Abstract
Multiple system atrophy is a rapidly progressive neurodegenerative disorder with a markedly reduced life expectancy. Failure of symptomatic treatment raises an urgent need for disease-modifying strategies. We have investigated the neuroprotective potential of erythropoietin in (proteolipid protein)-α-synuclein transgenic mice exposed to 3-nitropropionic acid featuring multiple system atrophy-like pathology including oligodendroglial α-synuclein inclusions and selective neuronal degeneration.
Mice were treated with erythropoietin starting before (early erythropoietin) and after (late erythropoietin) intoxication with 3-nitropropionic acid. Nonintoxicated animals receiving erythropoietin and intoxicated animals treated with saline served as control groups. Behavioral tests included pole test, open field activity, and motor behavior scale. Immunohistochemistry for tyrosine hydroxylase and dopamine and cyclic adenosine monophosphate-regulated phosphoprotein (DARPP-32) was analyzed stereologically.
Animals receiving erythropoietin before and after 3-nitropropionic acid intoxication scored significantly lower on the motor behavior scale and they performed better in the pole test than controls with no significant difference between early and late erythropoietin administration. Similarly, rearing scores were worse in 3-nitropropionic acid-treated animals with no difference between the erythropoietin subgroups. Immunohistochemistry revealed significant attenuation of 3-nitropropionic acid-induced loss of tyrosine hydroxylase and DARPP-32 positive neurons in substantia nigra pars compacta and striatum, respectively, in both erythropoietin-treated groups without significant group difference in the substantia nigra. However, at striatal level, a significant difference between early and late erythropoietin administration was observed.
In the combined (proteolipid protein)-α-synuclein 3-nitropropionic acid multiple system atrophy mouse model, erythropoietin appears to rescue dopaminergic and striatal gabaergic projection neurons. This effect is associated with improved motor function. Further studies are warranted to develop erythropoietin as a potential interventional therapy in multiple system atrophy.
Keywords: MSA, EPO, neuroprotection
Multiple system atrophy (MSA) is an atypical parkinsonian disorder clinically characterized by dysautonomia, parkinsonism, cerebellar symptoms, and pyramidal signs in any combination.1 Histopathological hallmarks are α-synuclein-positive glial cytoplasmic inclusions (GCIs) associated with a distinctive neuronal multisystem degeneration involving striatonigral, olivopontocerebellar, and central autonomic pathways.2 Because of loss of striatal projection neurons, parkinsonism in MSA is usually unresponsive to dopaminergic (DA) therapy. Disease is rapidly progressive, and life expectancy markedly reduced with a median survival of 7 to 9 years.3,4 Lack of symptomatic treatment combined with ongoing neurodegeneration raise an urgent need for disease-modifying strategies.
Both toxin-induced and transgenic MSA animal models are available to screen novel interventions.5 The combination of both genetic and toxic factors by exposing transgenic α-synuclein mice with GCI-like inclusions6 to mitochondrial stress [using 3-nitroproprionic acid (3-NP)] induces selective neurodegeneration in striatonigral and olivopontocerebellar pathways and results in a distinct motor phenotype.7 In this test bed, we have shown neuroprotective effects for minocycline8 and rasagiline.9
Circulating erythropoietin (EPO) is a large, highly glycosylated, and negatively charged molecule. Therefore, EPO was thought not to be able to cross the blood–brain barrier.10-12 However, more recent studies provided evidence that peripherally administered EPO indeed passes the blood–brain barrier in a dose-dependent fashion in several species including humans.13-17 After brain injury, increased cerebral concentrations of EPO were observed.17 EPO is mostly known for its effects in stimulating erythropoiesis. However, EPO protein and EPO receptors (EPO-R) are also expressed in the human brain throughout development.18,19 In response to hypoxia/ischemia, EPO expression is increased,20 and recombinant EPO protects against hypoxia/ischemia insults in rodent models.21-24 Exposure of rat embryonic mesencephalic DA precursor cells to higher environmental oxygen levels (18–20%) in vitro elicits a decrease in the number of tyrosine hydroxylase (TH) immunoreactive neurons when compared with cells grown at lower oxygen levels (1–2%).25 Addition of EPO to the culture medium of cells grown in the higher oxygen culture conditions results in a significant increase in the differentiation of precursor cells to TH-positive (TH+) neurons, an effect that is blocked by the addition of EPO-neutralizing antibodies.25 Genc et al.26 have shown in vivo that supranigral delivery of EPO provides neuroprotection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MTPT) insult in mice, which was associated with an upregulation of nitric oxide and glutathione peroxidase27 in the nigrostriatal pathway. Treatment of mesencephalic grafts with EPO enhances survival rate of TH+ cells by more than 100% corresponding with better behavioral recovery.28 Neuroprotective effects of EPO have also been observed in models of epilepsy,29 multiple sclerosis,30 and amytrophic lateral sclerosis.31 A clinical trial of EPO in patients with Friedreich’s ataxia showed a partial reversal of reduced frataxin levels and improved motor outcome.32,33 As opposed to that, a recently published double-blind, placebo-controlled, randomized, multicenter stroke trial failed to exert a beneficial effect of EPO.34 Whereas in MSA, EPO is known to be related to anemia and orthostatic hypotension,35,36 nothing is known about its neuroprotective potential in this atypical parkinsonian disorder. Long-term treatment with EPO may be limited due to the necessity of safety monitoring with regular blood cell counts and parameters of iron metabolism and of repeated bloodletting. Unfortunately, asialoEPO, which is a natural nonerythropoietic EPO metabolite, did not exert any beneficial effects in the R6/2 line of Huntington’s disease (HD) mice that share striatal pathology with MSA37 and was therefore not used in this study.
