hirsch and colleagues conducted PD post-mortem examinations and revealed massive astrogliosis and loss of dopaminergic neurons in the SN (Hirsch et al., 2005), whereas Vila et al. suggested a mild extent of reactive astroglia (Vila et al., 2001). At this time point regarding the current literature, it is not possible to conclude if astroglial activation occurs in PD brains, suggesting a large variability in human PD patients. Moreover, many different factors could be responsible for the activation of astroglial cells in PD and also the slightly different methods used for the analyses of astroglial activation in post-mortem brains could lead to these differing results. More neuropathological studies are necessary to evaluate astroglial activation in PD brains, however experimental data favor astroglial activation triggered by AS, as discussed further in the text.
Furthermore, in PD post-mortem brains, it was described that interferon-γ (IFN-γ) activation might lead to a neurotoxic reaction indicated by an increased amount of IFN-γ receptor on astroglia (Hashioka et al., 2009, Hashioka et al., 2010). In addition, an astroglial-dependent upregulation of the expression of myeloperoxidase, a key enzyme related to oxidative stress during inflammation, in the ventral midbrain of PD patients was found (Choi et al., 2005). However, also the release of beneficial factors by human astroglial cells was reported, including e.g. the brain-derived neurotrophic factors in SN of PD brains (Knott et al., 2002). Moreover, enhanced levels of glutathione peroxidase (GPx), a crucial protective enzyme against oxidative damage, in association with astroglial proliferation were reported in the SN of PD brains (Damier et al., 1993). Thus, the enhanced GPx activity was associated with elevated levels of the astroglial marker GFAP (Mythri et al., 2011) indicating that astroglial cells might be crucial for the protection of neurons against oxidative stress.
It is well known that neuronal depositions of AS serve as pathological hallmark of PD and DLB, however AS-positive protein aggregates were also described in human astroglial cells (Wakabayashi et al., 2000). Moreover, it was proposed that PD initiation starts inter alia with early nonfibrillized AS deposition in the cytoplasm of astroglia leading to the activation of microglial cells and neuronal cell death respectively (Halliday and Stevens 2011) as supported in two independent in vivo studies described further in the following section (Gu et al., 2010, Schmidt et al., 2011). Furthermore, it is suggested that altered AS, released by axon terminals, is taken up by astroglial cells surrounding the synapses (Braak et al., 2007), supporting the hypothesis of neuron-to-astroglia propagation of AS characterized in a different study (Lee et al., 2010). Moreover, Song and colleagues discovered that only human protoplasmic astroglia showed an elevated cytoplasmic AS accumulation in PD, whereas no obvious changes were seen in fibrous astroglia (Song et al., 2009). A more detailed characterization would be beneficial to clarify if and why only certain astroglial subgroups are accumulating AS and the impact of astroglial AS aggregation on neuron and other glial cell survival as well as disease progression.
Various experimental studies were able to shed light on different aspects of incorporation of AS by astroglial cells resulting in the release of pro-inflammatory but also anti-inflammatory molecules. Lee and colleagues confirmed that direct transfer of overexpressed AS from human derived SH-SY5Y neurons to rat astroglial cells takes place and furthermore, induces an inflammatory response in ASP suggesting a prion-like spread of the disease (Lee et al., 2010). Furthermore, uptake of neuronal-derived or recombinant AS in a time-dependent manner by human astroglial cells leading to impaired mitochondrial function was reported recently (Lee et al., 2010, Braidy et al., 2013). In another cell culture study, primary murine astroglial cells incorporated different forms of recombinant AS (soluble, fibrillized and truncated) by a TLR4-independent mechanism suggesting an endocytotic pathway of uptake (Fellner et al., 2013a) as also proposed by Lee and colleagues (Lee et al., 2010). The addition of extracellular AS to human astroglial cell cultures led to an accelerated production and release of pro-inflammatory cytokines including IL-6 and intercellular adhesion molecule 1 (ICAM-1), and in murine astroglia it induced the release of IL-6, TNF-α, the chemokine CXCL-1 and ROS (Klegeris et al., 2006, Fellner et al., 2013a). However, TLR4 ablation led to a decreased production of pro-inflammatory cytokines and ROS upon treatment with recombinant AS (Fellner et al., 2013a) indicating an important role for TLR4 in astroglial activation. Moreover, neuroprotective molecules might be released by astroglial cells when activated. It was found that hydrogen sulphide, a potential anti-inflammatory and neuroprotective agent, was downregulated upon astroglial activation indicating a possible role in neurodegeneration (Lee et al., 2009). In addition, the release of the glial cell line-derived neurotrophic factor (GDNF) by astroglia activated by selective dopaminergic neuronal damage was reported (Saavedra et al., 2006) suggesting a neuroprotective function for astroglial cells. Supporting the neuroprotective function of astroglia, the release of the antioxidant Glutathione by astroglia upon dopaminergic injury was described (Sandhu et al., 2009).
Different in vivo studies could show that astroglial activation or astrogliosis in combination with the secretion of pro-inflammatory cytokines contribute to the progression of PD and eventually also DLB confirming human post-mortem data. An increased expression of INF-γ receptor on astroglia, as well as TNF-α immunoreactivity related to astroglia were characterized in MPTP-treated monkeys (Parkinsonian macaques), suggesting that astroglial overactivation could play a crucial role in the progression of PD (Barcia et al., 2011). Furthermore, microgliosis and fast progressing paralysis triggered by widespread astrogliosis was the main finding in an inducible mouse model expressing the mutant A53T AS variant in astroglial cells. In addition, the overexpression of the mutant AS in astroglial cells in mice altered the normal function of astrocytes leading to a reduced integrity of the blood-brain barrier, a decreased homeostasis of extracellular glutamate and inducing a significant loss of dopaminergic neurons in the midbrain and motor neurons in the spinal cord (Gu et al., 2010). In a different study, the PD mouse model overexpressing mutant AS presented with morphological and functional alterations in astroglial mitochondria and a deranged secretion of factors fundamental for neuronal differentiation (Schmidt et al., 2011). These findings suggest that the accumulation of AS in astroglial cells might be of importance in the initiation of PD as also suggested by Halliday and Stevens in 2011 (Halliday and Stevens 2011). In a recent study, it was shown that PD mutant mice overexpressing human AS and the transglutaminase 2 (TG2) showed a promoted aggregation of AS and also an elevated astroglial activation compared to mice only overexpressing AS suggesting a significant contribution of TG2 to the accumulation of AS and pathogenesis of PD and other ASP and therefore a novel target regarding therapeutic approaches (Grosso et al., 2014).
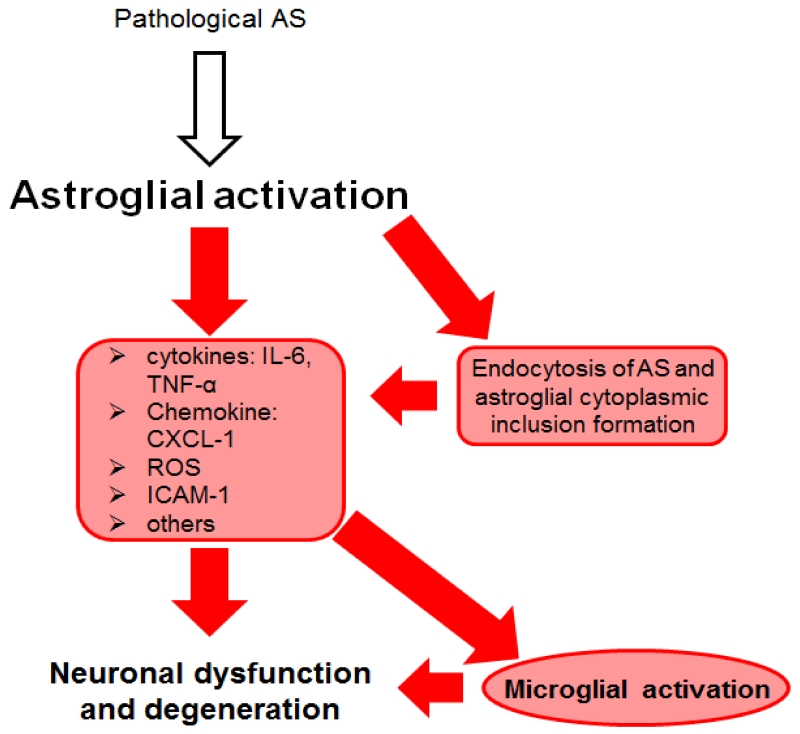
In summary, the accumulation of AS in astroglial cells may function as a crucial factor in the initiation of PD (Halliday and Stevens 2011). In addition, progression of disease might be driven by astroglial release of pro-inflammatory cytokines/chemokines, ROS and recruiting microglial cells (Figure 2). However, astroglial cells might also support neuronal survival through the secretion and production of neurotrophic and antioxidant factors induced by neuronal cell death. As there are not enough data supporting AS-dependent astroglial neuroprotection, more research will be necessary to identify the role of astroglial cells in the initiation and progression of PD and DLB.
Figure 2. Astroglial involvement in α-synucleinopathies (ASP).
Astroglial cells are activated by different forms of α-synuclein (AS). Different sources of these pathological AS species were proposed including release by neurons or cell-to-cell propagation (Braak et al., 2007, Lee et al., 2010). Thereby AS-induced release of intercellular adhesion molecule 1 (ICAM-1), reactive oxygen species (ROS) and pro-inflammatory cytokines [e.g. Interleukine-6 (IL-6) and tumor necrosis factor α (TNF-α)] was measured (Klegeris et al., 2006, Fellner et al., 2013a). An involvement of Toll-like receptor 4 (TLR4), myeloperoxidase (MPO, key enzyme related to oxidative stress during inflammation) and hydrogen sulphide in inflammation and oxidative stress has been suggested (Choi et al., 2005, Lee et al., 2009, Fellner et al., 2013a). Furthermore, various studies found that astroglial cells can internalize extracellular or neuron-derived AS via endocytosis (Lee et al., 2010, Braidy et al., 2013, Fellner et al., 2013a). As a consequence, AS-dependent inflammation and oxidative stress and the uptake and accumulation of AS might induce microglial activation as well as neuronal dysfunction and neurodegeneration (Lee et al., 2009, Gu et al., 2010). Astroglial cells are highly involved in inflammation and neuronal cell death. Some neuroprotective features were described for astroglial cells, yet not in an AS-dependent context (Saavedra et al., 2006, Sandhu et al., 2009). Further studies have to be completed to elucidate the role of AS-endocytosis and to understand the complete picture of astroglial involvement in ASP.
Oligodendroglia
It is suggested that oligodendroglial cells do not play a leading role in PD and DLB, however they might be involved in the late disease progression of these neuronal ASP (Halliday and Stevens 2011). Oligodendroglial AS-positive inclusions are present in the brains of clinical overt PD cases, yet with a rather low distribution that correlates with the degree of neurodegeneration in SN (Arai et al., 1999, Wakabayashi et al., 2000). However, no inclusions in oligodendroglial cells of preclinical Lewy body disease have been described (Wakabayashi et al., 2000). In addition, the occurrence of oligodendroglial cells showing complement-activation has been shown in some brain regions of PD and DLB cases (Yamada et al., 1992). Poor and protracted myelination due to neurodegeneration in PD and DLB led to a higher susceptibility of oligodendroglial cells (Braak and Del Tredici 2004). Moreover, a co-localization of AS-affected neurons with an enhanced number of nonmyelinating oligodendroglial cells has been described (Braak et al., 2003a, Braak and Del Tredici 2009). These findings highlight that oligodendroglial cells might not play a crucial role in the initiation and progression of disease pathology in PD and DLB. However, the involvement of oligodendroglial cells in the beginning of neuronal ASP has not been fully elucidated by now.
