Abstract
Multiple system atrophy (MSA) is a predominantly sporadic, adult-onset, fatal neurodegenerative disease of unknown etiology. MSA is characterized by autonomic failure, levodopa-unresponsive parkinsonism, cerebellar ataxia and pyramidal signs in any combination. MSA belongs to a group of neurodegenerative disorders termed α-synucleinopathies, which also include Parkinson’s disease and dementia with Lewy bodies. Their common pathological feature is the occurrence of abnormal α-synuclein positive inclusions in neurons or glial cells. In MSA, the main cell type presenting aggregates composed of α-synuclein are oligodendroglial cells. This pathological hallmark, also called glial cytoplasmic inclusions (GCIs), is associated with progressive and profound neuronal loss in various regions of the brain. The development of animal models of MSA is justified by the limited understanding of the mechanisms of neurodegeneration and GCIs formation, which is paralleled by a lack of therapeutic strategies. Two main types of rodent models have been generated to replicate different features of MSA neuropathology. On one hand, neurotoxin-based models have been produced to reproduce neuronal loss in substantia nigra pars compacta and striatum. On the other hand, transgenic mouse models with overexpression of α-synuclein in oligodendroglia have been used to reproduce GCIs-related pathology. This chapter gives an overview of the atypical Parkinson’s syndrome MSA and summarizes the currently available MSA animal models and their relevance for pre-clinical testing of disease-modifying therapies.
Keywords: Alpha-synuclein, Oligodendroglia, Striatonigral degeneration, Multiple system atrophy
1 Introduction to MSA
Graham and Oppenheimer first introduced the term multiple system atrophy (MSA) in 1969 (Graham and Oppenheimer 1969) to combine different clinicopathological disorders, including olivopontocerebellar atrophy (Déjérine and Thomas 1900), Shy Drager syndrome (Shy and Drager 1960), and striatonigral degeneration (SND) (Adams et al. 1964). MSA is now defined as a progressive neurodegenerative disorder which presents with autonomic failure, cerebellar ataxia, pyramidal signs, and parkinsonism in any combination (Wenning et al. 2004a). Due to the levodopa refractory Parkinsonism that is associated with different distinctive atypical features, MSA is categorized among atypical parkinsonian disorders (APD), including dementia with Lewy bodies (DLB), progressive supranuclear palsy (PSP), and corticobasal degeneration (CBD) (Wenning et al. 2011a, b). Disease onset is usually in the sixth decade with an annual worldwide incidence rate below 1 in 100,000. However, disease incidence increases to 3/100,000 in the population over 50 years (Schrag et al. 1999; Vanacore et al. 2001; Stefanova et al. 2009a). MSA patients have a poor prognosis compared to Parkinson’s disease (PD) patients. The mean survival rate ranges between 7 and 9 years following initial clinical presentation (Schrag et al. 2008). An early presentation of autonomic failure, as well as female gender, the parkinsonian variant of MSA, older age of onset, and shorter interval to reach clinical milestones (e.g., frequent falling, dysphagia, wheelchair dependency) predict shortened survival (Tada et al. 2007; O’Sullivan et al. 2008; Wenning et al. 2013).
MSA is clinically divided into a parkinsonian type (MSA-P) or a cerebellar type (MSA-C), which respectively relates to damage of either the basal ganglia (striatonigral degeneration, SND) or cerebellum (olivopontocerebellar atrophy, OPCA) (Ubhi et al. 2011). Parkinsonism is defined by bradykinesia, postural instability, rigidity, and tremor, whereas cerebellar dysfunction is associated with gait ataxia, ataxic dysarthria, limb ataxia, and sustained gaze evoked nystagmus (Gilman et al. 1998). Ethnic variations regarding the incidence of MSA-P and MSA-C were found in epidemiological studies of Europe, North America, and Japan. A pre-dominance of MSA-P was found for Europe and North America, where about 60 % of patients develop MSA-P (Gilman et al. 2005; Geser et al. 2006; May et al. 2007). However, in the Japanese population MSA-C is the more common MSA subtype with an incidence of about 83.8 % (Yabe et al. 2006). The cause of this variability remains unclear, but the involvement of environmental and/or genetic factors is suggested (Ubhi et al. 2011). In both forms of MSA the development of autonomic dysfunction, such as urogenital, gastrointestinal, and cardiovascular dysfunction, is common (Pfeiffer 2007; Ubhi et al. 2011). Furthermore, changes in behavior, including depression and executive dysfunction, may occur in MSA patients indicating an impairment of the frontal lobe (Fetoni et al. 1999; Dujardin et al. 2003; Benrud-Larson et al. 2005; Schrag et al. 2010).
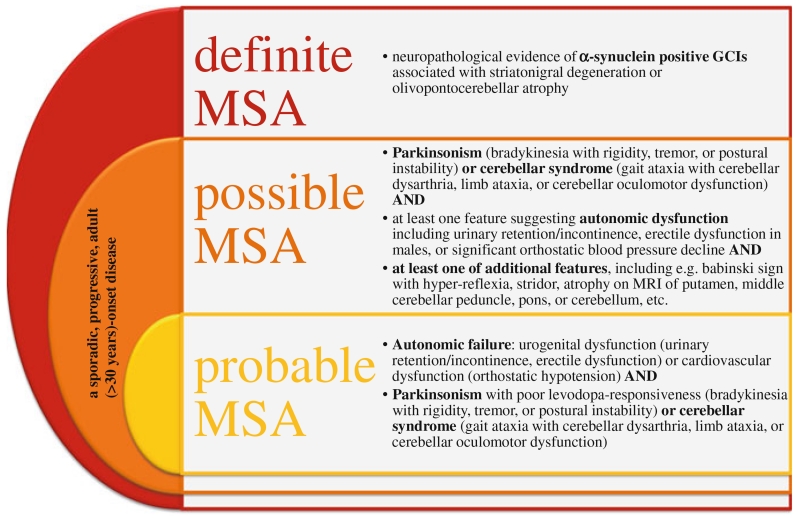
Clinical diagnosis of MSA is based on the consensus statement that specifies the clinical domains, features, and criteria to define three diagnostic categories of increasing certainty: possible, probable, and definite MSA (Gilman et al. 2008). Possible and probable MSA are diagnosed by means of specific clinical features (see Fig. 1), whereas a definite diagnosis of MSA depends on postmortem neuropathological evidence of glial cytoplasmic inclusions (GCIs) associated with SND or cerebellar ataxia (Trojanowski and Revesz 2007; Gilman et al. 2008). Furthermore, the unified MSA rating scale (UMSARS) was developed to help clinicians follow and assess disease progression in patients (Wenning et al. 2004b). Unfortunately, to date no treatment to stop the rapid disease progression exists. Only symptomatic treatment may be provided, however, treatment is moderately or poorly effective (Wenning et al. 2004a). A dopamine replacement therapy, i.e. levodopa, may be used to treat parkinsonism, yet only 28–65 % of pathological confirmed MSA cases presented with a positive response to levodopa treatment (Flabeau et al. 2010). Furthermore, in only 13 % of patients the effect of levodopa persists for some years and it may lead to pathological hypersexuality, the worsening of orthostatic hypotension, or early orofacial dyskinesias (Wenning et al. 1994; Klos et al. 2005). Other symptomatic treatments may be applied, including dopamine agonists (Wenning et al. 1994, 1997), amantadine (Wenning et al. 1997, 2005), and selective serotonin reuptake inhibitors (Friess et al. 2006) for the treatment of motor dysfunction, yet their efficiency is poor. Moreover, different treatments exist to alleviate autonomic symptoms, including, e.g., orthostatic hypotension, constipation, urinary retention, and breathing disorders [for review see (Flabeau et al. 2010)].
Fig. 1.
Consensus statement and criteria for the clinical diagnosis of MSA adapted from Gilman et al. (2008). Three different categories of increasing certainty were established to ease the diagnosis of MSA for clinicians. These include: possible, probable, and definite MSA which can be diagnosed by means of specific clinical features and postmortem neuropathological examination
In contrast to genetic studies of PD/DLB revealing SNCA gene duplications, triplications as well as pathogenic point mutations (Polymeropoulos et al. 1997; Singleton et al. 2003; Zarranz et al. 2004; Nishioka et al. 2006), genetic studies in MSA did not reveal a mutation in the entire coding region of the α-synuclein (AS) gene (SNCA) locus (Ozawa et al. 1999). However, other studies demonstrated that polymorphisms within the SNCA locus may correlate with the risk of developing MSA (Al-Chalabi et al. 2009; Scholz et al. 2009), strengthening the assumption that AS processing plays a major role in the MSA pathogenesis. Yet, another research group could not replicate these findings due to high variability in the control group and furthermore, in a genome-wide association study in 2012, polymorphisms within the SNCA gene locus could not be confirmed either (Yun et al. 2010; Sailer 2012). Some MSA pedigrees consistent with Mendelian disease have been described, but the identification of a single gene failed (Hara et al. 2007; Wullner et al. 2009). Polymorphisms in some genes involved in inflammatory processes (e.g., genes encoding interleukins one and eight) were proposed to be associated with enhanced risk to develop MSA (Nishimura et al. 2002; Combarros et al. 2003; Infante et al. 2005). Another attempt to identify a gene involved in MSA etiology was undertaken in a genome-wide association study resulting in no definite finding (Sailer 2012). In a very recent study mutations in the gene COQ2, encoding the parahydroxybenzoate-polyprenyl transferase which is essential for the biosynthesis of the coenzyme Q10, were identified in some familial and sporadic MSA cases. These mutations leading to functional impairment of COQ2 and therefore to an impairment of the mitochondrial respiratory chain and increased vulnerability to oxidative stress are suggested to be associated with an augmented risk of developing MSA (The Multiple-System Atrophy Research Collaboration 2013). These results lead to the assumption that genetic predisposition contributes to MSA etiology, but in most cases non-genetic factors may play a major role maybe in interaction with susceptibility genes such as mutations in the COQ2 gene (Jellinger 2012; The Multiple-System Atrophy Research Collaboration 2013).
Neuropathological examination of MSA brains reveals widespread neuronal loss in the striatum, substantia nigra pars compacta (SNpc), cerebellum, pons, inferior olives, and intermediolateral columns of the spinal cord (Stefanova et al. 2009a). Additionally, MSA brains exhibit prominent microglial and astroglial activation which may also play a role in the neurodegenerative process (Gerhard et al. 2003; Ishizawa et al. 2004; Ozawa et al. 2004). Moreover, the presence of argyrophilic filamentous GCIs in oligodendroglial cells throughout the brain is a major hallmark of MSA and was first described by Papp and colleagues (Papp et al. 1989). GCIs were found in pons, medulla, putamen, substantia nigra, cerebellum, and preganglionic autonomic structures (Papp and Lantos 1994; Beyer and Ariza 2007; Jellinger and Lantos 2010). The predominantly neuronal presynaptic protein (AS) was identified as major component of GCIs in 1998 (Spillantini et al. 1998; Wakabayashi et al. 1998). Therefore, the presence of AS-positive inclusions links MSA to PD and DLB, classifying them into the category of α-synucleinopathies (ASP). However, PD and DLB are identified as neuronal ASP due to AS-inclusions (Lewy bodies and Lewy neurites) occurring predominantly in neurons, whereas MSA is conceptualized as a primary oligodendrogliopathy or oligodendroglial ASP based on the presence of AS-positive aggregations mainly in oligodendroglial cells (Wenning et al. 2008; Fellner and Stefanova 2012). In addition to the aggregations in oligodendroglial cells, MSA brains feature neuronal and astroglial cytoplasmic AS-positive inclusions although in a decreased density (Wenning and Jellinger 2005).