Materials and Methods
Animals
A total of 40 homozygous transgenic mice with targeted overexpression of human α-synuclein in oligodendrocytes driven by the proteolipid protein (PLP) promoter [(PLP)-α-synuclein mice]6 were used. All animals were 6 months or older. Further, mice were housed under standard conditions with a 12-hour light/dark cycle and free access to food and water. All efforts were made to minimize the number of animals used and their suffering. In particular, all attempts were made to minimize discomfort from invasive procedures (e.g., intraperitoneal injections) to avoid a significant impact of pain on experimental results. The following in vivo protocols were approved by the Federal Ministry of Science and Research of Austria.
Animals were randomized into four experimental groups. Thirty mice were chronically intoxicated with 3-NP according to the following low-dose paradigm7: 4 × 10 mg/kg, 4 × 20 mg/kg, 4 × 40 mg/kg, 3 × 50 mg/kg—which results in a total dosage of 430 mg/kg 3-NP—were dissolved in saline and injected intraperitoneally (i.p.) every 12 hours during the intoxication period. Intoxicated animals were treated with saline, early EPO, and late EPO with treatment starting before (saline, early EPO) or after (late EPO) intoxication (each: n = 10). Nonintoxicated animals receiving EPO from the beginning served as control group (n = 10).
Commercially available EPO (Erypo; Janssen-Cilag, Vienna, Austria) was delivered i.p. three times per week during the whole study period of 4 weeks (i.e., 12 injections) in the early EPO group. The late EPO group was treated only following the last 3-NP administration for five injections.
Because of the necessity of long-term EPO treatment, when applied in neurodegenerative diseases a human dose of 100 IU/kg was chosen to be the long-term maintenance dose in chronic renal failure, which is considered safe in humans.38 The corresponding mouse dose (1,250 IU/kg) was calculated according to published Food and Drug Administration (FDA) Guidelines (http://www.fda.gov/cder/guidance/5541fnl.htm).
Behavioral Testing
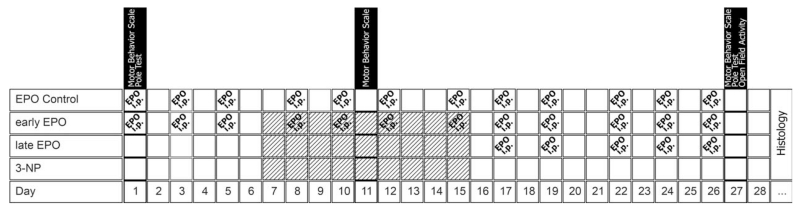
The following behavioral tests were performed blinded to the treatment status according to a standardized protocol: motor behavior scale (MBS), pole test, and spontaneous locomotor activity at the time points shown in the flowchart (Fig. 1).
FIG. 1.
Flowchart of the study. Diagonally shaded boxes represent injection of 3-NP intraperitoneally every 12 hours; dosage according to low-dose intoxication paradigm.
The MBS is used to score 3-NP-induced motor disability in mice39 and assesses general locomotor activity, hind limb clasping, hind limb dystonia, truncal dystonia, and postural challenge response. Higher scores indicate higher disability; the maximum total score is 10.
The pole test consists of a wooden vertical pole with rough surface, 1-cm wide and 50-cm high. After habituation, the animal is placed head up at the top of the pole, and both time for turning downwards (Tturn) as well as total time for climbing down (Ttotal) were taken in three trials. The best performance of all three trials was kept for statistical analysis.7
Open field activity was tested with the Flex Field Activity System (San Diego Instruments, CA), which allows monitoring and real-time counting of horizontal and vertical locomotor activity by 544 photobeam channels. Mice were tested for a 15-min period during the dark cycle.
The general observations (standardized MBS) were performed at the beginning, during the intoxication period, and on the day before scarification. The remaining tests (pole test and open field activity) were performed at the beginning of the study and on day 27 (day before sacrifice).
Immunohistochemistry
Animals were sacrificed at day 29 under at least three-fold thiopental (i.e., 120–150 mg/kg) overdose and perfused with 10 mL of 0.9% phosphate buffered saline (PBS) followed by 50 mL of ice-cold 4% paraformaldehyde (PFA) dissolved in PBS (pH = 7.4). After overnight postfixation in PFA and immersion in 25% sucrose (in PBS) until they sank, the brains were frozen in isopentane. Serial coronal sections were cut on a freezing microtome.