Glia in MSA
MSA is a progressive neurodegenerative disorder characterized by cerebellar ataxia, parkinsonism and autonomic dysfunction in any combination. MSA is categorized as a rare disease with a prevalence of about 4.4 per 100,000 cases (Schrag et al., 1999) and an onset of disease at about 52-57 years of age (Kollensperger et al., 2008, O’Sullivan et al., 2008). Furthermore, MSA is classified into 2 different clinical subtypes, being on the one hand MSAP presenting levodopa-unresponsive Parkinsonism due to SND and on the other hand MSAC mainly showing cerebellar ataxia reflecting olivopontocerebellar atrophy (Gilman et al., 1999, Wenning et al., 2004, Stefanova et al., 2009, Wenning and Stefanova 2009, Jecmenica-Lukic et al., 2012, Wenning et al., 2013). Both subtypes are characterized by progressive autonomic failure combined with degeneration in intermediolateral cell columns, Onuf’s nucleus in the spinal cord and autonomic brainstem centers (Wenning et al., 1997, Ozawa et al., 2004). Furthermore, MSA is hallmarked by so called Papp-Lantos bodies or GCIs which are located in the cytoplasm of oligodendroglia. The inclusions are histopathologically characterized by aggregated and phosphorylated (Ser129) AS similar to LB and reside in the movement, balance, and autonomic control centers of the brain (Jellinger and Lantos 2010). Due to these AS-positive GCI and their broad distribution throughout the CNS, MSA is conceptualized as a primary oligodendrogliopathy (Wenning et al., 2008).
Polymorphisms within the SNCA gene might induce the development of MSA (Al-Chalabi et al., 2009, Scholz et al., 2009). However, in different studies and in a genome-wide association study the polymorphisms in the SNCA gene in MSA could not be confirmed (Ozawa et al., 1999, Yun et al., 2010, Ahmed et al., 2012). Impairment of COQ2 and therefore inducing a functional impairment of the mitochondrial respiratory chain and enhanced vulnerability to oxidative stress were described in Japanese patients recently (Multiple-System Atrophy Research 2013), yet no such correlation between loss-of-function of COQ2 variants and increased risk of MSA in Europeans and Koreans was found (Jeon et al., 2014, Schottlaender et al., 2014, Sharma et al., 2014). These genetic data suggest that possible environmental risk factors and genetic predisposition might lead to MSA (Kuzdas-Wood et al., 2014). Furthermore, MSA is also characterized by microgliosis and astrogliosis in different affected regions of the brain (Gerhard et al., 2003, Ishizawa et al., 2004). However, the exact function of microglial and astroglial cells in MSA has not been completely elucidated to this date.
Similar to PD and DLB, biomarkers to distinguish MSA from other ASP and AD would increase the probability of an early diagnosis. A CSF study in MSA described a significant decrease of SNCA levels compared to controls and AD cases. However, no significant differences were determined between MSA and PD or DLB (Tateno et al., 2012). Furthermore, it was suggested that an altered ratio of phosphorylated AS CSF levels might serve as a biomarker to distinguish PD from MSA (Wang et al., 2012). Yet, others did not find significant differences between CSF samples of MSA patients and PD or DLB patients or controls (Mollenhauer et al., 2011, Shi et al., 2011, Aerts et al., 2012).
Microglia
Similar to PD and DLB microglial activation has been described repeatedly in MSA. Using [11C](R)-PK11195 PET imaging microglial activation was detected in the dorsolateral prefrontal cortex, putamen, pallidum, pons and SN in MSA patients (Gerhard et al., 2003). Additionally, an upregulation of activated microglial cells was found to be associated with GCI pathology in motor-related structures (Ishizawa et al., 2004).
Various in vitro data on AS-dependent microglial activation are of equal relevance for PD, DLB and MSA. The appropriate studies are already discussed in the first part of the review “Glia in PD and DLB” (Microglia) and AS-dependent microglial activation might contribute similarly to the initiation and progression in MSA compared to PD and DLB.
Age-dependent, region-specific chronic microglial activation was also demonstrated in the transgenic MSA mouse model overexpressing AS under an oligodendroglial promoter (Stefanova et al., 2007). It was shown that early microglial activation in SNpc of MSA mice was associated with an elevated expression of iNOS. The increased expression of iNOS correlated with dopaminergic neuronal loss (Stefanova et al., 2007, Fellner et al., 2013b). Furthermore, in this transgenic MSA mouse model and in human MSA brains, an upregulation of TLR4 was demonstrated, suggesting a possible attempt to increase phagocytotic activity in these brains (Stefanova et al., 2007, Brudek et al., 2013). In vivo experiments in a double transgenic mouse with a knock-out of TLR4 and oligodendroglial overexpression of AS under the proteolipid protein (PLP) promoter showed an impaired phagocytotic activity similar to the in vitro experiments which presented with increased motor disability and enhanced loss of nigrostriatal dopaminergic neurons in the mouse. In addition, increased brain levels of AS were linked to disturbed TLR4-mediated microglial phagocytosis of AS. Conclusively, TLR4 upregulation in microglial cells is suggested as a natural mechanism to promote the clearance of extracellular AS in MSA (Stefanova et al., 2011). In a very recent study, myeloperoxidase (MPO), a key enzyme important for the production of ROS by phagocytotic cells, was found to be upregulated in microglia of MSA post-mortem brains, as well as in a MSA mouse model (Stefanova et al., 2012a). Inhibition of this enzyme in the MSA mouse model revealed a rescue of neurons, a reduced amount of intracellular AS and suppressed microgliosis indicating that MPO might be involved in the AS-dependent activation of microglial cells as well as in the aggregation process of AS in MSA (Stefanova et al., 2012a). Microglial cells play an important role in the initiation and progression of MSA regarding phagocytosis, oxidative stress and inflammation. However, the complete mechanisms inducing AS-dependent microglial activation have not been elucidated to this date. The role of microglial activation might be equally relevant in MSA if compared to PD and DLB. Also in the context of MSA researchers have to resolve the complete involvement of microglia in the disease initiation and progression. Elucidation of the beneficial and detrimental functions of microglial activation on neuronal survival in ASP will remain a major challenge for research.
Astroglia
Astroglial activation is present in all ASP including MSA, and seems to play a role in disease initiation and progression respectively. In a Japanese study on the prognosis and progression of MSA, astrogliosis was demonstrated in the striatonigral, olivopontocerebellar and autonomic system, as well as in the corticospinal tract in MSA post-mortem brains (Watanabe et al., 2002). Furthermore, extensive astrogliosis has been confirmed in MSA brains (Ozawa et al., 2004, Jellinger et al., 2005) and moreover, AS-positive astroglial inclusions have been described in MSA brains, however in a decreased density compared to GCI (Wenning and Jellinger 2005). On the other hand Song et al. rejected the assumption that astroglial AS accumulation occurs in MSA cases (Song et al., 2009).
Different experimental data suggested that AS might be endocytosed by astroglial cells and furthermore, AS might induce astroglial activation, including the release of pro-inflammatory cytokines and increased oxidative stress (Lee et al., 2010, Fellner et al., 2013a). The summarized in vitro studies for PD and DLB on AS-dependent astroglial activation might be equally relevant for MSA (Figure 2). Moreover, in different MSA mouse models a role for astrogliosis in MSA-like neurodegeneration has been indicated. Astrogliosis in various brain areas as well as changes in cytokine and chemokine expression levels were detected in a MSA mouse model overexpressing AS in oligodendroglial cells under the myelin basic protein promoter (Shults et al., 2005, Valera et al., 2014). Moreover, astrogliosis has been also described in another MSA mouse model overexpressing AS under the 2’,3’-cyclic nucleotide 3’-phosphodiesterase (CNP) promoter (Yazawa et al., 2005). Furthermore, astroglial activation accompanying neurodegeneration was reported in a different MSA mouse model overexpressing AS under the PLP promoter exposed to 3-nitroproprionic acid (3-NP) (Stefanova et al., 2005a) (Table 1).
Table 1. MSA in vivo models with AS pathology.
In this table we summarize all in vivo models that were generated to imitate the main pathological hallmarks of MSA, including the accumulation of α-synuclein (AS) in oligodendroglial cells and neuronal loss. The replication of AS-positive accumulations in vivo was initiated by using various oligodendroglial specific promoters. Furthermore, different stressors [e.g. 3-nitroproprionic acid (3-NP), inducing mitochondrial dysfunction] were tested to induce a full-blown MSA pathology, including widespread GCI-like inclusions, microglial and astroglial activation as well as neuronal loss. For a more detailed description of in vivo MSA models see (Stefanova et al., 2005b, Ubhi et al., 2011, Fellner et al., 2013b, Kuzdas-Wood et al., 2014). This table is illustrative, but by no means complete. Additional abbreviations: SNpc – substantia nigra pars compacta, SND – striatonigral degeneration, OPCA – olivopontocerebellar atrophy.
| Promoter | Additional stressor |
Outcome | |
|---|---|---|---|
|
PLP-AS MSA mouse model |
proteolipid protein promoter |
|
|
|
PLP-AS MSA mouse model |
proteolipid protein promoter |
mitochondrial inhibition by systemic 3-NP administration |
|
|
PLP-AS MSA mouse model |
proteolipid protein promoter |
systemic proteasome inhibition |
|
|
CNP-AS MSA mouse model |
2’,3’-cyclic nucleotide 3’- phosphodiesterase promoter |
|
|
|
MBP-AS MSA mouse model |
myelin basic protein promoter |
|
|
|
MBP-AS MSA mouse model |
myelin basic protein promoter |
mitochondrial inhibition by systemic 3-NP administration |
|
Astroglial activation seems to be an important factor in the pathogenesis of MSA. However, to this date there is still insufficient data on the specific facets of astroglial responses related to MSA strengthening the necessity for further studies to understand astrogliosis in the pathogenesis of MSA.
Oligodendroglia
As mentioned above the hallmark of MSA are GCI which are mainly located in oligodendroglial cells characterized by their major component namely AS (Papp et al., 1989, Kato and Nakamura 1990, Kato et al., 1991, Papp and Lantos 1992, Arima et al., 1998, Spillantini et al., 1998, Tu et al., 1998, Wakabayashi et al., 1998). The occurrence and distribution of these GCI in MSA led to the assumption that oligodendroglial cells must play a leading role in the initiation and progression of MSA (Wenning and Quinn 1994, Ozawa et al., 2001, Wenning et al., 2008). In a postmortem study a correlation of GCI occurrence and neurodegeneration was reported suggesting the important role of oligodendroglial dysfunction in MSA progression (Ozawa et al., 2004). GCIs have an extensive distribution throughout the CNS including areas such as pons, medulla, putamen, SN, cerebellum and preganglionic autonomic brain structures (Papp and Lantos 1994, Nishie et al., 2004, Beyer and Ariza 2007, Jellinger and Lantos 2010).