In 2005, Jellinger and colleagues proposed a grading scale of MSA neuropathology by taking into account semiquantitative analyses of brain atrophy, neuronal loss, astrogliosis, and GCI pathology in different brain regions (Jellinger et al. 2005). Correlation of GCIs density in MSA brains with the disease duration and the degree of neuronal loss was established, thereby supporting the putative crucial role of oligodendroglial pathology in MSA (Ozawa et al. 2004). Interestingly, it was also found that the AS load is increased in MSA brains compared to PD and DLB (Tong et al. 2010).
GCIs present with different shapes, such as oval, sickle-shaped, or conical (Wenning and Jellinger 2005). Furthermore, GCIs were found to be immunoreactive to different other constituents in addition to the main component AS. These include tau, tubulin, ubiquitin, αB-crystallin, leucin-rich repeat serine/threonine-protein LRRK2, heat shock proteins, and prion disease-linked 14-3-3 protein among others (Wenning et al. 2008). The formation of AS-positive inclusions in oligodendroglial cells in MSA has not been fully elucidated yet. Two hypotheses on the formation of AS-positive inclusions exist: (1) the active uptake of AS by oligodendroglia or (2) selective upregulation of AS expression and slow degradation of AS in oligodendroglial cells (Fellner et al. 2011). Different aspects of recent research favor the first hypothesis, including that no AS mRNA expression has been detected in oligodendroglial cells of human control and MSA brains (Ozawa et al. 2001; Miller et al. 2005). Moreover, recent data provide evidence that cell-to-cell propagation of AS may be the mechanism of AS aggregation in ASP (Desplats et al. 2009; Lee et al. 2010; Luk et al. 2012). In different experiments, release of AS into the extracellular space (Emmanouilidou et al. 2010) and furthermore the uptake of AS into neurons and astroglial cells in vivo and in vitro were demonstrated (Desplats et al. 2009; Luk et al. 2009, 2012; Lee et al. 2010; Hansen et al. 2011; Fellner et al. 2013). However, transmission of AS to oligodendroglial cells has not been proven in any in vivo graft experiment to date (Stefanova et al. 2009b; Hansen et al. 2011). In recent experiments the uptake of AS by oligodendroglial cell lines and primary rat oligodendroglia in a time- and concentration-dependent manner was suggested (Kisos et al. 2012; Konno et al. 2012). Yet, further research is needed to elucidate the formation of AS-positive inclusion in oligodendroglial cells in the brains of MSA patients. Importantly, a recent study by the group of Stanley Prusiner (Watts et al. 2013) experimentally demonstrated that AS aggregates present in MSA brains are transmissible and may induce lethal disease in transgenic mice.
Although different pathomechanisms in MSA have been suggested, no effective treatment to stop neurodegeneration has been found to date. Symptomatic treatment may be provided to alleviate symptoms, but it remains moderately or poorly effective (Wenning et al. 2004a; Low and Singer 2008; Kollensperger et al. 2010). The lack of treatment as well as the limited understanding of the pathobiological mechanisms called for a development of animal models of MSA. Many of these models were used as pre-clinical test beds for new therapeutic approaches or to explore different pathogenic processes.
The use of neurotoxins was one of the first approaches to mimic the disease pathology especially in rodents, but also in nonhuman primates. The use of stereotaxic or systemic injections of neurotoxins leads to a combined deterioration of the nigrostriatal system mimicking L-DOPA unresponsive Parkinsonism. In addition to the neurotoxic models, transgenic models were developed to create the core pathology of MSA, oligodendroglial AS-positive inclusions respectively. These genetic models are interesting tools to investigate the underlying mechanisms of neurodegeneration caused by abnormal oligodendroglial aggregation of AS and secondary neuronal dysfunction.
2 First Steps: The Replication of SND
Neurotoxin models were established to generate SND, the key neuropathology of MSA-P. Based on the knowledge of PD and Huntington’s disease modeling through selective toxins, nigra and striatal toxins in combinations were used to create double-lesion SND/MSA-P models (Stefanova et al. 2005b). These double-lesion models reproduced L-DOPA unresponsive Parkinsonism typical for MSA-P. Two types of neurotoxin approaches can be distinguished: stereotaxic and systemic models.
The stereotaxic method induces a simultaneous or sequential unilateral degeneration of the SNpc and striatum and produces a dopamine unresponsive motor phenotype (Stefanova et al. 2005b; Fernagut and Tison 2012). The most widely used unilateral model applies 6-hydroxydopamine (6-OHDA) injected into the medial forebrain bundle (MFB) followed by quinolinic acid (QA) administrated into the striatum ipsilaterally (Wenning et al. 1996). This double lesion model presents with nigra and striatal neuronal loss, astrogliosis, microglial activation, and impaired motor behavior including amphetamine-induced ipsilateral rotations (but no apomorphine-induced rotation), severe impairment in paw-reaching and stepping tasks, side falling, and reduced overall activity in open field tests (Wenning et al. 1996; Scherfler et al. 2000, 2005; Stefanova et al. 2004b; Mantoan et al. 2005). The motor impairment does not respond to pulsatile L-DOPA administration, which can however induce dyskinetic behaviors (Stefanova et al. 2004a). It was found that the effect of the neurotoxins depended on the sequence of neurotoxin injections. The injection of 6-OHDA into the MFB prior to striatal lesion with QA weakened the neurotoxic effects of QA to the striatum as compared to animals with primary QA lesions (Scherfler et al. 2000). This well-characterized unilateral sequential double-toxin double-lesion rat model has been especially useful to test the role of neurotransplantation in SND.
A unilateral simultaneous injection of QA and 6-OHDA into the striatum was proposed to overcome the decreased vulnerability of striatal neurons due to preceding dopamine depletion (Ghorayeb et al. 2001). This strategy resulted in significant striatal degeneration due to QA, whereas the 6-OHDA-induced neuronal loss in the nigra region was less marked compared to 6-OHDA injection into the striatum alone suggesting that QA partly prevented retrograde dopaminergic denervation. In this simultaneous model astrogliosis was found, and behavioral analyses revealed reduced ipsilateral amphetamine-induced rotational behavior, attenuated contralateral apomorphine-induced rotational behavior compared to primary 6-OHDA or QA lesions, and severe impairment of paw reaching. The simultaneous strategy was suggested to serve as a model of mild, early SND that might qualify for early therapeutic strategies (Ghorayeb et al. 2001). A recent approach to mimic early MSA-P pathology applied a sequential striatal double-lesion approach in rats, whereby a partial 6-OHDA striatal lesion was followed by a QA injection into the striatum. The model presented severe nigral and moderate striatal degeneration combined with robust motor deficits (Kaindlstorfer et al. 2012).
Another strategy to model SND was the development of a unilateral “single toxin–double lesion” SND model to overcome the protection of striatal neurons as a result of a prior nigral lesion. For the generation of the lesions either the succinate dehydrogenase inhibitor 3-nitropropionic acid (3-NP) (Waldner et al. 2001) or the mitochondrial complex I inhibitor 1-methyl-4-phenylpyridinium ion (MPP+) (Ghorayeb et al. 2002a) were used. Both toxins when applied stereotaxically in the striatum cause neuronal loss in SNpc, extensive degeneration in the striatum, and astrogliosis. Moreover, motor impairment was also described for these stereotaxic models, including drug-induced rotation and severe deficits in paw reaching (Waldner et al. 2001; Ghorayeb et al. 2002a). In addition, MPP+ treated animals developed major motor deficits in side falling and thigmotactic scanning (Ghorayeb et al. 2002a).
The development of different degrees of nigral and/or striatal neuronal loss to model various stages of SND represents an important advantage of the stereotaxic rat models. Furthermore, they are an interesting tool to investigate various therapeutic strategies, including the evaluation of embryonic grafts as a possible therapy for MSA-P patients with the goal to achieve regeneration of L-DOPA responsiveness (Stefanova et al. 2005b). However, the stereotaxic rat models do not reproduce the pathological hallmark of MSA, namely AS-positive oligodendroglial inclusions, which is a major limitation of these models.
In addition to the unilateral stereotaxic models, systemic models were developed to mimic bilateral SND common for the human disease. The sub-chronic or chronic intoxication with systemic toxins induces a progressive neuronal dysfunction and temporal neurodegeneration similar to the development of neuronal deficits in MSA patients. SND in nonhuman primates (Macaca fascicularis) was modeled using intraperitoneal injections of the neurotoxin 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridin (MPTP) and 3-NP (Ghorayeb et al. 2000, 2002b). Thereby, MPTP treatment of monkeys was followed by administration of 3-NP inducing Parkinsonism with poor L-DOPA response, hind limb dystonia, as well as SND (Ghorayeb et al. 2000, 2002b). Systemic application of 3-NP in mice led to striatal neurodegeneration and mild loss of dopaminergic neurons associated with an impaired motor phenotype such as hind limb dystonia and clasping (Fernagut et al. 2002). Furthermore, the sequential administration of MPTP and 3-NP (MPTP prior to 3-NP and vice versa) in mice resulted in significant SND, astrogliosis and locomotor deficits. MPTP followed by 3-NP treatment decreased striatal damage, while prior 3-NP administration reduced MPTP-induced nigral degeneration (Stefanova et al. 2003). In a next step, a bilateral, simultaneous double-toxin double-lesion mouse model was developed to decrease the possibility of reduced neuronal vulnerability due to the sequential administration of MPTP and 3-NP. Thereby, the combined administration of MPTP and 3-NP induced striatal and nigral neuronal loss, as well as astrogliosis. The treated animals also showed disturbed balance, altered gait pattern, hindlimb and truncal dystonia, and severe motor symptoms including impairment in rotarod test, pole test, and traversing a beam (Fernagut et al. 2004; Diguet et al. 2005). Similar to the stereotaxic models, systemic neurotoxin models display different degrees of SND. Yet, they fail to reproduce the oligodendroglial inclusion pathology of MSA brains.
3 Reproducing the Specific Oligodendroglial AS Pathology of MSA in Transgenic Mice
As mentioned above, a disadvantage of the neurotoxin models is the lack of the core AS pathology of MSA (GCIs) which may play a fundamental role in the pathogenic mechanisms leading to neurodegeneration. Furthermore, these toxin models cannot reproduce various other cardinal features of MSA, including autonomic and cerebellar dysfunction. The finding that AS and its aggregation in oligodendroglia are strongly involved in the pathology and the categorization of MSA into the group of ASP increases the necessity to investigate the AS-dependent pathophysiological mechanisms of the disorder. Therefore, the generation of a transgenic mouse model characterized by the overexpression of human wild-type AS under a specific oligodendroglial promoter has been a major step forward to the elucidation of the pathogenic mechanisms linked to the AS aggregation in oligodendroglial cells.