Free-floating sections (40 μm) were stained with antibodies for TH (Sigma) and dopamine and cyclic adenosine monophosphate (cAMP)-regulated phosphoprotein (DARPP-32; kindly provided by Dr. Hugh Hemmings, Jr.; The New York Hospital-Cornell Medical Centre).6
Image and Data Analysis
Nikon E-800 microscope and computerized analysis system (Stereo Investigator software; MicroBrightField Europe e.K., Magdeburg, Germany) were used for image analysis.
The number of TH+ (substantia nigra) and DARPP-32 positive cells (striatum) were assessed using the stereology module of the Stereo Investigator software. To obtain unbiased estimates of cell numbers, five coronal sections containing comparable regions of the substantia nigra pars compacta as well as seven coronal sections containing comparable striatal structures were assessed using the optical dissector method. Structures of interest (striatum and substantia nigra) were outlined manually according to the Paxinos and Franklin Mouse Brain Atlas (1997, Academic Press, San Diego). Analysis was performed blinded to the treatment group. The quality of quantitative estimates was assessed using the Scheaffer coefficient of error (CE).
Statistical analysis was performed using SPSS 15.0 (SPSS). All data are expressed as mean ± standard deviation. Groups were subdivided into two strata comprising intoxicated and nonintoxicated animals. Within the intoxication group, group differences in behavioral and histology data were analyzed using a one-way ANOVA followed by Bonferroni post hoc correction. In cases of missing normality of distribution, a nonparametric Mann–Whitney U test was performed. Differences between the two strata were analyzed using a one-way ANOVA. Correlations between histopathology and behavioral variables were obtained using Spearman’s rho. The significance level was set at P < 0.05; all tests were two-sided.
Results
Survival
During the 3-NP intoxication period, four mice died in the 3-NP (treated with saline only) group, three mice in the early EPO group, and five mice in the late EPO group (no significant difference; 3-NP vs. early EPO, P = 0.648; 3-NP vs. late EPO, P = 0.661; early EPO vs. late EPO, P = 0.374). In the EPO control group (nonintoxicated animals receiving EPO from the beginning) all animals survived.
MBS
At the baseline assessment, no significant differences between the three intoxicated groups were observed (Fig. 2). During the intoxication period, early EPO-treated animals (0.00 ± 0.00) as well as late EPO-treated animals (0.00 ± 0.00) failed to score significantly lower than 3-NP-treated animals (0.67 ± 0.82, P = 0.759; Fig. 2).
FIG. 2.
Motor behavior scale. Motor scores at baseline assessment (A), during the intoxication period (B) and at the end of the study (C). Dark gray vertical line indicates segmentation into two strata. The box shows interquartile range (from lower to upper quartile, Q25 and Q75 respectively), a horizontal line within the box represents median. Whiskers extend box to minimum and maximum value. Discordant values are emphasized by symbols. Curly brackets indicate group composition. Statistical significance figured as *P < 0.05, **P < 0.01, and ***P ≤0.001.
At the end of the study, animals receiving early EPO (0.14 ± 0.38) performed significantly better than that of 3-NP animals (5.33 ± 1.03, P = 0.001). Animals receiving late EPO treatment (1.00 ± 1.22) also showed lower MBS scores than that of 3-NP animals (5.33 ± 1.03, P = 0.004). There was no significant difference between early and late EPO administration. Overall, EPO- and saline-treated 3-NP animals (2.11 ± 2.52) scored significantly higher than nonintoxicated animals (0.00 ± 0.00, P = 0.016; Fig. 2).
Pole Test
Pole test performance was good in all groups; no animal was unable to perform the test. At baseline, all animals showed homogenous pole test performance without any significant difference between groups. At the end of the study, 3-NP animals (5.83 ± 0.41) were significantly slower in turning downwards than either EPO-treated group (early EPO, 3.42 ± 1.72, P = 0.006; late EPO, 2.60 ± 0.55, P = 0.001). Total time to descend was also significantly longer within the 3-NP group (12.33 ± 0.52) compared with the EPO-treated groups (early EPO, 7.57 ± 2.23, P < 0.001; late EPO, 5.60 ± 0.89, P < 0.001). No differences were observed between both the EPO-treated groups (Fig. 3).
FIG. 3.
Pole test. Time until animals turned downwards (A and B) and total time to descend the pole (C and D). Left-sided box plots (A and C) represent baseline behavior. Dark gray vertical line indicates segmentation into two strata. The box shows interquartile range (from lower to upper quartile, Q25 and Q75, respectively), and a horizontal line within the box represents median. Whiskers extend box to minimum and maximum value. Discordant values are emphasized by symbols. Statistical significance after post hoc testing figured as *P < 0.05, **P < 0.01, and ***P ≤0.001
Open Field Activity
Comparison of nonintoxicated with intoxicated animals resulted in a significant difference in number of rearings (P = 0.048) but failed to differ significantly in central (P = 0.643), peripheral (P = 0.052), and total (P = 0.076) activity counts.
In the intoxicated group, no significant differences between different treatments were observed for number of rearings (P = 0.692) as well as central (P = 0.169), peripheral (P = 0.887), and total counts (P = 0.854) (Table 1).