It is still an ongoing debate whether oligodendroglial cells do actively incorporate and accumulate AS released by neurons or an elevated expression and slow degradation of AS in oligodendroglial cells occurs and leads to GCI formation in MSA (Fellner et al., 2011, Ubhi et al., 2011). Different studies demonstrated the active release of AS by neurons into the extracellular space (Emmanouilidou et al., 2010, Hansen et al., 2011). In a very recent study it was shown that grafted OLN-93 rat oligodendroglial cells can incorporate extracellular injected AS and AS from host rat brain neurons overexpressing human AS in vivo (Reyes et al., 2014) strengthening the assumption of cell-to-cell propagation mechanisms in MSA. A concentration-, time-, dynamin GTPase-, clathrin- and dynasore-dependent uptake mechanism of different forms of AS in oligodendroglial cells has been described in vitro (Kisos et al., 2012, Konno et al., 2012, Reyes et al., 2014). Furthermore, a role for oxidative stress regarding the uptake, accumulation and oligomerization of AS by OLN-93 oligodendroglial cells was demonstrated (Pukass and Richter-Landsberg 2014) (Table 2). Moreover, Nakamura and colleagues found an ectopic expression of Rab5 and Rabaptin-5 in GCIs of human oligodendrocytes. The enhanced expression of Rab5 may trigger endocytosis and lead to abnormal endocytotic activity resulting in the incorporation of elevated levels of AS into oligodendroglial cells (Nakamura et al., 2000). These recent data indicate that AS can be incorporated by oligodendroglial cells from the extracellular space or neurons.
Table 2. MSA in vitro models.
As the mechanisms of glial cytoplasmic inclusions (GCI) formation have not been elucidated to date, efforts are made to identify probable pathways in the initiation of this neurodegenerative disease in vitro. This table is illustrative, but by no means complete, of the various in vitro experiments trying to figure out the pathogenesis of MSA.
| Cell type | Type of AS | Outcome | |
|---|---|---|---|
|
Overexpression in vitro models | |||
|
U373 (Stefanova et al., 2001, Stefanova et al., 2003) |
Human astro- cytoma cell line |
wild type C-terminally truncated |
|
|
primary rat oligos (Stefanova et al., 2001, Stefanova et al., 2003) |
primary rat oligodendroglia |
||
|
CG-4 (Tsuboi et al., 2005) |
rat oligodendroglial progenitor cells |
wild type |
|
|
OLN-t40-AS (Kragh et al., 2009, Kragh et al., 2013) |
rat oligodendroglial cell line |
wild type |
|
|
primary mouse oligos (Kragh et al., 2009, Kragh et al., 2013) |
primary mouse oligodendroglia |
||
|
OLN-93 (Pukass and Richter-Landsberg 2014) |
rat oligodendroglial cell line |
wild type mutant A53T |
|
|
primary rat oligos (Pukass and Richter-Landsberg 2014) |
primary rat oligodendroglia |
||
|
Uptake in vitro models | |||
|
KG1C, MO3.13 (Konno et al., 2012) |
human oligodendroglial cell lines |
mutant A30P mutant A53T |
|
|
Oli-neu, OLN-93 (Kisos et al., 2012) |
rat oligodendroglial cell line |
wild type mutant A53T |
|
|
primary rat oligos (Kisos et al., 2012) |
primary rat oligodendroglia |
||
|
OLN-93 (Reyes et al., 2014) |
rat oligodendroglial cell line |
wild type (monomeric, oligomeric, fibrillized) |
|
Different studies have shown lack of SNCA mRNA in oligodendroglial cells of control and MSA brains (Ozawa et al., 2001, Miller et al., 2005). In a very recent study Asi and colleagues isolated oligodendroglia by laser-capture microdissection from MSA and control cases to perform cellular expression analysis and suggested that oligodendroglial SNCA mRNA expression had a tendency of elevation in MSA oligodendroglia however without reaching statistical significance as compared to healthy controls. Furthermore, no significant differences were found regarding the SNCA mRNA expression between MSA and control cases in tissue extracts of various brain regions (Asi et al., 2014).
It is suggested that oligodendroglial cells are primarily injured in MSA (Wenning et al., 2008) which might offer an explanation for the pathological accumulation of AS in these cells. One mechanism could be a defective degradation of AS in oligodendroglia in MSA inducing an enhanced accumulation in these cells (Ebrahimi-Fakhari et al., 2011, Schwarz et al., 2012, Stefanova et al., 2012b). A role for macroautophagy regarding the degradation of AS in human oligodendroglial cells was proposed given that the inhibition of the proteasomal system led to an increase of autophagy markers in cultured oligodendroglial cells. However, elevated levels of autophagy markers did not enhance the degradation of AS. Moreover, the autophagy protein LC3 was found in GCIs suggesting a major role for macroautophagy in MSA (Schwarz et al., 2012). Recently, it was suggested that the ubiquitin-proteasome system might contribute to the aggregation of AS in MSA (Stefanova et al., 2012b). Inhibition of the ubiquitin-proteasome system revealed enhanced aggregation of fibrillized AS in the cytoplasm of oligodendroglia inducing myelin disruption and demyelination in a MSA mouse model (Stefanova et al., 2012b). The histone deacetylase 6 (HDAC6) plays an important role in the regulation of the formation of aggresomes (Kawaguchi et al., 2003) and aggresome degradation (Iwata et al., 2005) regarding the transport of ubiquitinated misfolded proteins, as well as the control of autophagy pathways (Pan et al., 2008). The cytoplasmic enzyme HDAC6 was identified by Miki and colleagues in 98% of GCI in post-mortem MSA brains (Miki et al., 2011).This indicates that HDAC6 may promote the formation of fibrillized AS inclusions in oligodendroglial cells and suggests an important role for HDAC6 in MSA progression. Furthermore, AS seems to be a major factor in the initiation of the formation of protein inclusions, as in the absence of AS no accumulation of tau and αB-crystallin, further GCI components, occurs (Riedel et al., 2009), indicating that AS acts as a major initiator of GCI formation.
Additionally p25α, an oligodendroglial phosphoprotein (tubulin polymerization promoting protein) was shown to promote AS aggregation in vitro (Lindersson et al., 2005). Song and colleagues found that p25α is able to relocate to oligodendroglial soma in MSA cases, leading to an early oligodendroglial dysfunction and causing MSA initiation and GCI formation (Song et al., 2007). Furthermore, it was demonstrated that overexpression of p25α and AS in OLN-93 rat oligodendroglial cells led to disorganization of the microtubular cytoskeleton and the stimulation of the death domain receptor FAS as well as the activation of caspase-8 (Kragh et al., 2009, Kragh et al., 2013). In addition, more recently an upregulation of FAS receptor in MSA brains was found, indicating that oligodendroglial FAS ligand-mediated apoptosis might play an important role in MSA (Kragh et al., 2013). The inhibition of the phosphorylation of Ser129 of AS decreased the disorganization of the cytoskeleton and apoptosis suggesting that AS phosphorylation might be a key mechanism in the formation of AS oligomers and oligodendroglial cell death (Kragh et al., 2009).
Oligodendroglial cells featuring GCI pathology in vitro were found to have changed properties and they seem to be more vulnerable to different stimuli such as oxidative stress. In cell culture experiments, glial cells overexpressing AS were more susceptible to oxidative stress and TNF-α indicating that the higher oligodendroglial vulnerability to cytokines and stress plays an important role in MSA pathogenesis respectively (Stefanova et al., 2001, Stefanova et al., 2003). Moreover, a disturbed cell-extracellular matrix interaction was demonstrated by Tsuboi and colleagues who found that the overexpression of AS decreased the adhesion to fibronectin in CG-4 rat oligodendroglial cells (Tsuboi et al., 2005) (Table 1). In animal models the oligodendroglial overexpression of AS resulted in neuronal cell death in various regions of the brain such as SNpc, locus coeruleus, nucleus ambiguous, pedunculopontine tegmental nucleus, laterodorsal tegmental nucleus and Onuf’s nucleus (Stefanova et al., 2005a, Stemberger et al., 2010, Kuzdas et al., 2013). Furthermore, increased myelin disruption and mitochondrial dysfunction were found in the MSA mouse models overexpressing AS under an oligodendroglial promoter (Shults et al., 2005, Yazawa et al., 2005, Stefanova et al., 2007). Oligodendroglial AS overexpression but not neuronal AS overexpression led to a significant decrease of glial cell-derived neurotrophic factor (GDNF) as was also found in brain samples of MSA patients (Ubhi et al., 2010).
These findings indicate that aggregation of AS in oligodendroglia may lead to alterations of neurotrophic factors, oxidative stress and neuroinflammation, which all together promote MSA pathogenesis.
In summary, oligodendroglial cells play a crucial role in the pathogenesis of MSA including their vulnerability to different stress responses, the loss of trophic support and demyelination that further lead to neurodegeneration. Moreover, the formation of AS inclusions in these cells seems to be a key mechanism in disease initiation and progression. Unfortunately, the precise molecular and cellular mechanisms underlying GCI formation and altered oligodendroglial function still need to be unraveled. Additionally, detailed investigations using cell culture and transgenic MSA models will greatly enhance the understanding of the MSA pathogenesis and might lead to the development of new therapeutic targets.
Conclusion
Glial cells play an important role in the initiation and progression of ASP due to their multifaceted responses to AS aggregation in various brain areas. Especially microglial and astroglial cells respond to various brain insults and get activated which includes the release of pro-inflammatory cytokines or chemokines, ROS and NO. This stress response can lead to neuronal dysfunction and degeneration due to the chronic microgliosis and astrogliosis in brains of ASP patients. Furthermore, oligodendroglial cells develop AS-positive inclusions in MSA inducing oligodendroglial dysfunction including demyelination and reduced trophic support. All these detrimental features of glial cells affect neuronal viability and survival. However, glial cells also display beneficial functions, i.e. phagocytosis of debris and AS by microglial cells and the release of neurotrophic factors upon dopaminergic cell death by astroglial cells. Therefore, it is impossible to categorize the role of glial cells in the initiation and progression of ASP. To understand the full contribution of glial cells to the pathogenesis of ASP further studies are needed. To clarify the development of the inclusion bodies in glial cells and neurons should be a main focus for researchers regarding ASP. If we understand the mechanisms of the accumulation and aggregation of AS and the impact of these inclusions on the progression of these diseases, interventions with new therapeutic targets would be possible. Furthermore, the understanding of these basic mechanisms might also enable us to develop new biomarkers that help clinicians to overcome limitations of early diagnoses of ASP. Moreover, an early diagnosis would increase the chance to halt disease progression maybe even with now available therapeutics that contain inflammation. However, in vitro and in vivo experiments feature different limitations that have to be taken into account. Generated in vitro data in mouse or rat cells have to be confirmed in human tissue and have to be transferred successfully into an in vivo system. Moreover, animal models, especially rodent models, replicate rarely all aspects of the human diseases and therefore gained results have to be carefully considered and conclusions regarding the human disease have to be drawn cautiously.
Highlights.
Here we review the involvement of glial cells in different α-synucleinopathies.
Microgliosis and astrogliosis are key features in the initiation and progression.
Oligodendroglial involvement is crucial in the development of multiple system atrophy.
α-synuclein accumulation leads to dysfunction and death of glia and neurons.