The first transgenic MSA mouse model was introduced in 2002 by Kahle and colleagues (Kahle et al. 2002). Targeted AS overexpression in oligodendroglial cells was achieved using the proteolipid-protein (PLP) promoter. Hyperphosphorylation at serine 129 and insolubility of AS were found in this transgenic mouse, similar to the human disease (Kahle et al. 2002). Moderate dopaminergic neuronal loss in SNpc of the PLP-AS mice was associated with a reduced stride length in aged transgenic mice (Stefanova et al. 2005a). Furthermore, the PLP-AS overexpressing mouse model presented with microglial activation (Stefanova et al. 2007) similar to the human disease (Gerhard et al. 2003). In addition to the progressive motor phenotype the PLP-AS mouse model showed also features of autonomic dysfunction: (1) cardiovascular autonomic dysfunction was linked to degeneration in brainstem nuclei involved in autonomic control (Stemberger et al. 2010; Kuzdas et al. 2013), and (2) bladder dysfunction was linked to neuronal loss in the pontine micturition center as well as loss of parasympathetic preganglionic neurons in the intermediolateral columns and loss of motor neurons in the Onuf’s nucleus of the spinal cord (Boudes et al. 2013).
Another approach to generate oligodendroglial AS-overexpressing transgenic mice involved the application of the 2’,3’-cyclic nucleotide 3’-phosphodiesterase (CNP) promoter (Yazawa et al. 2005). The expression of AS in oligodendroglial cells led to axonal degeneration, brain atrophy, accumulation of endogenous mouse AS in axons and axon terminals predominantly in the spinal cord, astrogliosis, as well as neuronal and oligodendroglial loss in the spinal cord. Motor impairment was measured by a progressive reduction of rotarod performance (Yazawa et al. 2005). Moreover, in this model endogenous AS accumulation in neurons was dependent on the microtubule β-III tubulin protein and interfering with the synaptic vesicle release in GABAergic interneurons (Nakayama et al. 2009, 2012; Ito et al. 2012). Yet, the CNP-AS transgenic model could not replicate the selective neuropathology of MSA.
Overexpression of AS under the specific oligodendroglial promoter myelin basic protein (MBP) in mice was introduced by Shults and colleagues (Shults et al. 2005). The transgenic mice developed widespread insoluble human AS positive inclusion pathology characterized by phosphorylation of AS at serine 129. Furthermore, the MBP transgenic mice featured significant loss of dopaminergic terminals in striatum associated with an impaired motor phenotype as measured by rotarod and pole test (Shults et al. 2005). Reduced levels of several neurotrophic factors were found in the MBP-AS mouse model but not in neuronal overexpressors of AS, suggesting that AS expression in oligodendroglia might impact neuronal trophic support inducing MSA-like neurodegeneration (Ubhi et al. 2010).
MSA transgenic models recapitulate the hallmark pathology of the human disease, namely AS-positive inclusions in oligodendroglial cells and also other features of MSA, such as microglial activation and autonomic failure. Therefore, these mouse models are highly useful for studies on pathogenic mechanisms of neurodegeneration in MSA, as well as for testing candidate therapeutic interventions.
4 Getting Insights into the Pathogenic Pathways of MSA Through the Application of Animal Models
Mitochondrial dysfunction and MSA
As mentioned before, most likely both genetic and environmental factors contribute to the etiopathogenesis of MSA. The first attempt to combine genetic predisposition and environmental risk factors in a mouse model was achieved in 2005 providing an animal model presenting a full-blown MSA pathology (Stefanova et al. 2005a). The transgenic mouse overexpressing AS in oligodendroglia was exposed to chronic oxidative stress [mitochondrial inhibition relevant to the recently found COQ2 mutation in MSA cases (The Multiple-System Atrophy Research Collaboration 2013)] induced by intraperitoneal injections of 3-NP. These transgenic mice treated with 3-NP presented with widespread AS inclusion pathology in oligodendroglia associated with SND and olivopontocerebellar atrophy similar to the human disorder. In wild-type control mice treated with 3-NP only mild SND was found, but no OPCA, suggesting a major involvement of AS pathology in MSA-like neurodegeneration. Furthermore, mitochondrial inhibition induced profound astrogliosis and microgliosis in the brains of transgenic mice. The animals also presented with augmented motor and behavioral deficits as demonstrated by hindlimb and trunk dystonia, as well as decreased horizontal and vertical locomotor activity, impaired pole test performance, and shortened stride length (Stefanova et al. 2005a, b). A similar approach was conducted in the mouse overexpressing human AS under the MBP promoter. Systemic 3-NP application augmented neurodegeneration and motor deficits and moreover, altered levels of oxidized and nitrated AS were found in this combined model (Ubhi et al. 2009).
Proteolytic failure and MSA
Recently, an involvement of the ubiquitin–proteasome system in the pathology of MSA was tested experimentally in the MSA transgenic mouse overexpressing AS under the PLP promoter (Stefanova et al. 2012b). The ubiquitin–proteasome system is responsible for the degradation of unneeded or damaged proteins. Alterations in the ubiquitin–proteasome system may contribute to the formation of AS aggregations in ASP (Tofaris et al. 2003; Ebrahimi-Fakhari et al. 2011). Proteolytic failure induced by systemic proteasome inhibition (PSI) in transgenic MSA mice triggered impaired open field motor behavior associated to progressive SND and olivopontocerebellar neuronal degeneration. In contrast, PSI administration did not induce neurodegeneration or behavioral alterations in wild-type mice. Moreover, an increase of fibrillar human AS in the cytoplasm of oligodendroglia was found to lead to myelin disruption and demyelination in the PSI transgenic mice. Oligodendroglial dysfunction was followed by axonal degeneration (Stefanova et al. 2012b). These new data support the hypothesis that impaired protein degradation may play a major role in MSA pathology suggesting that a failure of the proteolytic system in the presence of oligodendroglial AS may induce MSA-like neurodegeneration.
Microglial activation and MSA
Increasing evidence suggests a role of microglial activation linked to AS pathology in the pathogenesis of MSA (Gerhard et al. 2003; Ishizawa et al. 2004; Fellner et al. 2013). Moreover, early progressive microglial activation was found to be associated with dopaminergic neuronal loss (Stefanova et al. 2007). Toll-like receptors (TLRs) are primarily expressed on cells of the innate immune system including microglia and they are important for the identification of conserved structural motifs on a wide array of pathogens (pathogen-associated molecular patterns) and for the recognition of endogenous molecules, including AS (Akira 2001). An upregulation of TLR4 was found in MSA brains and also MSA transgenic mice suggesting an involvement of TLR4 in the MSA pathogenesis (Stefanova et al. 2007). To identify the role of TLR4 in MSA-like ASP the PLP-AS overexpressing mouse model was crossbred with TLR4-deficient mice (Stefanova et al. 2011). TLR4 deficiency in MSA transgenic mice led to increased AS levels in the brains associated with augmented motor disability and enhanced loss of nigrostriatal dopaminergic neurons. Moreover, it was shown that enhanced AS levels were linked to disturbed microglial phagocytosis of AS mediated by TLR4 and to augmented tumor necrosis factor α (TNFα) release by astroglial cells (Stefanova et al. 2011; Fellner et al. 2013). These data reveal the importance of TLR4 in the clearance of AS by microglial cells and suggest that the upregulation of TLR4 may act as innate neuroprotective mechanism in MSA and other ASP (Letiembre et al. 2009).
In summary, transgenic MSA models emphasize the relevance of abnormal AS accumulation in oligodendroglial cells as a major key player in the pathogenesis of MSA. Moreover, different pathogenetic pathways, including mitochondrial dysfunction, impaired protein degradation, and microglial activation, are identified to be involved in disease mechanisms.
5 Screening Novel Therapeutic Interventions in MSA Animal Models
In light of the limited understanding of the pathomechanisms of MSA and the lack of effective treatments to slow down the rapid progression of the disease, the different animal models provide an invaluable tool to address these issues in pre-clinical conditions. Thereby, cell- and drug-based therapeutic approaches can be distinguished. Some of those therapeutic interventions were found to improve behavior and reduce neuronal loss in the described MSA animal models. Therefore, the successful treatments were transferred into clinical trials to test their efficacy in MSA patients.
5.1 Cell-Based Therapeutic Approaches
Neurorestorative approaches by transplantation of fetal allografts into the striatum aim to counterfeit the loss of dopaminergic neurons and to restore the responsiveness to L-DOPA. Especially, double-lesion animal models have been beneficial to evaluate the functionality of embryonic grafts as a probable therapeutic strategy for MSA-P patients (Stefanova et al. 2005b). These studies suggest striatal transplantation as possible therapeutic option for MSA-P patients to restore the lacking L-DOPA response and thus improve the symptomatic treatment of the motor symptoms (Wenning et al. 1996; Puschban et al. 2005; Kollensperger et al. 2009). However, the transplantation of a striatal allograft into the PLP-AS overexpressing mouse model of MSA intoxicated with 3-NP presented with reduced dopaminergic re-innervation and p-zone volume suggesting that the presence of MSA-like AS oligodendrogliopathy compromises the neurorestorative outcome of the graft (Stefanova et al. 2009b). The variable outcomes of different studies using embryonic grafts for striatal transplantation in MSA may be due to the different experimental settings and rodent models used. The neurorestorative potential of embryonic neuronal allografts remains therefore unclear. A standardized protocol and an increased number of experimental studies may help to evaluate the beneficial effects of neurorestorative approaches in MSA.
Furthermore, intravenous infusion of mesenchymal stem cells (MSCs) was tested as a therapeutic strategy in MSA rodent models. In a mouse model of double-toxin (MPTP and 3-NP) - induced MSA, human MSCs induced neuronal protection in SN and striatum associated with behavioral improvements (Park et al. 2011). Park and colleagues proposed that a great number of human MSCs invade the CNS and may exert neuroprotection by modulation of inflammation, cell survival and cell death signaling-pathways (Park et al. 2011). In a different study, intravenous infusion of mouse MSCs in a MSA transgenic mouse model resulted in neuroprotection in SNpc and immunomodulatory effects in the brain although no MSC invasion was found. Furthermore, no behavioral improvement was detected (Stemberger et al. 2011). In patients with MSA-C the intraarterial and intravenous injection of autologous MSCs slowed transiently the disease progression (Lee et al. 2008, 2012), thus suggesting MSCs as a potential therapy for MSA. Due to the fact that the study conducted in 2008 was an open-label trial and therefore earned critics on the strength of the clinical evidence, Lee and colleagues performed a randomized double-blind MSC study in 2012 to confirm the positive outcome from 2008 (Lee et al. 2008, 2012) (Table 1). However, further studies have to be performed to identify the mechanisms underlying these effects and to replicate the results of Lee and colleagues in a different population cohort or in MSA-P patients.
Table 1.