TABLE 1.
Results of spontaneous open field activity
| Rearing | Central | Peripheral | Total | |
|---|---|---|---|---|
| EPO control | 158.60 ± 31.75 | 598.50 ± 319.03 | 3944.90 ± 829.56 | 4702.00 ± 1077.00 |
| Early EPO | 137.71 ± 24.20 | 137.71 ± 213.09 | 3086.85 ± 583.45 | 3647.14 ± 798.80 |
| Late EPO | 116.80 ± 39.67 | 701.00 ± 172.41 | 3068.60 ± 915.16 | 3886.40 ± 1072.29 |
| 3-NP | 122.50 ± 58.20 | 564.67 ± 314.41 | 3346.67 ± 1509.23 | 4047.17 ± 1754.91 |
Data shown as mean ± standard deviation. Statistical significance is not shown.
EPO, erythropoietin; 3-NP, 3-nitropropionic acid.
Histology
All 3-NP-treated animals (3.96 × 103 ± 1.17 × 103) showed a significant loss of TH+ neurons in the substantia nigra pars compacta compared with nonintoxicated controls (6.69 × 103 ± 0.64 × 103, P 0.001; Fig. 4).
FIG. 4.
Cell counts. Cell counts in substantia nigra and striatum. Dark gray vertical line indicates segmentation into two strata. The box shows interquartile range (from lower to upper quartile, Q25 and Q75, respectively), a horizontal line within the box represents median. Whiskers extend box to minimum and maximum value. Discordant values are emphasized by symbols. Curly brackets indicate group composition. Statistical significance after post hoc testing figured as *P < 0.05, **P < 0.01, and ***P ≤0.001.
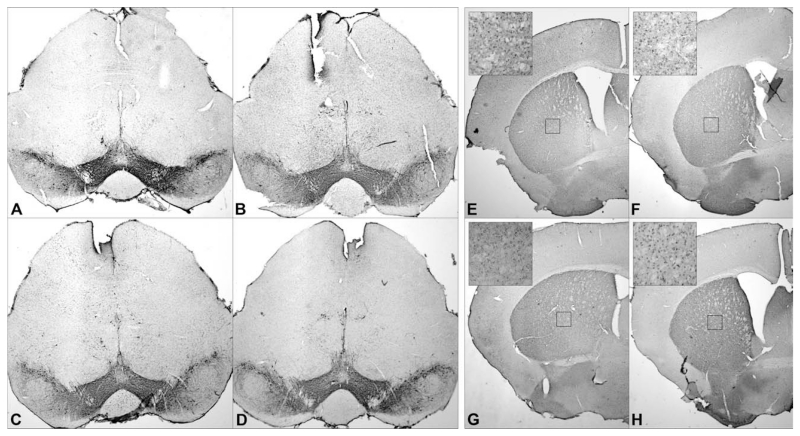
Both EPO treatment groups had significantly higher TH+ cell numbers (early EPO, 4.87 × 103 ± 0.69 × 103, P < 0.001; late EPO, 4.35 × 103 ± 0.64 × 103, P = 0.007) than the 3-NP group (2.72 × 103 ± 0.79 × 103). There was a numerical difference between the EPO groups favoring early administration; however, no significant difference was observed (Figs. 4 and 5).
FIG. 5.
Histology. TH staining of substantia nigra (A–D) and DARPP-32 staining of striatum (E–H). A and E showing EPO control; B and F, early EPO; C and G, late EPO; and D and H, indicating 3-NP controls.
In the striatum, intoxicated animals (2.97 × 105 ± 0.68 × 105) did not show significant loss of dopamine and cAMP-regulated phosphoprotein positive (DARPP-32+) neurons compared with nonintoxicated animals (3.48 × 105 ± 0.67 × 105, P = 0.062). The 3-NP group (2.17 × 105 ± 0.30 × 105) showed significant lower numbers of DARPP-32+ cells than animals receiving early (3.61 × 105 ± 0.29 × 105, P <0.001) or late (3.03 × 105 ± 0.23 × 105, P <0.001) EPO treatment. Additionally, a significant difference between both EPO treatment groups was observed (P = 0.007; Figs. 4 and 5).
Regarding the quality of quantitative estimates, a CE of 0.1741 ± 0.0515 was obtained within the substantia nigra. At striatal level, the CE was 0.0599 ± 0.0092.
In the nonintoxicated group, pole test performance correlated significantly with cell counts [Tturn with striatal DARPP-32+ cell number (r = 0.750, P = 0.012), Ttotal with nigral TH+ cell number (r = 0.675, P = 0.032)]. Intoxicated animals, EPO treated as well as saline treated, showed an inverse correlation between striatal DARPP-32+ cell number and pole test’s Ttotal (r= −0.478, P = 0.045). Additionally, in these three groups (early EPO, late EPO, and 3-NP) nigral TH+ cell number and pole test performance correlated inversely [nigral TH+ cells with Ttotal (r = −0.563, P = 0.019), nigral TH+ cells with Tturn (r = −0.535, P = 0.027)]. In the intoxicated group, an inverse correlation between MBS score and stereologically estimated cell number at striatal as well as nigral level was observed [striatal DARPP-32+ cell number with MBS (r = −0.839, P < 0.001); nigral TH+ cell number with MBS (r = −0.712, P = 0.001)].