Acknowledgements
This work was supported by grants of the Austrian Science Fund (FWF) P25161-B24, FWF SFB F4404, FWF DK SPIN W1206, Tyrolean Science Fund (TWF) UNI-0404/1660 and the European Community’s Seventh Framework Program (FP7/2007-2013) under grant agreement n°603646 Multisyn.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25(1):239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Aerts MB, Esselink RA, Abdo WF, Bloem BR, Verbeek MM. CSF alpha-synuclein does not differentiate between parkinsonian disorders. Neurobiol Aging. 2012;33(2):430, e431–433. doi: 10.1016/j.neurobiolaging.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Ahmed Z, Asi YT, Sailer A, Lees AJ, Houlden H, Revesz T, Holton JL. The neuropathology, pathophysiology and genetics of multiple system atrophy. Neuropathol Appl Neurobiol. 2012;38(1):4–24. doi: 10.1111/j.1365-2990.2011.01234.x. [DOI] [PubMed] [Google Scholar]
- Al-Chalabi A, Durr A, Wood NW, Parkinson MH, Camuzat A, Hulot JS, Morrison KE, Renton A, Sussmuth SD, Landwehrmeyer BG, Ludolph A, Agid Y, Brice A, Leigh PN, Bensimon G, Group, N. G. S. Genetic variants of the alpha-synuclein gene SNCA are associated with multiple system atrophy. PLoS One. 2009;4(9):e7114. doi: 10.1371/journal.pone.0007114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Couch Y, Richardson J, Cooper JM, Wood MJ. Alpha-synuclein release by neurons activates the inflammatory response in a microglial cell line. Neurosci Res. 2011;69(4):337–342. doi: 10.1016/j.neures.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Arai T, Ueda K, Ikeda K, Akiyama H, Haga C, Kondo H, Kuroki N, Niizato K, Iritani S, Tsuchiya K. Argyrophilic glial inclusions in the midbrain of patients with Parkinson’s disease and diffuse Lewy body disease are immunopositive for NACP/alpha-synuclein. Neurosci Lett. 1999;259(2):83–86. doi: 10.1016/s0304-3940(98)00890-8. [DOI] [PubMed] [Google Scholar]
- Arima K, Ueda K, Sunohara N, Arakawa K, Hirai S, Nakamura M, Tonozuka-Uehara H, Kawai M. NACP/alpha-synuclein immunoreactivity in fibrillary components of neuronal and oligodendroglial cytoplasmic inclusions in the pontine nuclei in multiple system atrophy. Acta Neuropathol. 1998;96(5):439–444. doi: 10.1007/s004010050917. [DOI] [PubMed] [Google Scholar]
- Asi YT, Simpson JE, Heath PR, Wharton SB, Lees AJ, Revesz T, Houlden H, Holton JL. Alpha-synuclein mRNA expression in oligodendrocytes in MSA. Glia. 2014;62(6):964–970. doi: 10.1002/glia.22653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auluck PK, Caraveo G, Lindquist S. alpha-Synuclein: membrane interactions and toxicity in Parkinson’s disease. Annu Rev Cell Dev Biol. 2010;26:211–233. doi: 10.1146/annurev.cellbio.042308.113313. [DOI] [PubMed] [Google Scholar]
- Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol. 1998;152(4):879–884. [PMC free article] [PubMed] [Google Scholar]
- Balasingam V, Dickson K, Brade A, Yong VW. Astrocyte reactivity in neonatal mice: apparent dependence on the presence of reactive microglia/macrophages. Glia. 1996;18(1):11–26. doi: 10.1002/(SICI)1098-1136(199609)18:1<11::AID-GLIA2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Barcia C, Ros CM, Annese V, Gomez A, Ros-Bernal F, Aguado-Yera D, Martinez-Pagan ME, de Pablos V, Fernandez-Villalba E, Herrero MT. IFN-gamma signaling, with the synergistic contribution of TNF-alpha, mediates cell specific microglial and astroglial activation in experimental models of Parkinson’s disease. Cell Death Dis. 2011;2:e142. doi: 10.1038/cddis.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer K, Ariza A. Protein aggregation mechanisms in synucleinopathies: commonalities and differences. J Neuropathol Exp Neurol. 2007;66(11):965–974. doi: 10.1097/nen.0b013e3181587d64. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299(5604):256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Boudes M, Uvin P, Pinto S, Voets T, Fowler CJ, Wenning GK, De Ridder D, Stefanova N. Bladder dysfunction in a transgenic mouse model of multiple system atrophy. Mov Disord. 2013;28(3):347–355. doi: 10.1002/mds.25336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Poor and protracted myelination as a contributory factor to neurodegenerative disorders. Neurobiol Aging. 2004;25(1):19–23. doi: 10.1016/j.neurobiolaging.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson’s disease. Adv Anat Embryol Cell Biol. 2009;201:1–119. [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rub U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages) J Neurol. 2002;249(Suppl 3):III/1–5. doi: 10.1007/s00415-002-1301-4. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003a;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003b;110(5):517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- Braak H, Sastre M, Del Tredici K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol. 2007;114(3):231–241. doi: 10.1007/s00401-007-0244-3. [DOI] [PubMed] [Google Scholar]
- Braidy N, Gai WP, Xu YH, Sachdev P, Guillemin GJ, Jiang XM, Ballard JW, Horan MP, Fang ZM, Chong BH, Chan DK. Uptake and mitochondrial dysfunction of alpha-synuclein in human astrocytes, cortical neurons and fibroblasts. Transl Neurodegener. 2013;2(1):20. doi: 10.1186/2047-9158-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudek T, Winge K, Agander TK, Pakkenberg B. Screening of Toll-like receptors expression in multiple system atrophy brains. Neurochem Res. 2013;38(6):1252–1259. doi: 10.1007/s11064-013-1020-5. [DOI] [PubMed] [Google Scholar]
- Burke RE, Dauer WT, Vonsattel JP. A critical evaluation of the Braak staging scheme for Parkinson’s disease. Ann Neurol. 2008;64(5):485–491. doi: 10.1002/ana.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22(20):8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson’s disease. Ann Neurol. 1992;32(Suppl):S125–127. doi: 10.1002/ana.410320721. [DOI] [PubMed] [Google Scholar]
- Cao S, Theodore S, Standaert DG. Fcgamma receptors are required for NF-kappaB signaling, microglial activation and dopaminergic neurodegeneration in an AAV-synuclein mouse model of Parkinson’s disease. Mol Neurodegener. 2010;5:42. doi: 10.1186/1750-1326-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123(3):383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Choi DK, Pennathur S, Perier C, Tieu K, Teismann P, Wu DC, Jackson-Lewis V, Vila M, Vonsattel JP, Heinecke JW, Przedborski S. Ablation of the inflammatory enzyme myeloperoxidase mitigates features of Parkinson’s disease in mice. J Neurosci. 2005;25(28):6594–6600. doi: 10.1523/JNEUROSCI.0970-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu R, Rosengren L, Johnels B, Zetterberg H, Holmberg B. Consecutive analyses of cerebrospinal fluid axonal and glial markers in Parkinson’s disease and atypical Parkinsonian disorders. Parkinsonism Relat Disord. 2010;16(2):142–145. doi: 10.1016/j.parkreldis.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Zhang P, Agid Y, Javoy-Agid F. Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience. 1993;52(1):1–6. doi: 10.1016/0306-4522(93)90175-f. [DOI] [PubMed] [Google Scholar]
- de Pablos RM, Herrera AJ, Espinosa-Oliva AM, Sarmiento M, Munoz MF, Machado A, Venero JL. Chronic stress enhances microglia activation and exacerbates death of nigral dopaminergic neurons under conditions of inflammation. J Neuroinflammation. 2014;11:34. doi: 10.1186/1742-2094-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JM, Wang X, Kaindl AM, Gressens P, Fleiss B, Hagberg H, Mallard C. Microglial MyD88 signaling regulates acute neuronal toxicity of LPS-stimulated microglia in vitro. Brain Behav Immun. 2010;24(5):776–783. doi: 10.1016/j.bbi.2009.10.018. [DOI] [PubMed] [Google Scholar]
- del Rio-Hortega P, Penfield W. Cytology and cellular pathology of the nervous system. Hoeber, PB; New York: 1932. Microglia; pp. 483–534. [Google Scholar]
- Deshpande M, Zheng J, Borgmann K, Persidsky R, Wu L, Schellpeper C, Ghorpade A. Role of activated astrocytes in neuronal damage: potential links to HIV-1-associated dementia. Neurotox Res. 2005;7(3):183–192. doi: 10.1007/BF03036448. [DOI] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106(31):13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev KK, Hofele K, Barbieri S, Buchman VL, van der Putten H. Part II: alpha-synuclein and its molecular pathophysiological role in neurodegenerative disease. Neuropharmacology. 2003;45(1):14–44. doi: 10.1016/s0028-3908(03)00140-0. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Lin W, Liu WK, Yen SH. Multiple system atrophy: a sporadic synucleinopathy. Brain Pathol. 1999;9(4):721–732. doi: 10.1111/j.1750-3639.1999.tb00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorn KJ, Moors T, Drukarch B, van de Berg W, Lucassen PJ, van Dam AM. Microglial phenotypes and toll-like receptor 2 in the substantia nigra and hippocampus of incidental Lewy body disease cases and Parkinson’s disease patients. Acta Neuropathol Commun. 2014;2:90. doi: 10.1186/s40478-014-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, Tanner CM. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z, Rockenstein E, Masliah E, Hyman BT, McLean PJ, Unni VK. Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of alpha-synuclein. J Neurosci. 2011;31(41):14508–14520. doi: 10.1523/JNEUROSCI.1560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards TL, Scott WK, Almonte C, Burt A, Powell EH, Beecham GW, Wang L, Zuchner S, Konidari I, Wang G, Singer C, Nahab F, Scott B, Stajich JM, Pericak-Vance M, Haines J, Vance JM, Martin ER. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann Hum Genet. 2010;74(2):97–109. doi: 10.1111/j.1469-1809.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Agnaf OM, Curran MD, Wallace A, Middleton D, Murgatroyd C, Curtis A, Perry R, Jaros E. Mutation screening in exons 3 and 4 of alpha-synuclein in sporadic Parkinson’s and sporadic and familial dementia with Lewy bodies cases. Neuroreport. 1998;9(17):3925–3927. doi: 10.1097/00001756-199812010-00029. [DOI] [PubMed] [Google Scholar]