List of drugs and treatments tested in pre-clinical and clinical trials
| Drug/Treatment | Pre-clinical studies | Clinical trial |
|---|---|---|
| Minocycline | Toxin MSA model (Stefanova et al. 2004b) anti-inflammatory, no neuroprotection Transgenic MSA model (Stefanova et al. 2007) anti-inflammatory, neuroprotection by early application |
MEMSA-trial (Dodel et al. 2010) anti-inflammatory, no change in progression |
| Riluzole | Toxin model (Diguet et al. 2005; Scherfler et al. 2005) partial striatal neuroprotection, no motor improvement | NNIPPS study (Bensimon et al. 2009) no neuroprotection |
| Rifampicin | Transgenic MSA model (Ubhi et al. 2008) protective | RDCRC (clinicaltrials.gov NCT01287221) no neuroprotection |
| Rasagiline | Transgenic MSA model (Stefanova et al. 2008) protective | Multi-center study (Poewe et al. 2012) no change in progression |
| Fluoxetine | Transgenic MSA model (Ubhi et al. 2012) protective | French clinical trial (clinicaltrial.gov NCT01146548) negative outcome |
| MSCs | Toxin MSA model (Park et al. 2011) Transgenic MSA model (Stemberger et al. 2011) protective, immunomodulatory |
South Korean clinical trial (Lee et al. 2008, 2012) delayed progression |
Summary of putative neuroprotective and neuroimmunomodulatory treatments that were tested in pre-clinical studies. Due to the positive outcome in pre-clinical studies, clinical trials were conducted with the listed promising target drugs, yet the positive results gained in animal studies could not be replicated in MSA patients. Only the Korean study using mesenchymal stem cells (MSCs) could replicate the positive outcome of the pre-clinical trials. MEMSA-trial, Minocycline European Multiple System Atrophy-trial; NNIPPS, Neuroprotection and Natural History in Parkinson Plus Syndromes; RDCRC, Rare Diseases Clinical Research Consortia
5.2 Neuroprotective Strategies
The accumulation of AS has been identified as a critical step in the pathogenesis of MSA (Wenning et al. 2008; Stefanova et al. 2009a; Jellinger and Lantos 2010). Yet, the exact mechanisms leading to progressive neurodegeneration in MSA still need to be elucidated. Different aspects, linked to the neuronal loss in MSA have to be taken into account, including the toxicity of AS, AS accumulation in oligodendroglial cells as well as oligodendroglial dysfunction, oxidative stress and neuroinflammatory processes (e.g., microgliosis, astrogliosis). The development of transgenic animals overexpressing AS in oligodendroglial cells allows the screening for candidate drugs before introducing them in clinical trials (Flabeau et al. 2010). Currently several drugs have completed both pre-clinical and clinical testing as listed below (and in Table 1). In spite of the negative clinical outcomes till date, these translational studies have been of importance to identify translational pitfalls and improve the design of the pre-clinical experiments with relevance to the clinics.
Minocycline is a tetracycline antibiotic with anti-inflammatory and anti-apoptotic properties that successfully crosses the blood–brain barrier (BBB) (Wang et al. 2003). Minocycline significantly decreased glial activation, but no neuroprotective effects were observed in a double-lesion rat model of SND (Stefanova et al. 2004b). However, early long-term treatment of PLP-AS transgenic mice with minocycline revealed protection of dopaminergic nigral neurons linked to decreased microglial activation (Stefanova et al. 2007). Parallel to the animal studies a randomized, double blind clinical trial with minocycline in MSA patients was performed. Similar to the animal experiments a significant reduction of microglial activation upon minocycline treatment was found as shown by [11C](R)-PK11195 PET, but no clinical effect on symptom severity was observed, probably due to the late start of the treatment in already advanced MSA cases (Dodel et al. 2010). Minocycline seems to be a promising agent to stop microglial inflammatory processes early in the disease progression leading to a potential rescue of dopaminergic neurons. However, late diagnosis of MSA is a pitfall for this kind of treatment. Another anti-inflammatory approach is the inhibition of the myeoloperoxidase (MPO), an enzyme involved in production of ROS by phagocytic cells, including microglia (Reynolds et al. 1999). An involvement of MPO in the pathogenesis of neurodegenerative diseases, such as PD and HD, was suggested recently (Choi et al. 2005). In the combined PLP-AS + 3-NP MSA mouse model the early inhibition of MPO, using the MPO-inhibitor 1-(2-Isopropoxyethyl)-2-thioxo-1,2,3,5-tetrahydropyrrolo[3,2-d]pyrimidin-4-one, ameliorated motor deficits which were associated to neuroprotection in striatum and SNpc, cerebellar cortex, pontine nuclei, and inferior olives (Stefanova et al. 2012a). It remains to be identified whether late-start therapy with MPOi may have the same protective potency.
The inhibition of AS aggregation is considered a candidate therapeutic approach relevant for the treatment of MSA. Rifampicin is an antibiotic routinely used for the treatment of tuberculosis and leprosy, which has shown propensity to lower AS fibrillization in vitro (Li et al. 2004). Rifampicin was tested on its ability to reduce AS aggregation and to act neuroprotective in a MBP-AS transgenic MSA mouse model (Li et al. 2004; Ubhi et al. 2008). Treatment with rifampicin lowered the aggregation of AS in the mouse brains resulting in reduced neuronal loss and suppressed astroglial activation (Ubhi et al. 2008). The promising effects of rifampicin were assessed in a clinical trial that started in 2011. The study has been completed; however the drug failed to demonstrate improvement or the tendency of improvement in MSA (clinicaltrials.gov NCT01287221; Philip Low, http://www.msaawareness.org). The ability to reduce AS aggregations by the antibiotic rifampicin is an interesting approach as especially AS fibrils are thought to be very toxic and increase microglial and astroglial activation (Lee et al. 2010; Fellner et al. 2013). The positive result of rifampicin in pre-clinical testing may be due to the high drug dose used which was too high for clinical use. For the translation of animal studies to clinical trials the drug dose given to the animals should relate to a clinical relevant dose.
The development of vaccines against AS aggregation is another very prospective approach to reduce AS levels and to slow down neurodegeneration in ASP. Active and passive immunization approaches targeting AS revealed attenuated AS accumulation and neurodegeneration in a mouse model of PD with LB pathology (Masliah et al. 2005, 2011). Furthermore, these results led to the first clinical immunization against AS in a PD cohort which started in 2012 (Schneeberger et al. 2010, 2012). Immunization studies targeting AS may also be an interesting target to slow down the progression of MSA.
Another therapeutic intervention tested both in pre-clinical and clinical MSA studies is the use of rasagiline which is a selective irreversible monoamine oxidase-B (MAO-B) inhibitor with certain anti-apoptotic and neurotrophic activity (Youdim et al. 2003; Bar-Am et al. 2004; Blandini et al. 2004; Eliash et al. 2005). In the PLP-AS transgenic mouse model intoxicated with 3-NP, rasagiline improved motor deficits as shown by pole test and stride length test associated with significant neuroprotection in various brain regions, such as striatum, SNpc, cerebellar cortex, pontine nuclei, and inferior olives (Stefanova et al. 2008). This drug entered a randomized, double-blind, placebo-controlled clinical trial in 2009. The study was completed in 2012, yet rasagiline had no effect on symptom severity as measured by using the UMSARS rating scale (Poewe et al. 2012). Similar to rifampicin, a higher dose of rasagiline was administered in the mouse study compared to the clinical trial. This might provide one explanation for the negative outcome of the clinical study. For further pre-clinical studies, the usage of a relevant drug dose should be considered.
The investigation of neuroprotective effects of riluzole was performed in a double-lesion rat model of SND. Riluzole is an anti-glutamatergic agent which is used for the treatment of amyotrophic lateral sclerosis (ALS) thereby prolonging survival (Bensimon et al. 1994; Lacomblez et al. 1996). Furthermore, riluzole was found to have a neuroprotective potential by the direct inhibition of protein kinase C (Koh et al. 1999; Noh et al. 2000; Obinu et al. 2002; Cheah et al. 2010; Carbone et al. 2012). In the double-lesion rat model of SND, riluzole treatment improved motor deficits and partially protected striatum (Scherfler et al. 2005). A similar result was achieved in a bilateral, simultaneous double-toxin double-lesion mouse model (using systemic intoxication with 3-NP and MPTP) treated with riluzole. Moderate behavioral improvements and neuroprotection were described in this mouse model (Diguet et al. 2005). Riluzole was also tested in a double-blind randomized placebo-controlled trial. However, no significant effect on survival or rate of functional deterioration in MSA was described (Bensimon et al. 2009). The achievement of a partial protection of the striatum is an interesting outcome in these animal studies; however, a partial pre-clinical protection proves not enough for the translation of a therapy into a clinical study.
Another interventional drug in the field of neurodegeneration is the use of fluoxetine. Fluoxetine is a selective serotonin reuptake inhibitor (SSRI) and commonly used as antidepressant (Ubhi et al. 2012). Moreover, fluoxetine is known for its influence on levels of neurotrophic factors such as glial-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF) (Mercier et al. 2004; Chang et al. 2010; Allaman et al. 2011) and for its pro-proliferative activity (Chang et al. 2010; Wang et al. 2011). The drug was also reported to protect against neuronal toxin-induced damage in 6-OHDA and MPTP models of PD (Suzuki et al. 2010; Chung et al. 2011). The effects of the target drug fluoxetine on neuronal loss and behavior was investigated in the MBP-AS transgenic mouse model lately. The administration of fluoxetine by gavage revealed neuroprotective effects in the basal ganglia, neocortex and hippocampus of these mice. Moreover, reduced behavioral deficits were observed (Ubhi et al. 2012). In parallel a clinical study on fluoxetine in MSA was conducted (see clinicaltrials.gov NCT01146548). However, the results have been negative (unpublished).
Various different neuroprotective therapies have been developed in recent years. Testing of the different drug candidates in pre-clinical test beds revealed promising effects, especially regarding neuroprotection. The positive effects on neuroprotection in animal models of MSA also improved the behavioral deficits common in these rodent models. Yet, none of the therapeutic drug approaches tested in clinical trials brought the desired effect in MSA patients. One reason that some of the clinical trials failed might be due to the inadequate drug dose used in pre-clinical studies compared to the drug dose applicable and used in clinical trials. Another reason might be that especially the neuroprotective or neuroimmunomodulatory treatments should be given in an early phase of the disease, yet MSA is usually diagnosed rather late when the progression and therefore neurodegeneration of the disease are advanced. Thus, the diagnosis of MSA has to be improved with regard to sensitivity and specificity. Furthermore, no specific cerebrospinal fluid or blood biomarkers exist to assess the effect of different neuroprotective drugs in clinical trials. However, these translational pitfalls may be helpful to develop pre-clinical study designs that are more relevant for clinical studies. An extended search for an effective therapy to slow the progression of MSA will be a challenge for researchers in the next years.
6 Conclusion
In the last few years, considerable progress has been made to extend the knowledge regarding the pathogenesis of MSA. The different animal models mimicking MSA substantially expedited the understanding of the mechanisms on the pathology and progression of this neurodegenerative disorder. Furthermore, animal models of MSA allowed the testing of numerous different promising therapeutic compounds. Without the results of these studies the application of these drugs in clinical trials would not have been possible. However, the outcome of most disease-modifying therapeutic strategies did not meet the expectations of researchers, clinicians, and patients. Therefore, increased effort investigating different therapeutic interventions has to be accomplished to increase the survival of MSA patients and the quality of life.
Acknowledgments
This work was supported by grants of the Austrian Science Fund (FWF) P25161 and F4404.