Locomotor activity failed to correlate with nigral and striatal cell counts.
Discussion
This study shows that EPO reduces both behavioral impairment and striatonigral pathology in an established transgenic MSA mouse model.7
The MBS used to assess the overall disease severity in MSA mice comprises different aspects related to clinical symptoms of human MSA. Reduced locomotor speed, impaired postural stability, dystonic postures of limbs, and especially truncal dystonia are characteristic for MSA.40 Hind limb clasping in rodents is characteristic for striatal lesions in models of HD and MSA.39 EPO had a significant effect on 3-NP toxicity; animals receiving EPO from the beginning did not show elevated MBS scores. Animals receiving EPO post-intoxication, although significantly worse than early EPO animals, scored significantly lower than 3-NP controls. The pole test animals receiving EPO performed significantly better than 3-NP controls with no difference between early and late EPO administration. In contrast to other studies, where animals were unable to perform the test and a default value of 120 seconds was taken into account,9,39 in the present experiments no animal took longer than 15 seconds to complete the test thus eliminating the bias introduced by nonperformers. Regarding spontaneous locomotor activity, animals receiving 3-NP (EPO treated as well as saline treated) showed a significant lower number of rearings compared with nonintoxicated animals. Similar behavior was observed in previous studies as impaired rearing is part of the transgenic MSA mouse model’s phenotype and is exacerbated by intoxication with 3-NP.6,7 Unfortunately, we were not able to observe significant differences between treatment groups.
The observed improvement of motor deficits may (partly) reflect peripheral effects on erythropoiesis and oxygen transport. Indeed, asialoEPO, a nonhematogenic variant of EPO, failed to provide neuroprotection in a transgenic model of HD which shares striatal pathology with MSA.37 EPO-R is also expressed in the cardiovascular system and skeletal muscle. It may promote vascularization via vascular endothelial growth factor.41 In a rat model, EPO was shown to induce changes in muscle fiber type42 and promote functional and histological recovery of traumatized skeletal muscle.43 However, no direct effects on skeletal fiber thickness and vascularization were observed in humans.44 The effect of EPO, however, in our study was not limited to motor improvement.
Stereological analysis of TH+ neurons in substantia nigra pars compacta and DARPP-32 neurons in the striatum demonstrated reduced cell loss in the EPO-treated animal groups. The oligodendroglial α-synucleinopathy in the (PLP)-α-synuclein mice increases vulnerability to 3-NP-induced oxidative stress.7 3-NP is a mitochondrial toxin which inhibits succinate dehydrogenase activity throughout the brain, but nevertheless causes selective neurodegeneration in the striatum, or in presence of GCIs, in striatum, substantia nigra pars compacta, cerebellum, and pontine nuclei. The exact mechanisms underlying this specificity are not yet understood. In HD models, it has been shown that 3-NP induces selective DNA fragmentation and increased bax/bcl-2 ratio in the centre of severe striatal lesions.45 Furthermore, bcl-2 overexpression has been shown to prevent 3-NP-induced cell death.46
The possible central mechanisms through which EPO may have provided neuroprotection in this study include upregulation of antiapoptotic signals (i.e., Bcl-2 and Bcl-XL), inhibition of caspases, inhibition of glutamate release, and upregulation of enzymes that scavenge oxygen radicals.47,48 On the other hand, MSA is thought to be a primary oligodendrogliopathy with secondary neurodegeneration.49 EPO and EPO-R are expressed in human astrocytes and play an important role in oligodendrocyte maturation.50 Astrocyte EPO expression is reduced by proinflammatory cytokines;51 exogenous EPO protects oligodendrocytes in vitro from cytotoxicity induced by inflammatory stimuli.52 In a model of spinal cord trauma, EPO has been shown to counteract secondary oligodendrocyte death enhancing functional recovery.53 These glioprotective effects might have contributed to neuroprotection observed in this study. Therefore, discrepancy between the above-mentioned study on asialoEPO in HD mice37 and this study might be a result of not only peripheral impacts but also oligodendroglial protection due to modulatory effects of EPO.
It is important to take into account that both EPO groups—the “early” group administering EPO ahead of 3-NP intoxication as well as the “late” group which started treatment 2 days after the end of the intoxication period—provided rescue of the DA phenotype. This is important, since the diagnosis of MSA is clinical and usually made 2 to 3 years after disease onset when patients may already be disabled. Thus, this study provides evidence that EPO might become a prospective disease-modifying drug candidate.
To sum up, striatonigral degeneration is the key neuropathological substrate of parkinsonism in MSA. Our finding of EPO-derived effects within the striatonigral pathway suggest that further studies to explore the underlying mechanisms and to define the preclinical rationale of EPO-based therapies in MSA are highly warranted.