- Elbaz A, Ross OA, Ioannidis JP, Soto-Ortolaza AI, Moisan F, Aasly J, Annesi G, Bozi M, Brighina L, Chartier-Harlin MC, Destee A, Ferrarese C, Ferraris A, Gibson JM, Gispert S, Hadjigeorgiou GM, Jasinska-Myga B, Klein C, Kruger R, Lambert JC, Lohmann K, van de Loo S, Loriot MA, Lynch T, Mellick GD, Mutez E, Nilsson C, Opala G, Puschmann A, Quattrone A, Sharma M, Silburn PA, Stefanis L, Uitti RJ, Valente EM, Vilarino-Guell C, Wirdefeldt K, Wszolek ZK, Xiromerisiou G, Maraganore DM, Farrer MJ, Genetic Epidemiology of Parkinson’s Disease, C. Independent and joint effects of the MAPT and SNCA genes in Parkinson disease. Ann Neurol. 2011;69(5):778–792. doi: 10.1002/ana.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30(20):6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen JL, Dawson TM, Dickson DW, Petrucelli L. Caught in the act: alpha-synuclein is the culprit in Parkinson’s disease. Neuron. 2003;40(3):453–456. doi: 10.1016/s0896-6273(03)00684-6. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, Wenning GK, Stefanova N. Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia. 2013a;61(3):349–360. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner L, Jellinger KA, Wenning GK, Stefanova N. Glial dysfunction in the pathogenesis of alpha-synucleinopathies: emerging concepts. Acta Neuropathol. 2011;121(6):675–693. doi: 10.1007/s00401-011-0833-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner L, Stefanova N. The role of glia in alpha-synucleinopathies. Mol Neurobiol. 2013;47(2):575–586. doi: 10.1007/s12035-012-8340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner L, Wenning GK, Stefanova N. Models of Multiple System Atrophy. Curr Top Behav Neurosci. 2013b doi: 10.1007/7854_2013_269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds PG, Mitchell JD, Parker A, Turner R, Green G, Diggle P, Hasegawa M, Taylor M, Mann D, Allsop D. Phosphorylated alpha-synuclein can be detected in blood plasma and is potentially a useful biomarker for Parkinson’s disease. FASEB J. 2011;25(12):4127–4137. doi: 10.1096/fj.10-179192. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4(2):160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- Gao L, Tang H, Nie K, Wang L, Zhao J, Gan R, Huang J, Zhu R, Feng S, Duan Z, Zhang Y, Wang L. Cerebrospinal fluid alpha-synuclein as a biomarker for Parkinson’s disease diagnosis: a systematic review and meta-analysis. Int J Neurosci. 2014 doi: 10.3109/00207454.2014.961454. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Banati RB, Goerres GB, Cagnin A, Myers R, Gunn RN, Turkheimer F, Good CD, Mathias CJ, Quinn N, Schwarz J, Brooks DJ. [11C](R)-PK11195 PET imaging of microglial activation in multiple system atrophy. Neurology. 2003;61(5):686–689. doi: 10.1212/01.wnl.0000078192.95645.e6. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Trender-Gerhard I, Turkheimer F, Quinn NP, Bhatia KP, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in progressive supranuclear palsy. Mov Disord. 2006;21(1):89–93. doi: 10.1002/mds.20668. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290(5493):985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- Gilman S, Low PA, Quinn N, Albanese A, Ben-Shlomo Y, Fowler CJ, Kaufmann H, Klockgether T, Lang AE, Lantos PL, Litvan I, Mathias CJ, Oliver E, Robertson D, Schatz I, Wenning GK. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci. 1999;163(1):94–98. doi: 10.1016/s0022-510x(98)00304-9. [DOI] [PubMed] [Google Scholar]
- Gitler AD, Shorter J. Prime time for alpha-synuclein. J Neurosci. 2007;27(10):2433–2434. doi: 10.1523/JNEUROSCI.0094-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M. Parkinson’s disease and other alpha-synucleinopathies. Clin Chem Lab Med. 2001;39(4):308–312. doi: 10.1515/CCLM.2001.047. [DOI] [PubMed] [Google Scholar]
- Goker-Alpan O, Giasson BI, Eblan MJ, Nguyen J, Hurtig HI, Lee VM, Trojanowski JQ, Sidransky E. Glucocerebrosidase mutations are an important risk factor for Lewy body disorders. Neurology. 2006;67(5):908–910. doi: 10.1212/01.wnl.0000230215.41296.18. [DOI] [PubMed] [Google Scholar]
- Grosso H, Woo JM, Lee KW, Im JY, Masliah E, Junn E, Mouradian MM. Transglutaminase 2 exacerbates alpha-synuclein toxicity in mice and yeast. FASEB J. 2014;28(10):4280–4291. doi: 10.1096/fj.14-251413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu XL, Long CX, Sun L, Xie C, Lin X, Cai H. Astrocytic expression of Parkinson’s disease-related A53T alpha-synuclein causes neurodegeneration in mice. Mol Brain. 2010;3:12. doi: 10.1186/1756-6606-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM, Stevens CH. Glia: initiators and progressors of pathology in Parkinson’s disease. Mov Disord. 2011;26(1):6–17. doi: 10.1002/mds.23455. [DOI] [PubMed] [Google Scholar]
- Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E, Kusel VI, Collura R, Roberts J, Griffith A, Samii A, Scott WK, Nutt J, Factor SA, Payami H. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genet. 2010;42(9):781–785. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40(2):140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, Li JY, Brundin P. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121(2):715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms AS, Cao S, Rowse AL, Thome AD, Li X, Mangieri LR, Cron RQ, Shacka JJ, Raman C, Standaert DG. MHCII is required for alpha-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci. 2013;33(23):9592–9600. doi: 10.1523/JNEUROSCI.5610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, Matsuzaki M, Takeda A, Kikuchi A, Akita H, Perry G, Smith MA, Itoyama Y. Accelerated alpha-synuclein aggregation after differentiation of SH-SY5Y neuroblastoma cells. Brain Res. 2004;1013(1):51–59. doi: 10.1016/j.brainres.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Hashioka S, Klegeris A, Schwab C, McGeer PL. Interferon-gamma-dependent cytotoxic activation of human astrocytes and astrocytoma cells. Neurobiol Aging. 2009;30(12):1924–1935. doi: 10.1016/j.neurobiolaging.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Hashioka S, Klegeris A, Schwab C, Yu S, McGeer PL. Differential expression of interferon-gamma receptor on human glial cells in vivo and in vitro. J Neuroimmunol. 2010;225(1-2):91–99. doi: 10.1016/j.jneuroim.2010.04.023. [DOI] [PubMed] [Google Scholar]
- Hauser DN, Cookson MR. Astrocytes in Parkinson’s disease and DJ-1. J Neurochem. 2011;117(3):357–358. doi: 10.1111/j.1471-4159.2011.07217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henchcliffe C, Dodel R, Beal MF. Biomarkers of Parkinson’s disease and Dementia with Lewy bodies. Prog Neurobiol. 2011;95(4):601–613. doi: 10.1016/j.pneurobio.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S, Hartmann A. Neuroinflammatory processes in Parkinson’s disease. Parkinsonism Relat Disord. 2005;11(Suppl 1):S9–S15. doi: 10.1016/j.parkreldis.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Iannaccone S, Cerami C, Alessio M, Garibotto V, Panzacchi A, Olivieri S, Gelsomino G, Moresco RM, Perani D. In vivo microglia activation in very early dementia with Lewy bodies, comparison with Parkinson’s disease. Parkinsonism Relat Disord. 2013;19(1):47–52. doi: 10.1016/j.parkreldis.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Ibanez P, Bonnet AM, Debarges B, Lohmann E, Tison F, Pollak P, Agid Y, Durr A, Brice A. Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet. 2004;364(9440):1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003;106(6):518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- Ishizawa K, Komori T, Sasaki S, Arai N, Mizutani T, Hirose T. Microglial activation parallels system degeneration in multiple system atrophy. J Neuropathol Exp Neurol. 2004;63(1):43–52. doi: 10.1093/jnen/63.1.43. [DOI] [PubMed] [Google Scholar]
- Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14(2):467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- Iwata A, Riley BE, Johnston JA, Kopito RR. HDAC6 and microtubules are required for autophagic degradation of aggregated huntingtin. J Biol Chem. 2005;280(48):40282–40292. doi: 10.1074/jbc.M508786200. [DOI] [PubMed] [Google Scholar]
- Jecmenica-Lukic M, Poewe W, Tolosa E, Wenning GK. Premotor signs and symptoms of multiple system atrophy. Lancet Neurol. 2012;11(4):361–368. doi: 10.1016/S1474-4422(12)70022-4. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Neuropathological spectrum of synucleinopathies. Mov Disord. 2003;18(Suppl 6):S2–12. doi: 10.1002/mds.10557. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. A critical evaluation of current staging of alpha-synuclein pathology in Lewy body disorders. Biochim Biophys Acta. 2009;1792(7):730–740. doi: 10.1016/j.bbadis.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Attems J. Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol. 2006;112(3):253–260. doi: 10.1007/s00401-006-0088-2. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Lantos PL. Papp-Lantos inclusions and the pathogenesis of multiple system atrophy: an update. Acta Neuropathol. 2010;119(6):657–667. doi: 10.1007/s00401-010-0672-3. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Seppi K, Wenning GK. Grading of neuropathology in multiple system atrophy: proposal for a novel scale. Mov Disord. 2005;20(Suppl 12):S29–36. doi: 10.1002/mds.20537. [DOI] [PubMed] [Google Scholar]
- Jeon BS, Farrer MJ, Bortnick SF, Korean Canadian Alliance on Parkinson’s, D. and Related, D. Mutant COQ2 in multiple-system atrophy. N Engl J Med. 2014;371(1):80. doi: 10.1056/NEJMc1311763. [DOI] [PubMed] [Google Scholar]
- Jurewicz A, Matysiak M, Tybor K, Kilianek L, Raine CS, Selmaj K. Tumour necrosis factor-induced death of adult human oligodendrocytes is mediated by apoptosis inducing factor. Brain. 2005;128(Pt 11):2675–2688. doi: 10.1093/brain/awh627. [DOI] [PubMed] [Google Scholar]
- Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Spooren W, Fuss B, Mallon B, Macklin WB, Fujiwara H, Hasegawa M, Iwatsubo T, Kretzschmar HA, Haass C. Hyperphosphorylation and insolubility of alpha-synuclein in transgenic mouse oligodendrocytes. EMBO Rep. 2002;3(6):583–588. doi: 10.1093/embo-reports/kvf109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Nakamura H. Cytoplasmic argyrophilic inclusions in neurons of pontine nuclei in patients with olivopontocerebellar atrophy: immunohistochemical and ultrastructural studies. Acta Neuropathol. 1990;79(6):584–594. doi: 10.1007/BF00294235. [DOI] [PubMed] [Google Scholar]
- Kato S, Nakamura H, Hirano A, Ito H, Llena JF, Yen SH. Argyrophilic ubiquitinated cytoplasmic inclusions of Leu-7-positive glial cells in olivopontocerebellar atrophy (multiple system atrophy) Acta Neuropathol. 1991;82(6):488–493. doi: 10.1007/BF00293383. [DOI] [PubMed] [Google Scholar]
- Katsuse O, Iseki E, Kosaka K. Immunohistochemical study of the expression of cytokines and nitric oxide synthases in brains of patients with dementia with Lewy bodies. Neuropathology. 2003;23(1):9–15. doi: 10.1046/j.1440-1789.2003.00483.x. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell. 2003;115(6):727–738. doi: 10.1016/s0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- Kim C, Ho DH, Suk JE, You S, Michael S, Kang J, Joong Lee S, Masliah E, Hwang D, Lee HJ, Lee SJ. Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun. 2013;4:1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisos H, Pukass K, Ben-Hur T, Richter-Landsberg C, Sharon R. Increased neuronal alpha-synuclein pathology associates with its accumulation in oligodendrocytes in mice modeling alpha-synucleinopathies. PLoS One. 2012;7(10):e46817. doi: 10.1371/journal.pone.0046817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klegeris A, Giasson BI, Zhang H, Maguire J, Pelech S, McGeer PL. Alpha-synuclein and its disease-causing mutants induce ICAM-1 and IL-6 in human astrocytes and astrocytoma cells. FASEB J. 2006;20(12):2000–2008. doi: 10.1096/fj.06-6183com. [DOI] [PubMed] [Google Scholar]