References
- Adams RD, Vanbogaert L, Vandereecken H. Striato-nigral degeneration. J Neuropathol Exp Neurol. 1964;23:584–608. [PubMed] [Google Scholar]
- Akira S. Toll-like receptors and innate immunity. Adv Immunol. 2001;78:1–56. doi: 10.1016/s0065-2776(01)78001-7. [DOI] [PubMed] [Google Scholar]
- Al-Chalabi A, Durr A, Wood NW, Parkinson MH, Camuzat A, Hulot JS, Morrison KE, Renton A, Sussmuth SD, Landwehrmeyer BG, Ludolph A, Agid Y, Brice A, Leigh PN, Bensimon G. Genetic variants of the alpha-synuclein gene SNCA are associated with multiple system atrophy. PLoS ONE. 2009;4(9):e7114. doi: 10.1371/journal.pone.0007114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaman I, Fiumelli H, Magistretti PJ, Martin JL. Fluoxetine regulates the expression of neurotrophic/growth factors and glucose metabolism in astrocytes. Psychopharmacology. 2011;216(1):75–84. doi: 10.1007/s00213-011-2190-y. [DOI] [PubMed] [Google Scholar]
- Bar-Am O, Yogev-Falach M, Amit T, Sagi Y, Youdim MB. Regulation of protein kinase C by the anti-Parkinson drug, MAO-B inhibitor, rasagiline and its derivatives. in vivo J Neurochem. 2004;89(5):1119–1125. doi: 10.1111/j.1471-4159.2004.02425.x. [DOI] [PubMed] [Google Scholar]
- Benrud-Larson LM, Sandroni P, Schrag A, Low PA. Depressive symptoms and life satisfaction in patients with multiple system atrophy. Mov Disord. 2005;20(8):951–957. doi: 10.1002/mds.20450. [DOI] [PubMed] [Google Scholar]
- Bensimon G, Lacomblez L, Meininger V, ALS/Riluzole study group A controlled trial of riluzole in amyotrophic lateral sclerosis. N Engl J Med. 1994;330(9):585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- Bensimon G, Ludolph A, Agid Y, Vidailhet M, Payan C, Leigh PN. Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study. Brain. 2009;132(Pt 1):156–171. doi: 10.1093/brain/awn291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer K, Ariza A. Protein aggregation mechanisms in synucleinopathies: commonalities and differences. J Neuropathol Exp Neurol. 2007;66(11):965–974. doi: 10.1097/nen.0b013e3181587d64. [DOI] [PubMed] [Google Scholar]
- Blandini F, Armentero MT, Fancellu R, Blaugrund E, Nappi G. Neuroprotective effect of rasagiline in a rodent model of Parkinson’s disease. Exp Neurol. 2004;187(2):455–459. doi: 10.1016/j.expneurol.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Boudes M, Uvin P, Pinto S, Voets T, Fowler CJ, Wenning GK, De Ridder D, Stefanova N. Bladder dysfunction in a transgenic mouse model of multiple system atrophy. Mov Disord. 2013;28(3):347–355. doi: 10.1002/mds.25336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone M, Duty S, Rattray M. Riluzole neuroprotection in a Parkinson’s disease model involves suppression of reactive astrocytosis but not GLT-1 regulation. BMC Neurosci. 2012;13:38. doi: 10.1186/1471-2202-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EA, Beyhan Z, Yoo MS, Siripattarapravat K, Ko T, Lookingland KJ, Madhukar BV, Cibelli JB. Increased cellular turnover in response to fluoxetine in neuronal precursors derived from human embryonic stem cells. Int J Dev Biol. 2010;54(4):707–715. doi: 10.1387/ijdb.092851ec. [DOI] [PubMed] [Google Scholar]
- Cheah BC, Vucic S, Krishnan AV, Kiernan MC. Riluzole, neuroprotection and amyotrophic lateral sclerosis. Curr Med Chem. 2010;17(18):1942–1999. doi: 10.2174/092986710791163939. [DOI] [PubMed] [Google Scholar]
- Choi DK, Pennathur S, Perier C, Tieu K, Teismann P, Wu DC, Jackson-Lewis V, Vila M, Vonsattel JP, Heinecke JW, Przedborski S. Ablation of the inflammatory enzyme myeloperoxidase mitigates features of Parkinson’s disease in mice. J Neurosci. 2005;25(28):6594–6600. doi: 10.1523/JNEUROSCI.0970-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YC, Kim SR, Park JY, Chung ES, Park KW, Won SY, Bok E, Jin M, Park ES, Yoon SH, Ko HW, Kim YS, Jin BK. Fluoxetine prevents MPTP-induced loss of dopaminergic neurons by inhibiting microglial activation. Neuropharmacology. 2011;60(6):963–974. doi: 10.1016/j.neuropharm.2011.01.043. [DOI] [PubMed] [Google Scholar]
- Combarros O, Infante J, Llorca J, Berciano J. Interleukin-1A (-889) genetic polymorphism increases the risk of multiple system atrophy. Mov Disord. 2003;18(11):1385–1386. doi: 10.1002/mds.10540. [DOI] [PubMed] [Google Scholar]
- Déjérine J, Thomas A. L’atrophie olivoponto-cérébelleuse. Nouvelle Iconographie de la Salpêtiére. 1900;13:330–370. [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106(31):13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diguet E, Fernagut PO, Scherfler C, Wenning G, Tison F. Effects of riluzole on combined MPTP + 3-nitropropionic acid-induced mild to moderate striatonigral degeneration in mice. J Neural Transm. 2005;112(5):613–631. doi: 10.1007/s00702-004-0206-z. [DOI] [PubMed] [Google Scholar]
- Dodel R, Spottke A, Gerhard A, Reuss A, Reinecker S, Schimke N, Trenkwalder C, Sixel-Doring F, Herting B, Kamm C, Gasser T, Sawires M, Geser F, Kollensperger M, Seppi K, Kloss M, Krause M, Daniels C, Deuschl G, Bottger S, Naumann M, Lipp A, Gruber D, Kupsch A, Du Y, Turkheimer F, Brooks DJ, Klockgether T, Poewe W, Wenning G, Schade-Brittinger C, Oertel WH, Eggert K. Minocycline 1-year therapy in multiple-system-atrophy: effect on clinical symptoms and [(11)C] (R)-PK11195 PET (MEMSA-trial) Mov Disord. 2010;25(1):97–107. doi: 10.1002/mds.22732. [DOI] [PubMed] [Google Scholar]
- Dujardin K, Defebvre L, Krystkowiak P, Degreef JF, Destee A. Executive function differences in multiple system atrophy and Parkinson’s disease. Parkinsonism Relat Disord. 2003;9(4):205–211. doi: 10.1016/s1353-8020(02)00050-0. [DOI] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z, Rockenstein E, Masliah E, Hyman BT, McLean PJ, Unni VK. Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of alpha-synuclein. J Neurosci. 2011;31(41):14508–14520. doi: 10.1523/JNEUROSCI.1560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliash S, Shteter N, Eilam R. Neuroprotective effect of rasagiline, a monoamine oxidase-B inhibitor, on spontaneous cell degeneration in a rat model. J Neural Transm. 2005;112(8):991–1003. doi: 10.1007/s00702-004-0254-4. [DOI] [PubMed] [Google Scholar]
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30(20):6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, Wenning GK, Stefanova N. Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia. 2013;61(3):349–360. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner L, Jellinger KA, Wenning GK, Stefanova N. Glial dysfunction in the pathogenesis of alpha-synucleinopathies: emerging concepts. Acta Neuropathol. 2011;121(6):675–693. doi: 10.1007/s00401-011-0833-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner L, Stefanova N. The role of glia in alpha-synucleinopathies. Mol Neurobiol. 2013;47(2):575–586. doi: 10.1007/s12035-012-8340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernagut PO, Diguet E, Bioulac B, Tison F. MPTP potentiates 3-nitropropionic acid-induced striatal damage in mice: reference to striatonigral degeneration. Exp Neurol. 2004;185(1):47–62. doi: 10.1016/j.expneurol.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Fernagut PO, Diguet E, Stefanova N, Biran M, Wenning GK, Canioni P, Bioulac B, Tison F. Subacute systemic 3-nitropropionic acid intoxication induces a distinct motor disorder in adult C57Bl/6 mice: behavioural and histopathological characterisation. Neuroscience. 2002;114(4):1005–1017. doi: 10.1016/s0306-4522(02)00205-1. [DOI] [PubMed] [Google Scholar]
- Fernagut PO, Tison F. Animal models of multiple system atrophy. Neuroscience. 2012;211:77–82. doi: 10.1016/j.neuroscience.2011.09.044. [DOI] [PubMed] [Google Scholar]
- Fetoni V, Soliveri P, Monza D, Testa D, Girotti F. Affective symptoms in multiple system atrophy and Parkinson’s disease: response to levodopa therapy. J Neurol Neurosurg Psychiatry. 1999;66(4):541–544. doi: 10.1136/jnnp.66.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flabeau O, Meissner WG, Tison F. Multiple system atrophy: current and future approaches to management. Ther Adv Neurol Disord. 2010;3(4):249–263. doi: 10.1177/1756285610375328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friess E, Kuempfel T, Modell S, Winkelmann J, Holsboer F, Ising M, Trenkwalder C. Paroxetine treatment improves motor symptoms in patients with multiple system atrophy. Parkinsonism Relat Disord. 2006;12(7):432–437. doi: 10.1016/j.parkreldis.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Banati RB, Goerres GB, Cagnin A, Myers R, Gunn RN, Turkheimer F, Good CD, Mathias CJ, Quinn N, Schwarz J, Brooks DJ. [11C](R)-PK11195 PET imaging of microglial activation in multiple system atrophy. Neurology. 2003;61(5):686–689. doi: 10.1212/01.wnl.0000078192.95645.e6. [DOI] [PubMed] [Google Scholar]
- Geser F, Wenning GK, Seppi K, Stampfer-Kountchev M, Scherfler C, Sawires M, Frick C, Ndayisaba JP, Ulmer H, Pellecchia MT, Barone P, Kim HT, Hooker J, Quinn NP, Cardozo A, Tolosa E, Abele M, Klockgether T, Ostergaard K, Dupont E, Schimke N, Eggert KM, Oertel W, Djaldetti R, Poewe W. Progression of multiple system atrophy (MSA): a prospective natural history study by the European MSA Study Group (EMSA SG) Mov Disord. 2006;21(2):179–186. doi: 10.1002/mds.20678. [DOI] [PubMed] [Google Scholar]
- Ghorayeb I, Fernagut PO, Aubert I, Bezard E, Poewe W, Wenning GK, Tison F. Toward a primate model of L-dopa-unresponsive parkinsonism mimicking striatonigral degeneration. Mov Disord. 2000;15(3):531–536. doi: 10.1002/1531-8257(200005)15:3<531::AID-MDS1017>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Ghorayeb I, Fernagut PO, Hervier L, Labattu B, Bioulac B, Tison F. A ‘single toxin-double lesion’ rat model of striatonigral degeneration by intrastriatal 1-methyl-4-phenylpyridinium ion injection: a motor behavioural analysis. Neuroscience. 2002a;115(2):533–546. doi: 10.1016/s0306-4522(02)00401-3. [DOI] [PubMed] [Google Scholar]
- Ghorayeb I, Fernagut PO, Stefanova N, Wenning GK, Bioulac B, Tison F. Dystonia is predictive of subsequent altered dopaminergic responsiveness in a chronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine + 3-nitropropionic acid model of striatonigral degeneration in monkeys. Neurosci Lett. 2002b;335(1):34–38. doi: 10.1016/s0304-3940(02)01137-0. [DOI] [PubMed] [Google Scholar]