Some limitations have to be acknowledged. The model used in this study combines 3-NP-induced oxidative stress and transgenic overexpression of synuclein to induce MSA-like pathology. However, the relative contribution of genetic and environmental factors in the pathogenesis of MSA remains unknown at present and therefore the EPO effects observed in the mouse model may not be reproducible in humans. In addition, we have not investigated whether EPO interferes with synuclein aggregation itself as it has been demonstrated for rifampicin. Further, we cannot exclude nonspecific effects of EPO on motor behavior and neuronal integrity due to lack of a non-EPO nonintoxicated control group.
In summary, we have shown that EPO improves motor deficits and rescues TH+ as well as DARPP32 neurons in the combined toxin/transgenic model of MSA. Further studies are warranted to develop EPO as a potential interventional therapy in MSA.
Acknowledgments
The study was supported by MFI Grant 9441 and by FWF Grant P19989-B05.
Footnotes
Relevant conflict of interest/financial disclosure: Nothing to report.
Full financial disclosures and author roles may be found in the online version of this article.
References
- 1.Wenning GK, Colosimo C, Geser F, Poewe W. Multiple system atrophy. Lancet Neurol. 2004;3:93–103. doi: 10.1016/s1474-4422(03)00662-8. [DOI] [PubMed] [Google Scholar]
- 2.Trojanowski JQ, Revesz T. Proposed neuropathological criteria for the post mortem diagnosis of multiple system atrophy. Neuropathol Appl Neurobiol. 2007;33:615–620. doi: 10.1111/j.1365-2990.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe H, Saito Y, Terao S, et al. Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain. 2002;125(Part 5):1070–1083. doi: 10.1093/brain/awf117. [DOI] [PubMed] [Google Scholar]
- 4.Schrag A, Wenning GK, Quinn N, Ben-Shlomo Y. Survival in multiple system atrophy. Mov Disord. 2008;23:294–296. doi: 10.1002/mds.21839. [DOI] [PubMed] [Google Scholar]
- 5.Stefanova N, Tison F, Reindl M, Poewe W, Wenning GK. Animal models of multiple system atrophy. Trends Neurosci. 2005;28:501–506. doi: 10.1016/j.tins.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Kahle PJ, Neumann M, Ozmen L, et al. Hyperphosphorylation and insolubility of alpha-synuclein in transgenic mouse oligodendrocytes. EMBO Rep. 2002;3:583–588. doi: 10.1093/embo-reports/kvf109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stefanova N, Reindl M, Neumann M, et al. Oxidative stress in transgenic mice with oligodendroglial alpha-synuclein overexpression replicates the characteristic neuropathology of multiple system atrophy. AmJ Pathol. 2005;166:869–876. doi: 10.1016/s0002-9440(10)62307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stefanova N, Reindl M, Neumann M, Kahle PJ, Poewe W, Wenning GK. Microglial activation mediates neurodegeneration related to oligodendroglial alpha-synucleinopathy: implications for multiple system atrophy. Mov Disord. 2007;22:2196–2203. doi: 10.1002/mds.21671. [DOI] [PubMed] [Google Scholar]
- 9.Stefanova N, Poewe W, Wenning GK. Rasagiline is neuroprotective in a transgenic model of multiple system atrophy. Exp Neurol. 2008;210:421–427. doi: 10.1016/j.expneurol.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Marti HH, Bernaudin M, Petit E, Bauer C. Neuroprotection and angiogenesis: dual role of erythropoietin in brain ischemia. News Physiol Sci. 2000;15:225–229. doi: 10.1152/physiologyonline.2000.15.5.225. [DOI] [PubMed] [Google Scholar]
- 11.Cerami A. Beyond erythropoiesis: novel applications for recombinant human erythropoietin. Semin Hematol. 2001;38(3 Suppl 7):33–39. doi: 10.1016/s0037-1963(01)90128-3. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki R, Masuda S, Nagao M. Erythropoietin: multiple physiological functions and regulation of biosynthesis. Biosci Biotechnol Biochem. 2000;64:1775–1793. doi: 10.1271/bbb.64.1775. [DOI] [PubMed] [Google Scholar]
- 13.Brines ML, Ghezzi P, Keenan S, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banks WA, Jumbe NL, Farrell CL, Niehoff ML, Heatherington AC. Passage of erythropoietic agents across the blood-brain barrier: a comparison of human and murine erythropoietin and the analog darbepoetin alfa. Eur J Pharmacol. 2004;505:93–101. doi: 10.1016/j.ejphar.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 15.Ehrenreich H, Degner D, Meller J, et al. Erythropoietin: a candidate compound for neuroprotection in schizophrenia. Mol Psychiatry. 2004;9:42–54. doi: 10.1038/sj.mp.4001442. [DOI] [PubMed] [Google Scholar]