- Klegeris A, Pelech S, Giasson BI, Maguire J, Zhang H, McGeer EG, McGeer PL. Alpha-synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol Aging. 2008;29(5):739–752. doi: 10.1016/j.neurobiolaging.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Knott C, Stern G, Kingsbury A, Welcher AA, Wilkin GP. Elevated glial brain-derived neurotrophic factor in Parkinson’s diseased nigra. Parkinsonism Relat Disord. 2002;8(5):329–341. doi: 10.1016/s1353-8020(02)00008-1. [DOI] [PubMed] [Google Scholar]
- Kollensperger M, Geser F, Seppi K, Stampfer-Kountchev M, Sawires M, Scherfler C, Boesch S, Mueller J, Koukouni V, Quinn N, Pellecchia MT, Barone P, Schimke N, Dodel R, Oertel W, Dupont E, Ostergaard K, Daniels C, Deuschl G, Gurevich T, Giladi N, Coelho M, Sampaio C, Nilsson C, Widner H, Sorbo FD, Albanese A, Cardozo A, Tolosa E, Abele M, Klockgether T, Kamm C, Gasser T, Djaldetti R, Colosimo C, Meco G, Schrag A, Poewe W, Wenning GK, European, M. S. A. S. G. Red flags for multiple system atrophy. Mov Disord. 2008;23(8):1093–1099. doi: 10.1002/mds.21992. [DOI] [PubMed] [Google Scholar]
- Konno M, Hasegawa T, Baba T, Miura E, Sugeno N, Kikuchi A, Fiesel FC, Sasaki T, Aoki M, Itoyama Y, Takeda A. Suppression of dynamin GTPase decreases alpha-synuclein uptake by neuronal and oligodendroglial cells: a potent therapeutic target for synucleinopathy. Mol Neurodegener. 2012;7:38. doi: 10.1186/1750-1326-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragh CL, Fillon G, Gysbers A, Hansen HD, Neumann M, Richter-Landsberg C, Haass C, Zalc B, Lubetzki C, Gai WP, Halliday GM, Kahle PJ, Jensen PH. FAS-dependent cell death in alpha-synuclein transgenic oligodendrocyte models of multiple system atrophy. PLoS One. 2013;8(1):e55243. doi: 10.1371/journal.pone.0055243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragh CL, Lund LB, Febbraro F, Hansen HD, Gai WP, El-Agnaf O, Richter-Landsberg C, Jensen PH. Alpha-synuclein aggregation and Ser-129 phosphorylation-dependent cell death in oligodendroglial cells. J Biol Chem. 2009;284(15):10211–10222. doi: 10.1074/jbc.M809671200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzdas-Wood D, Stefanova N, Jellinger KA, Seppi K, Schlossmacher MG, Poewe W, Wenning GK. Towards translational therapies for multiple system atrophy. Prog Neurobiol. 2014;118:19–35. doi: 10.1016/j.pneurobio.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzdas D, Stemberger S, Gaburro S, Stefanova N, Singewald N, Wenning GK. Oligodendroglial alpha-synucleinopathy and MSA-like cardiovascular autonomic failure: experimental evidence. Exp Neurol. 2013;247:531–536. doi: 10.1016/j.expneurol.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Bae EJ, Lee JH, Paik SR, Lee SJ. Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol. 2008;40(9):1835–1849. doi: 10.1016/j.biocel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285(12):9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Schwab C, Yu S, McGeer E, McGeer PL. Astrocytes produce the antiinflammatory and neuroprotective agent hydrogen sulfide. Neurobiol Aging. 2009;30(10):1523–1534. doi: 10.1016/j.neurobiolaging.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Liberto CM, Albrecht PJ, Herx LM, Yong VW, Levison SW. Pro-regenerative properties of cytokine-activated astrocytes. J Neurochem. 2004;89(5):1092–1100. doi: 10.1111/j.1471-4159.2004.02420.x. [DOI] [PubMed] [Google Scholar]
- Lindersson E, Lundvig D, Petersen C, Madsen P, Nyengaard JR, Hojrup P, Moos T, Otzen D, Gai WP, Blumbergs PC, Jensen PH. p25alpha Stimulates alpha-synuclein aggregation and is co-localized with aggregated alpha-synuclein in alpha-synucleinopathies. J Biol Chem. 2005;280(7):5703–5715. doi: 10.1074/jbc.M410409200. [DOI] [PubMed] [Google Scholar]
- Luk KC, Song C, O’Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A. 2009;106(47):20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR. Activated microglia in dementia with Lewy bodies. Neurology. 2000;55(1):132–134. doi: 10.1212/wnl.55.1.132. [DOI] [PubMed] [Google Scholar]
- Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DM, Hasegawa M. Prion-like spreading of pathological alpha-synuclein in brain. Brain. 2013;136(Pt 4):1128–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith I. Dementia with Lewy bodies. Dialogues Clin Neurosci. 2004;6(3):333–341. doi: 10.31887/DCNS.2004.6.3/imckeith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki Y, Mori F, Tanji K, Kakita A, Takahashi H, Wakabayashi K. Accumulation of histone deacetylase 6, an aggresome-related protein, is specific to Lewy bodies and glial cytoplasmic inclusions. Neuropathology. 2011;31(6):561–568. doi: 10.1111/j.1440-1789.2011.01200.x. [DOI] [PubMed] [Google Scholar]
- Miller DW, Johnson JM, Solano SM, Hollingsworth ZR, Standaert DG, Young AB. Absence of alpha-synuclein mRNA expression in normal and multiple system atrophy oligodendroglia. J Neural Transm. 2005;112(12):1613–1624. doi: 10.1007/s00702-005-0378-1. [DOI] [PubMed] [Google Scholar]
- Mirza B, Hadberg H, Thomsen P, Moos T. The absence of reactive astrocytosis is indicative of a unique inflammatory process in Parkinson’s disease. Neuroscience. 2000;95(2):425–432. doi: 10.1016/s0306-4522(99)00455-8. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Kuno R, Nitta A, Nabeshima T, Zhang G, Kawanokuchi J, Wang J, Jin S, Takeuchi H, Suzumura A. Protective effects of nicergoline against neuronal cell death induced by activated microglia and astrocytes. Brain Res. 2005;1066(1-2):78–85. doi: 10.1016/j.brainres.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Cullen V, Kahn I, Krastins B, Outeiro TF, Pepivani I, Ng J, Schulz-Schaeffer W, Kretzschmar HA, McLean PJ, Trenkwalder C, Sarracino DA, Vonsattel JP, Locascio JJ, El-Agnaf OM, Schlossmacher MG. Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol. 2008;213(2):315–325. doi: 10.1016/j.expneurol.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Esselmann H, Roeber S, Schulz-Schaeffer WJ, Trenkwalder C, Bibl M, Steinacker P, Kretzschmar HA, Wiltfang J, Otto M. Different CSF beta-amyloid processing in Alzheimer’s and Creutzfeldt-Jakob disease. J Neural Transm. 2011;118(5):691–697. doi: 10.1007/s00702-010-0543-z. [DOI] [PubMed] [Google Scholar]
- Multiple-System Atrophy Research, C. Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med. 2013;369(3):233–244. doi: 10.1056/NEJMoa1212115. [DOI] [PubMed] [Google Scholar]
- Mythri RB, Venkateshappa C, Harish G, Mahadevan A, Muthane UB, Yasha TC, Srinivas Bharath MM, Shankar SK. Evaluation of markers of oxidative stress, antioxidant function and astrocytic proliferation in the striatum and frontal cortex of Parkinson’s disease brains. Neurochem Res. 2011;36(8):1452–1463. doi: 10.1007/s11064-011-0471-9. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Sawada M. Inflammatory process in Parkinson’s disease: role for cytokines. Curr Pharm Des. 2005;11(8):999–1016. doi: 10.2174/1381612053381620. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Kawamoto Y, Nakano S, Akiguchi I. Expression of the endocytosis regulatory proteins Rab5 and Rabaptin-5 in glial cytoplasmic inclusions from brains with multiple system atrophy. Clin Neuropathol. 2000;19(2):51–56. [PubMed] [Google Scholar]
- Neumann H, Schweigreiter R, Yamashita T, Rosenkranz K, Wekerle H, Barde YA. Tumor necrosis factor inhibits neurite outgrowth and branching of hippocampal neurons by a rho-dependent mechanism. J Neurosci. 2002;22(3):854–862. doi: 10.1523/JNEUROSCI.22-03-00854.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nishie M, Mori F, Fujiwara H, Hasegawa M, Yoshimoto M, Iwatsubo T, Takahashi H, Wakabayashi K. Accumulation of phosphorylated alpha-synuclein in the brain and peripheral ganglia of patients with multiple system atrophy. Acta Neuropathol. 2004;107(4):292–298. doi: 10.1007/s00401-003-0811-1. [DOI] [PubMed] [Google Scholar]
- Nishioka K, Hayashi S, Farrer MJ, Singleton AB, Yoshino H, Imai H, Kitami T, Sato K, Kuroda R, Tomiyama H, Mizoguchi K, Murata M, Toda T, Imoto I, Inazawa J, Mizuno Y, Hattori N. Clinical heterogeneity of alpha-synuclein gene duplication in Parkinson’s disease. Ann Neurol. 2006;59(2):298–309. doi: 10.1002/ana.20753. [DOI] [PubMed] [Google Scholar]
- Norris EH, Giasson BI, Lee VM. Alpha-synuclein: normal function and role in neurodegenerative diseases. Curr Top Dev Biol. 2004;60:17–54. doi: 10.1016/S0070-2153(04)60002-0. [DOI] [PubMed] [Google Scholar]
- O’Sullivan SS, Massey LA, Williams DR, Silveira-Moriyama L, Kempster PA, Holton JL, Revesz T, Lees AJ. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain. 2008;131(Pt 5):1362–1372. doi: 10.1093/brain/awn065. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yagi S, Yokokura M, Sakamoto M. Neuroinflammation in the living brain of Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S200–204. doi: 10.1016/S1353-8020(09)70814-4. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, Torizuka T. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann Neurol. 2005;57(2):168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Okuizumi K, Ikeuchi T, Wakabayashi K, Takahashi H, Tsuji S. Analysis of the expression level of alpha-synuclein mRNA using postmortem brain samples from pathologically confirmed cases of multiple system atrophy. Acta Neuropathol. 2001;102(2):188–190. doi: 10.1007/s004010100367. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Paviour D, Quinn NP, Josephs KA, Sangha H, Kilford L, Healy DG, Wood NW, Lees AJ, Holton JL, Revesz T. The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain. 2004;127(Pt 12):2657–2671. doi: 10.1093/brain/awh303. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Takano H, Onodera O, Kobayashi H, Ikeuchi T, Koide R, Okuizumi K, Shimohata T, Wakabayashi K, Takahashi H, Tsuji S. No mutation in the entire coding region of the alpha-synuclein gene in pathologically confirmed cases of multiple system atrophy. Neurosci Lett. 1999;270(2):110–112. doi: 10.1016/s0304-3940(99)00475-9. [DOI] [PubMed] [Google Scholar]
- Pan T, Kondo S, Zhu W, Xie W, Jankovic J, Le W. Neuroprotection of rapamycin in lactacystin-induced neurodegeneration via autophagy enhancement. Neurobiol Dis. 2008;32(1):16–25. doi: 10.1016/j.nbd.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Papa M, De Luca C, Petta F, Alberghina L, Cirillo G. Astrocyte-neuron interplay in maladaptive plasticity. Neurosci Biobehav Rev. 2014;42:35–54. doi: 10.1016/j.neubiorev.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J Neurol Sci. 1989;94(1-3):79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- Papp MI, Lantos PL. Accumulation of tubular structures in oligodendroglial and neuronal cells as the basic alteration in multiple system atrophy. J Neurol Sci. 1992;107(2):172–182. doi: 10.1016/0022-510x(92)90286-t. [DOI] [PubMed] [Google Scholar]