- Ghorayeb I, Puschban Z, Fernagut PO, Scherfler C, Rouland R, Wenning GK, Tison F. Simultaneous intrastriatal 6-hydroxydopamine and quinolinic acid injection: a model of early-stage striatonigral degeneration. Exp Neurol. 2001;167(1):133–147. doi: 10.1006/exnr.2000.7535. [DOI] [PubMed] [Google Scholar]
- Gilman S, Low P, Quinn N, Albanese A, Ben-Shlomo Y, Fowler C, Kaufmann H, Klockgether T, Lang A, Lantos P, Litvan I, Mathias C, Oliver E, Robertson D, Schatz I, Wenning G. Consensus statement on the diagnosis of multiple system atrophy. American autonomic society and American academy of neurology. Clin Auton Res. 1998;8(6):359–362. doi: 10.1007/BF02309628. [DOI] [PubMed] [Google Scholar]
- Gilman S, May SJ, Shults CW, Tanner CM, Kukull W, Lee VM, Masliah E, Low P, Sandroni P, Trojanowski JQ, Ozelius L, Foroud T. The north American multiple system atrophy study group. J Neural Transm. 2005;112(12):1687–1694. doi: 10.1007/s00702-005-0381-6. [DOI] [PubMed] [Google Scholar]
- Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JG, Oppenheimer DR. Orthostatic hypotension and nicotine sensitivity in a case of multiple system atrophy. J Neurol Neurosurg Psychiatry. 1969;32(1):28–34. doi: 10.1136/jnnp.32.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, Li JY, Brundin P. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121(2):715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Momose Y, Tokiguchi S, Shimohata M, Terajima K, Onodera O, Kakita A, Yamada M, Takahashi H, Hirasawa M, Mizuno Y, Ogata K, Goto J, Kanazawa I, Nishizawa M, Tsuji S. Multiplex families with multiple system atrophy. Arch Neurol. 2007;64(4):545–551. doi: 10.1001/archneur.64.4.545. [DOI] [PubMed] [Google Scholar]
- Infante J, Llorca J, Berciano J, Combarros O. Interleukin-8, intercellular adhesion molecule-1 and tumour necrosis factor-alpha gene polymorphisms and the risk for multiple system atrophy. J Neurol Sci. 2005;228(1):11–13. doi: 10.1016/j.jns.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Ishizawa K, Komori T, Sasaki S, Arai N, Mizutani T, Hirose T. Microglial activation parallels system degeneration in multiple system atrophy. J Neuropathol Exp Neurol. 2004;63(1):43–52. doi: 10.1093/jnen/63.1.43. [DOI] [PubMed] [Google Scholar]
- Ito H, Nakayama K, Jin C, Suzuki Y, Yazawa I. alpha-Synuclein accumulation reduces GABAergic inhibitory transmission in a model of multiple system atrophy. Biochem Biophys Res Commun. 2012;428(3):348–353. doi: 10.1016/j.bbrc.2012.10.057. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. The role of a-synuclein in neurodegeneration—an update. Translat Neurosci. 2012;3(2):75–122. [Google Scholar]
- Jellinger KA, Lantos PL. Papp-Lantos inclusions and the pathogenesis of multiple system atrophy: an update. Acta Neuropathol. 2010;119(6):657–667. doi: 10.1007/s00401-010-0672-3. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Seppi K, Wenning GK. Grading of neuropathology in multiple system atrophy: proposal for a novel scale. Mov Disord. 2005;20(Suppl 12):S29–S36. doi: 10.1002/mds.20537. [DOI] [PubMed] [Google Scholar]
- Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Spooren W, Fuss B, Mallon B, Macklin WB, Fujiwara H, Hasegawa M, Iwatsubo T, Kretzschmar HA, Haass C. Hyperphosphorylation and insolubility of alpha-synuclein in transgenic mouse oligodendrocytes. EMBO Rep. 2002;3(6):583–588. doi: 10.1093/embo-reports/kvf109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaindlstorfer C, Garcia J, Winkler C, Wenning GK, Nikkhah G, Dobrossy MD. Behavioral and histological analysis of a partial double-lesion model of parkinson-variant multiple system atrophy. J Neurosci Res. 2012;90(6):1284–1295. doi: 10.1002/jnr.23021. [DOI] [PubMed] [Google Scholar]
- Kisos H, Pukass K, Ben-Hur T, Richter-Landsberg C, Sharon R. Increased neuronal alpha-synuclein pathology associates with its accumulation in oligodendrocytes in mice modeling alpha-synucleinopathies. PLoS ONE. 2012;7(10):e46817. doi: 10.1371/journal.pone.0046817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klos KJ, Bower JH, Josephs KA, Matsumoto JY, Ahlskog JE. Pathological hypersexuality predominantly linked to adjuvant dopamine agonist therapy in Parkinson’s disease and multiple system atrophy. Parkinsonism Relat Disord. 2005;11(6):381–386. doi: 10.1016/j.parkreldis.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Koh JY, Kim DK, Hwang JY, Kim YH, Seo JH. Antioxidative and proapoptotic effects of riluzole on cultured cortical neurons. J Neurochem. 1999;72(2):716–723. doi: 10.1046/j.1471-4159.1999.0720716.x. [DOI] [PubMed] [Google Scholar]
- Kollensperger M, Geser F, Ndayisaba JP, Boesch S, Seppi K, Ostergaard K, Dupont E, Cardozo A, Tolosa E, Abele M, Klockgether T, Yekhlef F, Tison F, Daniels C, Deuschl G, Coelho M, Sampaio C, Bozi M, Quinn N, Schrag A, Mathias CJ, Fowler C, Nilsson CF, Widner H, Schimke N, Oertel W, Del Sorbo F, Albanese A, Pellecchia MT, Barone P, Djaldetti R, Colosimo C, Meco G, Gonzalez-Mandly A, Berciano J, Gurevich T, Giladi N, Galitzky M, Rascol O, Kamm C, Gasser T, Siebert U, Poewe W, Wenning GK. Presentation, diagnosis, and management of multiple system atrophy in Europe: final analysis of the European multiple system atrophy registry. Mov Disord. 2010;25(15):2604–2612. doi: 10.1002/mds.23192. [DOI] [PubMed] [Google Scholar]
- Kollensperger M, Stefanova N, Pallua A, Puschban Z, Dechant G, Hainzer M, Reindl M, Poewe W, Nikkhah G, Wenning GK. Striatal transplantation in a rodent model of multiple system atrophy: effects on L-Dopa response. J Neurosci Res. 2009;87(7):1679–1685. doi: 10.1002/jnr.21972. [DOI] [PubMed] [Google Scholar]
- Konno M, Hasegawa T, Baba T, Miura E, Sugeno N, Kikuchi A, Fiesel FC, Sasaki T, Aoki M, Itoyama Y, Takeda A. Suppression of dynamin GTPase decreases alpha-synuclein uptake by neuronal and oligodendroglial cells: a potent therapeutic target for synucleinopathy. Mol Neurodegener. 2012;7:38. doi: 10.1186/1750-1326-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzdas D, Stemberger S, Gaburro S, Stefanova N, Singewald N, Wenning GK. Oligodendroglial alpha-synucleinopathy and MSA-like cardiovascular autonomic failure: experimental evidence. Exp Neurol. 2013;247:531–536. doi: 10.1016/j.expneurol.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V, Amyotrophic lateral sclerosis/riluzole study group II Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Lancet. 1996;347(9013):1425–1431. doi: 10.1016/s0140-6736(96)91680-3. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285(12):9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, Kim JW, Bang OY, Ahn YH, Joo IS, Huh K. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther. 2008;83(5):723–730. doi: 10.1038/sj.clpt.6100386. [DOI] [PubMed] [Google Scholar]
- Lee PH, Lee JE, Kim HS, Song SK, Lee HS, Nam HS, Cheong JW, Jeong Y, Park HJ, Kim DJ, Nam CM, Lee JD, Kim HO, Sohn YH. A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann Neurol. 2012;72(1):32–40. doi: 10.1002/ana.23612. [DOI] [PubMed] [Google Scholar]
- Letiembre M, Liu Y, Walter S, Hao W, Pfander T, Wrede A, Schulz-Schaeffer W, Fassbender K. Screening of innate immune receptors in neurodegenerative diseases: a similar pattern. Neurobiol Aging. 2009;30(5):759–768. doi: 10.1016/j.neurobiolaging.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Li J, Zhu M, Rajamani S, Uversky VN, Fink AL. Rifampicin inhibits alpha-synuclein fibrillation and disaggregates fibrils. Chem Biol. 2004;11(11):1513–1521. doi: 10.1016/j.chembiol.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Low PA, Singer W. Management of neurogenic orthostatic hypotension: an update. Lancet Neurol. 2008;7(5):451–458. doi: 10.1016/S1474-4422(08)70088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338(6109):949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Song C, O’Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci USA. 2009;106(47):20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantoan L, Stefanova N, Egger KE, Jellinger KA, Poewe W, Wenning GK. Failure of caspase inhibition in the double-lesion rat model of striatonigral degeneration (multiple system atrophy) Acta Neuropathol. 2005;109(2):191–197. doi: 10.1007/s00401-004-0931-2. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, Seubert P, Lee M, Goldstein J, Chilcote T, Games D, Schenk D. Effects of alpha-synuclein immunization in a mouse model of Parkinson’s disease. Neuron. 2005;46(6):857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Mante M, Crews L, Spencer B, Adame A, Patrick C, Trejo M, Ubhi K, Rohn TT, Mueller-Steiner S, Seubert P, Barbour R, McConlogue L, Buttini M, Games D, Schenk D. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS ONE. 2011;6(4):e19338. doi: 10.1371/journal.pone.0019338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- May S, Gilman S, Sowell BB, Thomas RG, Stern MB, Colcher A, Tanner CM, Huang N, Novak P, Reich SG, Jankovic J, Ondo WG, Low PA, Sandroni P, Lipp A, Marshall FJ, Wooten F, Shults CW. Potential outcome measures and trial design issues for multiple system atrophy. Mov Disord. 2007;22(16):2371–2377. doi: 10.1002/mds.21734. [DOI] [PubMed] [Google Scholar]
- Mercier G, Lennon AM, Renouf B, Dessouroux A, Ramauge M, Courtin F, Pierre M. MAP kinase activation by fluoxetine and its relation to gene expression in cultured rat astrocytes. J Mol Neurosci. 2004;24(2):207–216. doi: 10.1385/JMN:24:2:207. [DOI] [PubMed] [Google Scholar]
- Miller DW, Johnson JM, Solano SM, Hollingsworth ZR, Standaert DG, Young AB. Absence of alpha-synuclein mRNA expression in normal and multiple system atrophy oligodendroglia. J Neural Transm. 2005;112(12):1613–1624. doi: 10.1007/s00702-005-0378-1. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Suzuki Y, Yazawa I. Microtubule depolymerization suppresses alpha-synuclein accumulation in a mouse model of multiple system atrophy. Am J Pathol. 2009;174(4):1471–1480. doi: 10.2353/ajpath.2009.080503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Suzuki Y, Yazawa I. Binding of neuronal alpha-synuclein to beta-III tubulin and accumulation in a model of multiple system atrophy. Biochem Biophys Res Commun. 2012;417(4):1170–1175. doi: 10.1016/j.bbrc.2011.12.092. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Kawakami H, Komure O, Maruyama H, Morino H, Izumi Y, Nakamura S, Kaji R, Kuno S. Contribution of the interleukin-1beta gene polymorphism in multiple system atrophy. Mov Disord. 2002;17(4):808–811. doi: 10.1002/mds.10124. [DOI] [PubMed] [Google Scholar]