- 16.Ehrenreich H, Hasselblatt M, Dembowski C, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- 17.Statler PA, McPherson RJ, Bauer LA, Kellert BA, Juul SE. Pharmacokinetics of high-dose recombinant erythropoietin in plasma and brain of neonatal rats. Pediatr Res. 2007;61:671–675. doi: 10.1203/pdr.0b013e31805341dc. [DOI] [PubMed] [Google Scholar]
- 18.Juul SE, Anderson DK, Li Y, Christensen RD. Erythropoietin and erythropoietin receptor in the developing human central nervous system. Pediatr Res. 1998;43:40–49. doi: 10.1203/00006450-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Juul SE, Yachnis AT, Rojiani AM, Christensen RD. Immunohistochemical localization of erythropoietin and its receptor in the developing human brain. Pediatr Dev Pathol. 1999;2:148–158. doi: 10.1007/s100249900103. [DOI] [PubMed] [Google Scholar]
- 20.Digicaylioglu M, Bichet S, Marti HH, et al. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc Natl Acad Sci USA. 1995;92:3717–3720. doi: 10.1073/pnas.92.9.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akdemir Ozisik P, Oruckaptan H, Ozdemir Geyik P, et al. Effect of erythropoietin on brain tissue after experimental head trauma in rats. Surg Neurol. 2007;68:547–555. doi: 10.1016/j.surneu.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 22.Cherian L, Goodman JC, Robertson C. Neuroprotection with erythropoietin administration following controlled cortical impact injury in rats. J Pharmacol Exp Ther. 2007;322:789–794. doi: 10.1124/jpet.107.119628. [DOI] [PubMed] [Google Scholar]
- 23.Iwai M, Cao G, Yin W, Stetler RA, Liu J, Chen J. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke. 2007;38:2795–2803. doi: 10.1161/STROKEAHA.107.483008. [DOI] [PubMed] [Google Scholar]
- 24.Mahmood A, Lu D, Qu C, et al. Treatment of traumatic brain injury in rats with erythropoietin and carbamylated erythropoietin. J Neurosurg. 2007;107:392–397. doi: 10.3171/JNS-07/08/0392. [DOI] [PubMed] [Google Scholar]
- 25.Studer L, Csete M, Lee SH, et al. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genc S, Kuralay F, Genc K, et al. Erythropoietin exerts neuroprotection in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated C57/BL mice via increasing nitric oxide production. Neurosci Lett. 2001;298:139–141. doi: 10.1016/s0304-3940(00)01716-x. [DOI] [PubMed] [Google Scholar]
- 27.Genc S, Akhisaroglu M, Kuralay F, Genc K. Erythropoietin restores glutathione peroxidase activity in 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine-induced neurotoxicity in C57BL mice and stimulates murine astroglial glutathione peroxidase production in vitro. Neurosci Lett. 2002;321:73–76. doi: 10.1016/s0304-3940(02)00041-1. [DOI] [PubMed] [Google Scholar]
- 28.Kanaan NM, Collier TJ, Marchionini DM, McGuire SO, Fleming MF, Sortwell CE. Exogenous erythropoietin provides neuroprotection of grafted dopamine neurons in a rodent model of Parkinson’s disease. Brain Res. 2006;1068:221–229. doi: 10.1016/j.brainres.2005.10.078. [DOI] [PubMed] [Google Scholar]
- 29.Nadam J, Navarro F, Sanchez P, et al. Neuroprotective effects of erythropoietin in the rat hippocampus after pilocarpine-induced status epilepticus. Neurobiol Dis. 2007;25:412–426. doi: 10.1016/j.nbd.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Sattler MB, Merkler D, Maier K, et al. Neuroprotective effects and intracellular signaling pathways of erythropoietin in a rat model of multiple sclerosis. Cell Death Differ. 2004;11(Suppl 2):S181–S192. doi: 10.1038/sj.cdd.4401504. [DOI] [PubMed] [Google Scholar]
- 31.Grunfeld JF, Barhum Y, Blondheim N, Rabey JM, Melamed E, Offen D. Erythropoietin delays disease onset in an amyotrophic lateral sclerosis model. Exp Neurol. 2007;204:260–263. doi: 10.1016/j.expneurol.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Boesch S, Sturm B, Hering S, et al. Neurological effects of recombinant human erythropoietin in Friedreich’s ataxia: a clinical pilot trial. Mov Disord. 2008;23:1940–1944. doi: 10.1002/mds.22294. [DOI] [PubMed] [Google Scholar]
- 33.Boesch S, Sturm B, Hering S, Goldenberg H, Poewe W, Scheiber-Mojdehkar B. Friedreich’s ataxia: clinical pilot trial with recombinant human erythropoietin. Ann Neurol. 2007;62:521–524. doi: 10.1002/ana.21177. [DOI] [PubMed] [Google Scholar]
- 34.Ehrenreich H, Weissenborn K, Prange H, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40:e647–e656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- 35.Winkler AS, Marsden J, Parton M, Watkins PJ, Chaudhuri KR. Erythropoietin deficiency and anaemia in multiple system atrophy. Mov Disord. 2001;16:233–239. doi: 10.1002/mds.1063. [DOI] [PubMed] [Google Scholar]