- Papp MI, Lantos PL. The distribution of oligodendroglial inclusions in multiple system atrophy and its relevance to clinical symptomatology. Brain. 1994;117(Pt 2):235–243. doi: 10.1093/brain/117.2.235. [DOI] [PubMed] [Google Scholar]
- Park JY, Paik SR, Jou I, Park SM. Microglial phagocytosis is enhanced by monomeric alpha-synuclein, not aggregated alpha-synuclein: implications for Parkinson’s disease. Glia. 2008;56(11):1215–1223. doi: 10.1002/glia.20691. [DOI] [PubMed] [Google Scholar]
- Parkkinen L, Kauppinen T, Pirttila T, Autere JM, Alafuzoff I. Alpha-synuclein pathology does not predict extrapyramidal symptoms or dementia. Ann Neurol. 2005;57(1):82–91. doi: 10.1002/ana.20321. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Pukass K, Richter-Landsberg C. Oxidative stress promotes uptake, accumulation, and oligomerization of extracellular alpha-synuclein in oligodendrocytes. J Mol Neurosci. 2014;52(3):339–352. doi: 10.1007/s12031-013-0154-x. [DOI] [PubMed] [Google Scholar]
- Qian L, Flood PM. Microglial cells and Parkinson’s disease. Immunol Res. 2008;41(3):155–164. doi: 10.1007/s12026-008-8018-0. [DOI] [PubMed] [Google Scholar]
- Qian L, Flood PM, Hong JS. Neuroinflammation is a key player in Parkinson’s disease and a prime target for therapy. J Neural Transm. 2010;117(8):971–979. doi: 10.1007/s00702-010-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes JF, Rey NL, Bousset L, Melki R, Brundin P, Angot E. Alpha-synuclein transfers from neurons to oligodendrocytes. Glia. 2014;62(3):387–398. doi: 10.1002/glia.22611. [DOI] [PubMed] [Google Scholar]
- Reynolds AD, Glanzer JG, Kadiu I, Ricardo-Dukelow M, Chaudhuri A, Ciborowski P, Cerny R, Gelman B, Thomas MP, Mosley RL, Gendelman HE. Nitrated alpha-synuclein-activated microglial profiling for Parkinson’s disease. J Neurochem. 2008;104(6):1504–1525. doi: 10.1111/j.1471-4159.2007.05087.x. [DOI] [PubMed] [Google Scholar]
- Riedel M, Goldbaum O, Richter-Landsberg C. alpha-Synuclein promotes the recruitment of tau to protein inclusions in oligodendroglial cells: effects of oxidative and proteolytic stress. J Mol Neurosci. 2009;39(1-2):226–234. doi: 10.1007/s12031-009-9190-y. [DOI] [PubMed] [Google Scholar]
- Rohl C, Lucius R, Sievers J. The effect of activated microglia on astrogliosis parameters in astrocyte cultures. Brain Res. 2007;1129(1):43–52. doi: 10.1016/j.brainres.2006.10.057. [DOI] [PubMed] [Google Scholar]
- Rojanathammanee L, Murphy EJ, Combs CK. Expression of mutant alpha-synuclein modulates microglial phenotype in vitro. J Neuroinflammation. 2011;8:44. doi: 10.1186/1742-2094-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodveldt C, Labrador-Garrido A, Gonzalez-Rey E, Fernandez-Montesinos R, Caro M, Lachaud CC, Waudby CA, Delgado M, Dobson CM, Pozo D. Glial innate immunity generated by non-aggregated alpha-synuclein in mouse: differences between wild-type and Parkinson’s disease-linked mutants. PLoS One. 2010;5(10):e13481. doi: 10.1371/journal.pone.0013481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros-Bernal F, Hunot S, Herrero MT, Parnadeau S, Corvol JC, Lu L, Alvarez-Fischer D, Carrillo-de Sauvage MA, Saurini F, Coussieu C, Kinugawa K, Prigent A, Hoglinger G, Hamon M, Tronche F, Hirsch EC, Vyas S. Microglial glucocorticoid receptors play a pivotal role in regulating dopaminergic neurodegeneration in parkinsonism. Proc Natl Acad Sci U S A. 2011;108(16):6632–6637. doi: 10.1073/pnas.1017820108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra A, Baltazar G, Santos P, Carvalho CM, Duarte EP. Selective injury to dopaminergic neurons up-regulates GDNF in substantia nigra postnatal cell cultures: role of neuron-glia crosstalk. Neurobiol Dis. 2006;23(3):533–542. doi: 10.1016/j.nbd.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Sacino AN, Brooks M, Thomas MA, McKinney AB, Lee S, Regenhardt RW, McGarvey NH, Ayers JI, Notterpek L, Borchelt DR, Golde TE, Giasson BI. Intramuscular injection of alpha-synuclein induces CNS alpha-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc Natl Acad Sci U S A. 2014;111(29):10732–10737. doi: 10.1073/pnas.1321785111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Guajardo V, Febbraro F, Kirik D, Romero-Ramos M. Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PLoS One. 2010;5(1):e8784. doi: 10.1371/journal.pone.0008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu JK, Gardaneh M, Iwasiow R, Lanthier P, Gangaraju S, Ribecco-Lutkiewicz M, Tremblay R, Kiuchi K, Sikorska M. Astrocyte-secreted GDNF and glutathione antioxidant system protect neurons against 6OHDA cytotoxicity. Neurobiol Dis. 2009;33(3):405–414. doi: 10.1016/j.nbd.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Savitt JM, Dawson VL, Dawson TM. Diagnosis and treatment of Parkinson disease: molecules to medicine. J Clin Invest. 2006;116(7):1744–1754. doi: 10.1172/JCI29178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S, Linnartz B, Mendritzki S, Sczepan T, Lubbert M, Stichel CC, Lubbert H. Genetic mouse models for Parkinson’s disease display severe pathology in glial cell mitochondria. Hum Mol Genet. 2011;20(6):1197–1211. doi: 10.1093/hmg/ddq564. [DOI] [PubMed] [Google Scholar]
- Scholz SW, Houlden H, Schulte C, Sharma M, Li A, Berg D, Melchers A, Paudel R, Gibbs JR, Simon-Sanchez J, Paisan-Ruiz C, Bras J, Ding J, Chen H, Traynor BJ, Arepalli S, Zonozi RR, Revesz T, Holton J, Wood N, Lees A, Oertel W, Wullner U, Goldwurm S, Pellecchia MT, Illig T, Riess O, Fernandez HH, Rodriguez RL, Okun MS, Poewe W, Wenning GK, Hardy JA, Singleton AB, Del Sorbo F, Schneider S, Bhatia KP, Gasser T. SNCA variants are associated with increased risk for multiple system atrophy. Ann Neurol. 2009;65(5):610–614. doi: 10.1002/ana.21685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottlaender LV, Houlden H, Multiple-System Atrophy Brain Bank, C. Mutant COQ2 in multiple-system atrophy. N Engl J Med. 2014;371(1):81. doi: 10.1056/NEJMc1311763. [DOI] [PubMed] [Google Scholar]
- Schrag A, Ben-Shlomo Y, Quinn NP. Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet. 1999;354(9192):1771–1775. doi: 10.1016/s0140-6736(99)04137-9. [DOI] [PubMed] [Google Scholar]
- Schwarz L, Goldbaum O, Bergmann M, Probst-Cousin S, Richter-Landsberg C. Involvement of macroautophagy in multiple system atrophy and protein aggregate formation in oligodendrocytes. J Mol Neurosci. 2012;47(2):256–266. doi: 10.1007/s12031-012-9733-5. [DOI] [PubMed] [Google Scholar]
- Sharma M, Wenning G, Kruger R, European Multiple-System Atrophy Study, G. Mutant COQ2 in multiple-system atrophy. N Engl J Med. 2014;371(1):80–81. doi: 10.1056/NEJMc1311763. [DOI] [PubMed] [Google Scholar]
- Shi M, Bradner J, Hancock AM, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP, Kim HM, Leverenz JB, Montine TJ, Ginghina C, Kang UJ, Cain KC, Wang Y, Aasly J, Goldstein D, Zhang J. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann Neurol. 2011;69(3):570–580. doi: 10.1002/ana.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shults CW, Rockenstein E, Crews L, Adame A, Mante M, Larrea G, Hashimoto M, Song D, Iwatsubo T, Tsuboi K, Masliah E. Neurological and neurodegenerative alterations in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. J Neurosci. 2005;25(46):10689–10699. doi: 10.1523/JNEUROSCI.3527-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Kruger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, Gwinn K, van der Brug M, Lopez G, Chanock SJ, Schatzkin A, Park Y, Hollenbeck A, Gao J, Huang X, Wood NW, Lorenz D, Deuschl G, Chen H, Riess O, Hardy JA, Singleton AB, Gasser T. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41(12):1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Song YJ, Halliday GM, Holton JL, Lashley T, O’Sullivan SS, McCann H, Lees AJ, Ozawa T, Williams DR, Lockhart PJ, Revesz TR. Degeneration in different parkinsonian syndromes relates to astrocyte type and astrocyte protein expression. J Neuropathol Exp Neurol. 2009;68(10):1073–1083. doi: 10.1097/NEN.0b013e3181b66f1b. [DOI] [PubMed] [Google Scholar]
- Song YJ, Lundvig DM, Huang Y, Gai WP, Blumbergs PC, Hojrup P, Otzen D, Halliday GM, Jensen PH. p25alpha relocalizes in oligodendroglia from myelin to cytoplasmic inclusions in multiple system atrophy. Am J Pathol. 2007;171(4):1291–1303. doi: 10.2353/ajpath.2007.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett. 1998;251(3):205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Goedert M. The alpha-synucleinopathies: Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy. Ann N Y Acad Sci. 2000;920:16–27. doi: 10.1111/j.1749-6632.2000.tb06900.x. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Bucke P, Duerr S, Wenning GK. Multiple system atrophy: an update. Lancet Neurol. 2009;8(12):1172–1178. doi: 10.1016/S1474-4422(09)70288-1. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Fellner L, Reindl M, Masliah E, Poewe W, Wenning GK. Toll-like receptor 4 promotes alpha-synuclein clearance and survival of nigral dopaminergic neurons. Am J Pathol. 2011;179(2):954–963. doi: 10.1016/j.ajpath.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N, Georgievska B, Eriksson H, Poewe W, Wenning GK. Myeloperoxidase inhibition ameliorates multiple system atrophy-like degeneration in a transgenic mouse model. Neurotox Res. 2012a;21(4):393–404. doi: 10.1007/s12640-011-9294-3. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Kaufmann WA, Humpel C, Poewe W, Wenning GK. Systemic proteasome inhibition triggers neurodegeneration in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. Acta Neuropathol. 2012b;124(1):51–65. doi: 10.1007/s00401-012-0977-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N, Klimaschewski L, Poewe W, Wenning GK, Reindl M. Glial cell death induced by overexpression of alpha-synuclein. J Neurosci Res. 2001;65(5):432–438. doi: 10.1002/jnr.1171. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Reindl M, Neumann M, Haass C, Poewe W, Kahle PJ, Wenning GK. Oxidative stress in transgenic mice with oligodendroglial alpha-synuclein overexpression replicates the characteristic neuropathology of multiple system atrophy. Am J Pathol. 2005a;166(3):869–876. doi: 10.1016/s0002-9440(10)62307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N, Reindl M, Neumann M, Kahle PJ, Poewe W, Wenning GK. Microglial activation mediates neurodegeneration related to oligodendroglial alpha-synucleinopathy: implications for multiple system atrophy. Mov Disord. 2007;22(15):2196–2203. doi: 10.1002/mds.21671. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Schanda K, Klimaschewski L, Poewe W, Wenning GK, Reindl M. Tumor necrosis factor-alpha-induced cell death in U373 cells overexpressing alpha-synuclein. J Neurosci Res. 2003;73(3):334–340. doi: 10.1002/jnr.10662. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Tison F, Reindl M, Poewe W, Wenning GK. Animal models of multiple system atrophy. Trends Neurosci. 2005b;28(9):501–506. doi: 10.1016/j.tins.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Stemberger S, Poewe W, Wenning GK, Stefanova N. Targeted overexpression of human alpha-synuclein in oligodendroglia induces lesions linked to MSA-like progressive autonomic failure. Exp Neurol. 2010;224(2):459–464. doi: 10.1016/j.expneurol.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Federoff HJ, Maguire-Zeiss KA. Mutant alpha-synuclein overexpression mediates early proinflammatory activity. Neurotox Res. 2009;16(3):238–254. doi: 10.1007/s12640-009-9053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol Aging. 2008;29(11):1690–1701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji K, Mori F, Kakita A, Takahashi H, Wakabayashi K. Alteration of autophagosomal proteins (LC3, GABARAP and GATE-16) in Lewy body disease. Neurobiol Dis. 2011;43(3):690–697. doi: 10.1016/j.nbd.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Tateno F, Sakakibara R, Kawai T, Kishi M, Murano T. Alpha-synuclein in the cerebrospinal fluid differentiates synucleinopathies (Parkinson Disease, dementia with Lewy bodies, multiple system atrophy) from Alzheimer disease. Alzheimer Dis Assoc Disord. 2012;26(3):213–216. doi: 10.1097/WAD.0b013e31823899cc. [DOI] [PubMed] [Google Scholar]
- Theodore S, Cao S, McLean PJ, Standaert DG. Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J Neuropathol Exp Neurol. 2008;67(12):1149–1158. doi: 10.1097/NEN.0b013e31818e5e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburne SK, Juurlink BH. Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem. 1996;67(3):1014–1022. doi: 10.1046/j.1471-4159.1996.67031014.x. [DOI] [PubMed] [Google Scholar]
- Tofaris GK, Razzaq A, Ghetti B, Lilley KS, Spillantini MG. Ubiquitination of alpha-synuclein in Lewy bodies is a pathological event not associated with impairment of proteasome function. J Biol Chem. 2003;278(45):44405–44411. doi: 10.1074/jbc.M308041200. [DOI] [PubMed] [Google Scholar]
- Trotta L, Guella I, Solda G, Sironi F, Tesei S, Canesi M, Pezzoli G, Goldwurm S, Duga S, Asselta R. SNCA and MAPT genes: Independent and joint effects in Parkinson disease in the Italian population. Parkinsonism Relat Disord. 2012;18(3):257–262. doi: 10.1016/j.parkreldis.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi K, Grzesiak JJ, Bouvet M, Hashimoto M, Masliah E, Shults CW. Alpha-synuclein overexpression in oligodendrocytic cells results in impaired adhesion to fibronectin and cell death. Mol Cell Neurosci. 2005;29(2):259–268. doi: 10.1016/j.mcn.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Tsuboi Y, Dickson DW. Dementia with Lewy bodies and Parkinson’s disease with dementia: are they different? Parkinsonism Relat Disord. 2005;11(Suppl 1):S47–51. doi: 10.1016/j.parkreldis.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T, Trojanowski JQ, Lee VM. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44(3):415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- Ubhi K, Lee PH, Adame A, Inglis C, Mante M, Rockenstein E, Stefanova N, Wenning GK, Masliah E. Mitochondrial inhibitor 3-nitroproprionic acid enhances oxidative modification of alpha-synuclein in a transgenic mouse model of multiple system atrophy. J Neurosci Res. 2009;87(12):2728–2739. doi: 10.1002/jnr.22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubhi K, Low P, Masliah E. Multiple system atrophy: a clinical and neuropathological perspective. Trends Neurosci. 2011;34(11):581–590. doi: 10.1016/j.tins.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubhi K, Rockenstein E, Mante M, Inglis C, Adame A, Patrick C, Whitney K, Masliah E. Neurodegeneration in a transgenic mouse model of multiple system atrophy is associated with altered expression of oligodendroglial-derived neurotrophic factors. J Neurosci. 2010;30(18):6236–6246. doi: 10.1523/JNEUROSCI.0567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky VN. Alpha-synuclein misfolding and neurodegenerative diseases. Curr Protein Pept Sci. 2008;9(5):507–540. doi: 10.2174/138920308785915218. [DOI] [PubMed] [Google Scholar]
- Valera E, Ubhi K, Mante M, Rockenstein E, Masliah E. Antidepressants reduce neuroinflammatory responses and astroglial alpha-synuclein accumulation in a transgenic mouse model of multiple system atrophy. Glia. 2014;62(2):317–337. doi: 10.1002/glia.22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum D, Hanisch UK. Microglia. Metab Brain Dis. 2004;19(3-4):393–411. doi: 10.1023/b:mebr.0000043984.73063.d8. [DOI] [PubMed] [Google Scholar]
- Vila M, Jackson-Lewis V, Guegan C, Wu DC, Teismann P, Choi DK, Tieu K, Przedborski S. The role of glial cells in Parkinson’s disease. Curr Opin Neurol. 2001;14(4):483–489. doi: 10.1097/00019052-200108000-00009. [DOI] [PubMed] [Google Scholar]
- Vilar M, Chou HT, Luhrs T, Maji SK, Riek-Loher D, Verel R, Manning G, Stahlberg H, Riek R. The fold of alpha-synuclein fibrils. Proc Natl Acad Sci U S A. 2008;105(25):8637–8642. doi: 10.1073/pnas.0712179105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Hayashi S, Kakita A, Yamada M, Toyoshima Y, Yoshimoto M, Takahashi H. Accumulation of alpha-synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta Neuropathol. 1998;96(5):445–452. doi: 10.1007/s004010050918. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Hayashi S, Yoshimoto M, Kudo H, Takahashi H. NACP/alpha-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson’s disease brains. Acta Neuropathol. 2000;99(1):14–20. doi: 10.1007/pl00007400. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shi M, Chung KA, Zabetian CP, Leverenz JB, Berg D, Srulijes K, Trojanowski JQ, Lee VM, Siderowf AD, Hurtig H, Litvan I, Schiess MC, Peskind ER, Masuda M, Hasegawa M, Lin X, Pan C, Galasko D, Goldstein DS, Jensen PH, Yang H, Cain KC, Zhang J. Phosphorylated alpha-synuclein in Parkinson’s disease. Sci Transl Med. 2012;4(121):121ra120. doi: 10.1126/scitranslmed.3002566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Saito Y, Terao S, Ando T, Kachi T, Mukai E, Aiba I, Abe Y, Tamakoshi A, Doyu M, Hirayama M, Sobue G. Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain. 2002;125(Pt 5):1070–1083. doi: 10.1093/brain/awf117. [DOI] [PubMed] [Google Scholar]
- Watts JC, Giles K, Oehler A, Middleton L, Dexter DT, Gentleman SM, DeArmond SJ, Prusiner SB. Transmission of multiple system atrophy prions to transgenic mice. Proc Natl Acad Sci U S A. 2013;110(48):19555–19560. doi: 10.1073/pnas.1318268110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster H, Astrom KE. Gliogenesis: historical perspectives, 1839-1985. Adv Anat Embryol Cell Biol. 2009;202:1–109. [PubMed] [Google Scholar]
- Wenning G, Quinn N. Are Lewy bodies non-specific epiphenomena of nigral damage? Mov Disord. 1994;9(3):378–379. doi: 10.1002/mds.870090327. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Colosimo C, Geser F, Poewe W. Multiple system atrophy. Lancet Neurol. 2004;3(2):93–103. doi: 10.1016/s1474-4422(03)00662-8. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Geser F, Krismer F, Seppi K, Duerr S, Boesch S, Kollensperger M, Goebel G, Pfeiffer KP, Barone P, Pellecchia MT, Quinn NP, Koukouni V, Fowler CJ, Schrag A, Mathias CJ, Giladi N, Gurevich T, Dupont E, Ostergaard K, Nilsson CF, Widner H, Oertel W, Eggert KM, Albanese A, del Sorbo F, Tolosa E, Cardozo A, Deuschl G, Hellriegel H, Klockgether T, Dodel R, Sampaio C, Coelho M, Djaldetti R, Melamed E, Gasser T, Kamm C, Meco G, Colosimo C, Rascol O, Meissner WG, Tison F, Poewe W, European Multiple System Atrophy Study, G. The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol. 2013;12(3):264–274. doi: 10.1016/S1474-4422(12)70327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning GK, Jellinger KA. The role of alpha-synuclein in the pathogenesis of multiple system atrophy. Acta Neuropathol. 2005;109(2):129–140. doi: 10.1007/s00401-004-0935-y. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Stefanova N. Recent developments in multiple system atrophy. J Neurol. 2009;256(11):1791–1808. doi: 10.1007/s00415-009-5173-8. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Stefanova N, Jellinger KA, Poewe W, Schlossmacher MG. Multiple system atrophy: a primary oligodendrogliopathy. Ann Neurol. 2008;64(3):239–246. doi: 10.1002/ana.21465. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Tison F, Ben Shlomo Y, Daniel SE, Quinn NP. Multiple system atrophy: a review of 203 pathologically proven cases. Mov Disord. 1997;12(2):133–147. doi: 10.1002/mds.870120203. [DOI] [PubMed] [Google Scholar]
- Wennstrom M, Surova Y, Hall S, Nilsson C, Minthon L, Bostrom F, Hansson O, Nielsen HM. Low CSF levels of both alpha-synuclein and the alpha-synuclein cleaving enzyme neurosin in patients with synucleinopathy. PLoS One. 2013;8(1):e53250. doi: 10.1371/journal.pone.0053250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, Ellisman MH, Pekny M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci U S A. 2006;103(46):17513–17518. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow AR, Chen CW, Corrochano S, Acevedo-Arozena A, Gordon DE, Peden AA, Lichtenberg M, Menzies FM, Ravikumar B, Imarisio S, Brown S, O’Kane CJ, Rubinsztein DC. alpha-Synuclein impairs macroautophagy: implications for Parkinson’s disease. J Cell Biol. 2010;190(6):1023–1037. doi: 10.1083/jcb.201003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xilouri M, Stefanis L. Autophagic pathways in Parkinson disease and related disorders. Expert Rev Mol Med. 2011;13:e8. doi: 10.1017/S1462399411001803. [DOI] [PubMed] [Google Scholar]
- Yamada T, McGeer PL, McGeer EG. Lewy bodies in Parkinson’s disease are recognized by antibodies to complement proteins. Acta Neuropathol. 1992;84(1):100–104. doi: 10.1007/BF00427222. [DOI] [PubMed] [Google Scholar]
- Yazawa I, Giasson BI, Sasaki R, Zhang B, Joyce S, Uryu K, Trojanowski JQ, Lee VM. Mouse model of multiple system atrophy alpha-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron. 2005;45(6):847–859. doi: 10.1016/j.neuron.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Yun JY, Lee WW, Lee JY, Kim HJ, Park SS, Jeon BS. SNCA variants and multiple system atrophy. Ann Neurol. 2010;67(4):554–555. doi: 10.1002/ana.21889. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55(2):164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19(6):533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44(4):601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]