- Nishioka K, Hayashi S, Farrer MJ, Singleton AB, Yoshino H, Imai H, Kitami T, Sato K, Kuroda R, Tomiyama H, Mizoguchi K, Murata M, Toda T, Imoto I, Inazawa J, Mizuno Y, Hattori N. Clinical heterogeneity of alpha-synuclein gene duplication in Parkinson’s disease. Ann Neurol. 2006;59(2):298–309. doi: 10.1002/ana.20753. [DOI] [PubMed] [Google Scholar]
- Noh KM, Hwang JY, Shin HC, Koh JY. A novel neuroprotective mechanism of riluzole: direct inhibition of protein kinase C. Neurobiol Dis. 2000;7(4):375–383. doi: 10.1006/nbdi.2000.0297. [DOI] [PubMed] [Google Scholar]
- O’Sullivan SS, Massey LA, Williams DR, Silveira-Moriyama L, Kempster PA, Holton JL, Revesz T, Lees AJ. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain. 2008;131(Pt 5):1362–1372. doi: 10.1093/brain/awn065. [DOI] [PubMed] [Google Scholar]
- Obinu MC, Reibaud M, Blanchard V, Moussaoui S, Imperato A. Neuroprotective effect of riluzole in a primate model of Parkinson’s disease: behavioral and histological evidence. Mov Disord. 2002;17(1):13–19. doi: 10.1002/mds.1272. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Okuizumi K, Ikeuchi T, Wakabayashi K, Takahashi H, Tsuji S. Analysis of the expression level of alpha-synuclein mRNA using postmortem brain samples from pathologically confirmed cases of multiple system atrophy. Acta Neuropathol. 2001;102(2):188–190. doi: 10.1007/s004010100367. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Paviour D, Quinn NP, Josephs KA, Sangha H, Kilford L, Healy DG, Wood NW, Lees AJ, Holton JL, Revesz T. The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain. 2004;127(Pt 12):2657–2671. doi: 10.1093/brain/awh303. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Takano H, Onodera O, Kobayashi H, Ikeuchi T, Koide R, Okuizumi K, Shimohata T, Wakabayashi K, Takahashi H, Tsuji S. No mutation in the entire coding region of the alpha-synuclein gene in pathologically confirmed cases of multiple system atrophy. Neurosci Lett. 1999;270(2):110–112. doi: 10.1016/s0304-3940(99)00475-9. [DOI] [PubMed] [Google Scholar]
- Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J Neurol Sci. 1989;94(1-3):79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- Papp MI, Lantos PL. The distribution of oligodendroglial inclusions in multiple system atrophy and its relevance to clinical symptomatology. Brain. 1994;117(Pt 2):235–243. doi: 10.1093/brain/117.2.235. [DOI] [PubMed] [Google Scholar]
- Park HJ, Bang G, Lee BR, Kim HO, Lee PH. Neuroprotective effect of human mesenchymal stem cells in an animal model of double toxin-induced multiple system atrophy parkinsonism. Cell Transplant. 2011;20(6):827–835. doi: 10.3727/096368910X540630. [DOI] [PubMed] [Google Scholar]
- Pfeiffer RF. Multiple system atrophy. Handb Clin Neurol. 2007;84:305–326. doi: 10.1016/S0072-9752(07)84046-2. [DOI] [PubMed] [Google Scholar]
- Poewe W, Barone P, Gliadi N, Gilman S, Low PA, Sampaio C, Seppi K. A randomized, placebo-controlled clinical trial to assess the effects of rasagiline in patients with multiple system atrophy of the parkinsonian subtype [abstract] Mov Disord. 2012;27(Suppl 1):1182. [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Puschban Z, Stefanova N, Petersen A, Winkler C, Brundin P, Poewe W, Wenning GK. Evidence for dopaminergic re-innervation by embryonic allografts in an optimized rat model of the Parkinsonian variant of multiple system atrophy. Brain Res Bull. 2005;68(1-2):54–58. doi: 10.1016/j.brainresbull.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Reynolds WF, Rhees J, Maciejewski D, Paladino T, Sieburg H, Maki RA, Masliah E. Myeloperoxidase polymorphism is associated with gender specific risk for Alzheimer’s disease. Exp Neurol. 1999;155(1):31–41. doi: 10.1006/exnr.1998.6977. [DOI] [PubMed] [Google Scholar]
- Sailer A, on behalf of the MSA GWAS Consortium First genome-wide association study in multiple system atrophy [abstract] Mov Disord. 2012;27(Suppl 1):1425. [Google Scholar]
- Scherfler C, Puschban Z, Ghorayeb I, Goebel GP, Tison F, Jellinger K, Poewe W, Wenning GK. Complex motor disturbances in a sequential double lesion rat model of striatonigral degeneration (multiple system atrophy) Neuroscience. 2000;99(1):43–54. doi: 10.1016/s0306-4522(00)00171-8. [DOI] [PubMed] [Google Scholar]
- Scherfler C, Sather T, Diguet E, Stefanova N, Puschban Z, Tison F, Poewe W, Wenning GK. Riluzole improves motor deficits and attenuates loss of striatal neurons in a sequential double lesion rat model of striatonigral degeneration (parkinson variant of multiple system atrophy) J Neural Transm. 2005;112(8):1025–1033. doi: 10.1007/s00702-004-0245-5. [DOI] [PubMed] [Google Scholar]
- Schneeberger A, Mandler M, Mattner F, Schmidt W. AFFITOME(R) technology in neurodegenerative diseases: the doubling advantage. Hum Vaccin. 2010;6(11):948–952. doi: 10.4161/hv.6.11.13217. [DOI] [PubMed] [Google Scholar]
- Schneeberger A, Mandler M, Mattner F, Schmidt W. Vaccination for Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S11–S13. doi: 10.1016/S1353-8020(11)70006-2. [DOI] [PubMed] [Google Scholar]
- Scholz SW, Houlden H, Schulte C, Sharma M, Li A, Berg D, Melchers A, Paudel R, Gibbs JR, Simon-Sanchez J, Paisan-Ruiz C, Bras J, Ding J, Chen H, Traynor BJ, Arepalli S, Zonozi RR, Revesz T, Holton J, Wood N, Lees A, Oertel W, Wullner U, Goldwurm S, Pellecchia MT, Illig T, Riess O, Fernandez HH, Rodriguez RL, Okun MS, Poewe W, Wenning GK, Hardy JA, Singleton AB, Del Sorbo F, Schneider S, Bhatia KP, Gasser T. SNCA variants are associated with increased risk for multiple system atrophy. Ann Neurol. 2009;65(5):610–614. doi: 10.1002/ana.21685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag A, Ben-Shlomo Y, Quinn NP. Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet. 1999;354(9192):1771–1775. doi: 10.1016/s0140-6736(99)04137-9. [DOI] [PubMed] [Google Scholar]
- Schrag A, Sheikh S, Quinn NP, Lees AJ, Selai C, Mathias C, Litvan I, Lang AE, Bower JH, Burn DJ, Low P, Jahanshahi M. A comparison of depression, anxiety, and health status in patients with progressive supranuclear palsy and multiple system atrophy. Mov Disord. 2010;25(8):1077–1081. doi: 10.1002/mds.22794. [DOI] [PubMed] [Google Scholar]
- Schrag A, Wenning GK, Quinn N, Ben-Shlomo Y. Survival in multiple system atrophy. Mov Disord. 2008;23(2):294–296. doi: 10.1002/mds.21839. [DOI] [PubMed] [Google Scholar]
- Shults CW, Rockenstein E, Crews L, Adame A, Mante M, Larrea G, Hashimoto M, Song D, Iwatsubo T, Tsuboi K, Masliah E. Neurological and neurodegenerative alterations in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. J Neurosci. 2005;25(46):10689–10699. doi: 10.1523/JNEUROSCI.3527-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shy GM, Drager GA. A neurological syndrome associated with orthostatic hypotension: a clinical-pathologic study. Arch Neurol. 1960;2:511–527. doi: 10.1001/archneur.1960.03840110025004. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. Alpha-synuclein locus triplication causes Parkinson’s disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett. 1998;251(3):205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Bucke P, Duerr S, Wenning GK. Multiple system atrophy: an update. Lancet Neurol. 2009a;8(12):1172–1178. doi: 10.1016/S1474-4422(09)70288-1. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Fellner L, Reindl M, Masliah E, Poewe W, Wenning GK. Toll-like receptor 4 promotes alpha-synuclein clearance and survival of nigral dopaminergic neurons. Am J Pathol. 2011;179(2):954–963. doi: 10.1016/j.ajpath.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N, Georgievska B, Eriksson H, Poewe W, Wenning GK. Myeloperoxidase inhibition ameliorates multiple system atrophy-like degeneration in a transgenic mouse model. Neurotox Res. 2012a;21(4):393–404. doi: 10.1007/s12640-011-9294-3. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Hainzer M, Stemberger S, Couillard-Despres S, Aigner L, Poewe W, Wenning GK. Striatal transplantation for multiple system atrophy—are grafts affected by alpha-synucleinopathy? Exp Neurol. 2009b;219(1):368–371. doi: 10.1016/j.expneurol.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Kaufmann WA, Humpel C, Poewe W, Wenning GK. Systemic proteasome inhibition triggers neurodegeneration in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. Acta Neuropathol. 2012b;124(1):51–65. doi: 10.1007/s00401-012-0977-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N, Lundblad M, Tison F, Poewe W, Cenci MA, Wenning GK. Effects of pulsatile L-DOPA treatment in the double lesion rat model of striatonigral degeneration (multiple system atrophy) Neurobiol Dis. 2004a;15(3):630–639. doi: 10.1016/j.nbd.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Mitschnigg M, Ghorayeb I, Diguet E, Geser F, Tison F, Poewe W, Wenning GK. Failure of neuronal protection by inhibition of glial activation in a rat model of striatonigral degeneration. J Neurosci Res. 2004b;78(1):87–91. doi: 10.1002/jnr.20233. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Poewe W, Wenning GK. Rasagiline is neuroprotective in a transgenic model of multiple system atrophy. Exp Neurol. 2008;210(2):421–427. doi: 10.1016/j.expneurol.2007.11.022. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Puschban Z, Fernagut PO, Brouillet E, Tison F, Reindl M, Jellinger KA, Poewe W, Wenning GK. Neuropathological and behavioral changes induced by various treatment paradigms with MPTP and 3-nitropropionic acid in mice: towards a model of striatonigral degeneration (multiple system atrophy) Acta Neuropathol. 2003;106(2):157–166. doi: 10.1007/s00401-003-0717-y. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Reindl M, Neumann M, Haass C, Poewe W, Kahle PJ, Wenning GK. Oxidative stress in transgenic mice with oligodendroglial alpha-synuclein overexpression replicates the characteristic neuropathology of multiple system atrophy. Am J Pathol. 2005a;166(3):869–876. doi: 10.1016/s0002-9440(10)62307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N, Reindl M, Neumann M, Kahle PJ, Poewe W, Wenning GK. Microglial activation mediates neurodegeneration related to oligodendroglial alpha-synucleinopathy: implications for multiple system atrophy. Mov Disord. 2007;22(15):2196–2203. doi: 10.1002/mds.21671. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Tison F, Reindl M, Poewe W, Wenning GK. Animal models of multiple system atrophy. Trends Neurosci. 2005b;28(9):501–506. doi: 10.1016/j.tins.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Stemberger S, Jamnig A, Stefanova N, Lepperdinger G, Reindl M, Wenning GK. Mesenchymal stem cells in a transgenic mouse model of multiple system atrophy: immunomodulation and neuroprotection. PLoS ONE. 2011;6(5):e19808. doi: 10.1371/journal.pone.0019808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemberger S, Poewe W, Wenning GK, Stefanova N. Targeted overexpression of human alpha-synuclein in oligodendroglia induces lesions linked to MSA-like progressive autonomic failure. Exp Neurol. 2010;224(2):459–464. doi: 10.1016/j.expneurol.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Okada K, Wakuda T, Shinmura C, Kameno Y, Iwata K, Takahashi T, Suda S, Matsuzaki H, Iwata Y, Hashimoto K, Mori N. Destruction of dopaminergic neurons in the midbrain by 6-hydroxydopamine decreases hippocampal cell proliferation in rats: reversal by fluoxetine. PLoS ONE. 2010;5(2):e9260. doi: 10.1371/journal.pone.0009260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Onodera O, Ozawa T, Piao YS, Kakita A, Takahashi H, Nishizawa M. Early development of autonomic dysfunction may predict poor prognosis in patients with multiple system atrophy. Arch Neurol. 2007;64(2):256–260. doi: 10.1001/archneur.64.2.256. [DOI] [PubMed] [Google Scholar]