- 36.Winkler AS, Landau S, Watkins P, Chaudhuri KR. Observations on haematological and cardiovascular effects of erythropoietin treatment in multiple system atrophy with sympathetic failure. Clin Auton Res. 2002;12:203–206. doi: 10.1007/s10286-002-0009-y. [DOI] [PubMed] [Google Scholar]
- 37.Gil JM, Leist M, Popovic N, Brundin P, Petersen A. Asialoerythropoietin is not effective in the R6/2 line of Huntington’s disease mice. BMC Neurosci. 2004;5:17. doi: 10.1186/1471-2202-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilcox CS, Tisher CC. Handbook of nephrology and hypertension. Sixth ed. Lippincott Williams & Wilkins; Philadelphia: 2008. pp. 340–344. [Google Scholar]
- 39.Fernagut PO, Diguet E, Stefanova N, et al. Subacute systemic 3-nitropropionic acid intoxication induces a distinct motor disorder in adult C57Bl/6 mice: behavioural and histopathological characterisation. Neuroscience. 2002;114:1005–1017. doi: 10.1016/s0306-4522(02)00205-1. [DOI] [PubMed] [Google Scholar]
- 40.Kollensperger M, Geser F, Seppi K, et al. Red flags for multiple system atrophy. Mov Disord. 2008;23:1093–1099. doi: 10.1002/mds.21992. [DOI] [PubMed] [Google Scholar]
- 41.Alvarez Arroyo MV, Castilla MA, Gonzalez Pacheco FR, et al. Role of vascular endothelial growth factor on erythropoietin-related endothelial cell proliferation. J Am Soc Nephrol. 1998;9:1998–2004. doi: 10.1681/ASN.V9111998. [DOI] [PubMed] [Google Scholar]
- 42.Cayla JL, Maire P, Duvallet A, Wahrmann JP. Erythropoietin induces a shift of muscle phenotype from fast glycolytic to slow oxidative. Int J Sports Med. 2008;29:460–465. doi: 10.1055/s-2007-965359. [DOI] [PubMed] [Google Scholar]
- 43.Rotter R, Menshykova M, Winkler T, et al. Erythropoietin improves functional and histological recovery of traumatized skeletal muscle tissue. J Orthop Res. 2008;26:1618–1626. doi: 10.1002/jor.20692. [DOI] [PubMed] [Google Scholar]
- 44.Lundby C, Hellsten Y, Jensen MB, Munch AS, Pilegaard H. Erythropoietin receptor in human skeletal muscle and the effects ofacute and long-term injections with recombinant human erythropoietin on the skeletal muscle. J Appl Physiol. 2008;104:1154–1160. doi: 10.1152/japplphysiol.01211.2007. [DOI] [PubMed] [Google Scholar]
- 45.Vis JC, Verbeek MM, de Waal RM, ten Donkelaar HJ, Kremer B. The mitochondrial toxin 3-nitropropionic acid induces differential expression patterns of apoptosis-related markers in rat striatum. Neuropathol Appl Neurobiol. 2001;27:68–76. doi: 10.1046/j.0305-1846.2001.00305.x. [DOI] [PubMed] [Google Scholar]
- 46.Mandavilli BS, Boldogh I, Van Houten B. 3-Nitropropionic acid-induced hydrogen peroxide, mitochondrial DNA damage, and cell death are attenuated by Bcl-2 overexpression in PC12 cells. Brain Res. 2005;133:215–223. doi: 10.1016/j.molbrainres.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 47.Chong ZZ, Lin SH, Kang JQ, Maiese K. Erythropoietin prevents early and late neuronal demise through modulation of Akt1 and induction of caspase 1, 3, and 8. J Neurosci Res. 2003;71:659–669. doi: 10.1002/jnr.10528. [DOI] [PubMed] [Google Scholar]
- 48.Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003;138:1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wenning GK, Stefanova N, Jellinger KA, Poewe W, Schlossmacher MG. Multiple system atrophy: a primary oligodendrogliopathy. Ann Neurol. 2008;64:239–246. doi: 10.1002/ana.21465. [DOI] [PubMed] [Google Scholar]
- 50.Sugawa M, Sakurai Y, Ishikawa-Ieda Y, Suzuki H, Asou H. Effects of erythropoietin on glial cell development; oligodendrocyte maturation and astrocyte proliferation. Neurosci Res. 2002;44:391–403. doi: 10.1016/s0168-0102(02)00161-x. [DOI] [PubMed] [Google Scholar]
- 51.Nagai A, Nakagawa E, Choi HB, Hatori K, Kobayashi S, Kim SU. Erythropoietin and erythropoietin receptors in human CNS neurons, astrocytes, microglia, and oligodendrocytes grown in culture. J Neuropathol Exp Neurol. 2001;60:386–392. doi: 10.1093/jnen/60.4.386. [DOI] [PubMed] [Google Scholar]
- 52.Genc K, Genc S, Baskin H, Semin I. Erythropoietin decreases cytotoxicity and nitric oxide formation induced by inflammatory stimuli in rat oligodendrocytes. Physiol Res. 2006;55:33–38. doi: 10.33549/physiolres.930749. [DOI] [PubMed] [Google Scholar]
- 53.Gorio A, Gokmen N, Erbayraktar S, et al. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc Natl Acad Sci USA. 2002;99:9450–9455. doi: 10.1073/pnas.142287899. [DOI] [PMC free article] [PubMed] [Google Scholar]