- The Multiple-System Atrophy Research Collaboration Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med. 2013;369(3):233–244. doi: 10.1056/NEJMoa1212115. [DOI] [PubMed] [Google Scholar]
- Tofaris GK, Razzaq A, Ghetti B, Lilley KS, Spillantini MG. Ubiquitination of alpha-synuclein in Lewy bodies is a pathological event not associated with impairment of proteasome function. J Biol Chem. 2003;278(45):44405–44411. doi: 10.1074/jbc.M308041200. [DOI] [PubMed] [Google Scholar]
- Tong J, Wong H, Guttman M, Ang LC, Forno LS, Shimadzu M, Rajput AH, Muenter MD, Kish SJ, Hornykiewicz O, Furukawa Y. Brain alpha-synuclein accumulation in multiple system atrophy, Parkinson’s disease and progressive supranuclear palsy: a comparative investigation. Brain. 2010;133(Pt 1):172–188. doi: 10.1093/brain/awp282. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Revesz T. Proposed neuropathological criteria for the post mortem diagnosis of multiple system atrophy. Neuropathol Appl Neurobiol. 2007;33(6):615–620. doi: 10.1111/j.1365-2990.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- Ubhi K, Inglis C, Mante M, Patrick C, Adame A, Spencer B, Rockenstein E, May V, Winkler J, Masliah E. Fluoxetine ameliorates behavioral and neuropathological deficits in a transgenic model mouse of alpha-synucleinopathy. Exp Neurol. 2012;234(2):405–416. doi: 10.1016/j.expneurol.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubhi K, Lee PH, Adame A, Inglis C, Mante M, Rockenstein E, Stefanova N, Wenning GK, Masliah E. Mitochondrial inhibitor 3-nitroproprionic acid enhances oxidative modification of alpha-synuclein in a transgenic mouse model of multiple system atrophy. J Neurosci Res. 2009;87(12):2728–2739. doi: 10.1002/jnr.22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubhi K, Low P, Masliah E. Multiple system atrophy: a clinical and neuropathological perspective. Trends Neurosci. 2011;34(11):581–590. doi: 10.1016/j.tins.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubhi K, Rockenstein E, Mante M, Inglis C, Adame A, Patrick C, Whitney K, Masliah E. Neurodegeneration in a transgenic mouse model of multiple system atrophy is associated with altered expression of oligodendroglial-derived neurotrophic factors. J Neurosci. 2010;30(18):6236–6246. doi: 10.1523/JNEUROSCI.0567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubhi K, Rockenstein E, Mante M, Patrick C, Adame A, Thukral M, Shults C, Masliah E. Rifampicin reduces alpha-synuclein in a transgenic mouse model of multiple system atrophy. NeuroReport. 2008;19(13):1271–1276. doi: 10.1097/WNR.0b013e32830b3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacore N, Bonifati V, Fabbrini G, Colosimo C, De Michele G, Marconi R, Nicholl D, Locuratolo N, Talarico G, Romano S, Stocchi F, Bonuccelli U, De Mari M, Vieregge P, Meco G, ESGAP consortium. European study group on atypical Parkinsonisms Epidemiology of multiple system atrophy. Neurol Sci. 2001;22(1):97–99. doi: 10.1007/s100720170064. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Hayashi S, Kakita A, Yamada M, Toyoshima Y, Yoshimoto M, Takahashi H. Accumulation of alpha-synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta Neuropathol. 1998;96(5):445–452. doi: 10.1007/s004010050918. [DOI] [PubMed] [Google Scholar]
- Waldner R, Puschban Z, Scherfler C, Seppi K, Jellinger K, Poewe W, Wenning GK. No functional effects of embryonic neuronal grafts on motor deficits in a 3-nitropropionic acid rat model of advanced striatonigral degeneration (multiple system atrophy) Neuroscience. 2001;102(3):581–592. doi: 10.1016/s0306-4522(00)00500-5. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, Ferrante RJ, Kristal BS, Friedlander RM. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington’s disease. Proc Natl Acad Sci USA. 2003;100(18):10483–10487. doi: 10.1073/pnas.1832501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Neumann M, Hansen K, Hong SM, Kim S, Noble-Haeusslein LJ, Liu J. Fluoxetine increases hippocampal neurogenesis and induces epigenetic factors but does not improve functional recovery after traumatic brain injury. J Neurotrauma. 2011;28(2):259–268. doi: 10.1089/neu.2010.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JC, Giles K, Oehler A, Middleton L, Dexter DT, Gentleman SM, Dearmond SJ, Prusiner SB. Transmission of multiple system atrophy prions to transgenic mice. Proc Natl Acad Sci USA. 2013;110(48):19555–19560. doi: 10.1073/pnas.1318268110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning GK. Placebo-controlled trial of amantadine in multiple-system atrophy. Clin Neuropharmacol. 2005;28(5):225–227. doi: 10.1097/01.wnf.0000183240.47960.f0. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Ben Shlomo Y, Magalhaes M, Daniel SE, Quinn NP. Clinical features and natural history of multiple system atrophy. An analysis of 100 cases. Brain. 1994;117(Pt 4):835–845. doi: 10.1093/brain/117.4.835. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Colosimo C, Geser F, Poewe W. Multiple system atrophy. Lancet Neurol. 2004a;3(2):93–103. doi: 10.1016/s1474-4422(03)00662-8. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Geser F, Krismer F, Seppi K, Duerr S, Boesch S, Kollensperger M, Goebel G, Pfeiffer KP, Barone P, Pellecchia MT, Quinn NP, Koukouni V, Fowler CJ, Schrag A, Mathias CJ, Giladi N, Gurevich T, Dupont E, Ostergaard K, Nilsson CF, Widner H, Oertel W, Eggert KM, Albanese A, del Sorbo F, Tolosa E, Cardozo A, Deuschl G, Hellriegel H, Klockgether T, Dodel R, Sampaio C, Coelho M, Djaldetti R, Melamed E, Gasser T, Kamm C, Meco G, Colosimo C, Rascol O, Meissner WG, Tison F, Poewe W. The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol. 2013;12(3):264–274. doi: 10.1016/S1474-4422(12)70327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning GK, Granata R, Laboyrie PM, Quinn NP, Jenner P, Marsden CD. Reversal of behavioural abnormalities by fetal allografts in a novel rat model of striatonigral degeneration. Mov Disord. 1996;11(5):522–532. doi: 10.1002/mds.870110507. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Jellinger KA. The role of alpha-synuclein in the pathogenesis of multiple system atrophy. Acta Neuropathol. 2005;109(2):129–140. doi: 10.1007/s00401-004-0935-y. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Krismer F, Poewe W. New insights into atypical parkinsonism. Curr Opin Neurol. 2011a;24(4):331–338. doi: 10.1097/WCO.0b013e3283480569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning GK, Litvan I, Tolosa E. Milestones in atypical and secondary Parkinsonisms. Mov Disord. 2011b;26(6):1083–1095. doi: 10.1002/mds.23713. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Stefanova N, Jellinger KA, Poewe W, Schlossmacher MG. Multiple system atrophy: a primary oligodendrogliopathy. Ann Neurol. 2008;64(3):239–246. doi: 10.1002/ana.21465. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Tison F, Ben Shlomo Y, Daniel SE, Quinn NP. Multiple system atrophy: a review of 203 pathologically proven cases. Mov Disord. 1997;12(2):133–147. doi: 10.1002/mds.870120203. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Tison F, Seppi K, Sampaio C, Diem A, Yekhlef F, Ghorayeb I, Ory F, Galitzky M, Scaravilli T, Bozi M, Colosimo C, Gilman S, Shults CW, Quinn NP, Rascol O, Poewe W. Development and validation of the unified multiple system atrophy rating scale (UMSARS) Mov Disord. 2004b;19(12):1391–1402. doi: 10.1002/mds.20255. [DOI] [PubMed] [Google Scholar]
- Wullner U, Schmitt I, Kammal M, Kretzschmar HA, Neumann M. Definite multiple system atrophy in a German family. J Neurol Neurosurg Psychiatry. 2009;80(4):449–450. doi: 10.1136/jnnp.2008.158949. [DOI] [PubMed] [Google Scholar]
- Yabe I, Soma H, Takei A, Fujiki N, Yanagihara T, Sasaki H. MSA-C is the predominant clinical phenotype of MSA in Japan: analysis of 142 patients with probable MSA. J Neurol Sci. 2006;249(2):115–121. doi: 10.1016/j.jns.2006.05.064. [DOI] [PubMed] [Google Scholar]
- Yazawa I, Giasson BI, Sasaki R, Zhang B, Joyce S, Uryu K, Trojanowski JQ, Lee VM. Mouse model of multiple system atrophy alpha-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron. 2005;45(6):847–859. doi: 10.1016/j.neuron.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Amit T, Falach-Yogev M, Bar Am O, Maruyama W, Naoi M. The essentiality of Bcl-2, PKC and proteasome-ubiquitin complex activations in the neuroprotective-antiapoptotic action of the anti-Parkinson drug, rasagiline. Biochem Pharmacol. 2003;66(8):1635–1641. doi: 10.1016/s0006-2952(03)00535-5. [DOI] [PubMed] [Google Scholar]
- Yun JY, Lee WW, Lee JY, Kim HJ, Park SS, Jeon BS. SNCA variants and multiple system atrophy. Ann Neurol. 2010;67(4):554–555. doi: 10.1002/ana.21889. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46 K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55(2):164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]