Abstract
Neisserial surface protein A (NspA) is a highly conserved outer membrane protein previously investigated as a meningococcal vaccine candidate. Despite eliciting serum bactericidal activity in mice, a recombinant NspA vaccine failed to elicit serum bactericidal antibodies in a phase 1 clinical trial in humans. The discordant results may be explained by the recent discovery that NspA is a human-specific ligand of the complement inhibitor factor H (FH). Therefore, in humans but not mice, NspA would be expected to form a complex with FH, which could impair human anti-NspA protective antibody responses. To investigate this question, we immunized human FH transgenic BALB/c mice with three doses of recombinant NspA expressed in Escherichia coli microvesicles, with each dose being separated by 3 weeks. Three of 12 (25%) transgenic mice and 13 of 14 wild-type mice responded with bactericidal titers of ≥1:10 in postimmunization sera (P = 0.0008, Fisher's exact test). In contrast, human FH transgenic and wild-type mice immunized with a control meningococcal native outer membrane vesicle vaccine had similar serum bactericidal antibody responses directed at PorA, which is not known to bind human FH, and a mutant factor H binding protein (FHbp) antigen with a >50-fold lower level of FH binding than wild-type FHbp antigen binding.Thus, human FH can impair anti-NspA serum bactericidal antibody responses, which may explain the poor immunogenicity of the NspA vaccine previously tested in humans. A mutant NspA vaccine engineered to have decreased binding to human FH may increase protective antibody responses in humans.
INTRODUCTION
The 1990s witnessed the discovery of two promising recombinant protein meningococcal vaccine candidates capable of eliciting broad serum bactericidal antibody responses in mice: neisserial surface protein A (NspA) (1–3) and transferrin-binding protein (TbpB) (4–6). However, in phase 1 clinical trials, both recombinant protein vaccines failed to elicit serum bactericidal antibody responses in humans (7, 8). NspA was subsequently shown to bind specifically to human complement factor H (FH) (9), and transferrin-binding protein was known to bind specifically to human and not mouse transferrin (10). The impaired serum bactericidal antibody responses to these vaccines in humans may have resulted in part from binding of the host proteins to the vaccine antigens. For example, human FH transgenic mice immunized with meningococcal factor H binding protein (FHbp) vaccines that bound human FH had lower serum anti-FHbp bactericidal antibody responses than wild-type mice whose mouse FH did not bind to FHbp (11, 12). Rhesus macaques with FH that bound with a high avidity to FHbp also had lower serum anti-FHbp bactericidal antibody responses than macaques with FH containing a polymorphism associated with low-avidity binding to FHbp (13). Pigs immunized with a mutant Haemophilus parasuis TbpB vaccine with decreased binding to porcine transferrin elicited higher T- and B-cell responses than pigs immunized with a native TbpB that bound porcine transferrin (12).
NspA is highly conserved in Neisseria meningitidis (1) and, therefore, would be of interest for use as a component of a serogroup B vaccine if the antigen were capable of eliciting protective antibodies in humans. In the present study, we immunized wild-type and human FH transgenic BALB/c mice with a prototype recombinant NspA vaccine expressed in Escherichia coli microvesicles and measured serum anti-NspA bactericidal antibody responses. We report that human FH impaired the immunogenicity of NspA, which provides another example of the effect of binding of a host protein to a vaccine antigen on impairment of a protective serum antibody response.
(E.L. performed this work as part of a master's program in cell and molecular biology at San Francisco State University, San Francisco, CA.)
MATERIALS AND METHODS
Recombinant NspA expressed in E. coli microvesicles.
In previous studies, purified recombinant NspA vaccines failed to elicit serum bactericidal antibody responses in wild-type mice, which were thought to result from the failure of the recombinant protein to be properly folded (14, 15). In contrast, microvesicles released from E. coli cells that had been transformed with the NspA gene elicited serum anti-NspA bactericidal activity (15). Therefore, in the present study we transformed E. coli strain BL21(DE3) competent cells (Life Technologies) with a pUC19-based plasmid construct (pEHNspA; see Fig. S1 in the supplemental material) containing an NspA gene cloned from serogroup W strain Sudan 1/06 (Su 1/06) (16), an upstream EH promoter described previously (16), and an ampicillin resistance marker. The NspA gene, which was identical to that from group B strain LNP21362 described previously (17), including its natural signal peptide-coding segment, was cloned for surface expression in E. coli. The E. coli cells were grown overnight in SB broth (18) containing 50 μg/ml of ampicillin. The culture was centrifuged for 40 min at 4,000 × g, and the cell pellet was discarded. The supernatant containing the microvesicles was passed through a 0.45-μm-pore-size Millipore filter (Thermo Fisher Scientific) to remove the remaining bacteria and concentrated to ∼100 ml by ultrafiltration (model 8400; Millipore). After ultracentrifugation at 100,000 × g for 2 h, the microvesicles containing recombinant NspA were resuspended in 3% sucrose and 0.2 M glycine, pH 8.0, and stored at −20°C.
Meningococcal NOMV vaccine.
To determine whether the human FH transgenic mice had impaired serum antibody responses to antigens that did not bind human FH, we prepared a control meningococcal native outer membrane vesicle (NOMV) vaccine, which was derived from a previously described mutant of N. meningitidis strain Su 1/06 (W:P1.5,2: sequence type 11 [ST-11; clonal complex 11 {CC11}]) (16). The mutant vaccine strain had a genetically attenuated endotoxin (lpxL1 knockout), its capsular expression was deleted, and it overexpressed mutant subfamily B FHbp (peptide identification number [ID] 9) containing one amino acid substitution (R41S) that decreased the binding of FH >50-fold compared to the level of FH binding by the respective wild-type FHbp (11). By Western blotting, expression of the mutant subfamily B FHbp in the vaccine strain was 10-fold higher than that in the parent strain (16). For the present study, we inserted an additional copy of a mutant subfamily A FHbp (ID 22) with one amino acid substitution (D211A) that also decreased the binding of FH >50-fold (19). However, in wild-type CD1 mice, the NOMV vaccine elicited low serum IgG anti-FHbp ID 22 antibody responses (data not shown), which were attributed to insufficient overexpression of the antigen (see, for example, reference 20). Therefore, the meningococcal NOMV vaccine generated serum bactericidal antibody responses primarily against meningococcal strains with subfamily B FHbp and/or PorA P1.5,2 (which was the PorA variable region [VR] sequence type of the vaccine strain). The control meningococcal NOMV vaccine was resuspended in 3% sucrose and 0.2 M glycine, pH 8.0, and stored at −20°C.
SDS-PAGE and Western blotting.
The major outer membrane proteins were analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and Western blotting as described previously (15, 16). In the Western blotting assay, NspA was detected with a previously described anti-NspA monoclonal antibody (MAb), AL12 (21).
Flow cytometry.
Expression of NspA on the surface of E. coli cells was measured by flow cytometry with an anti-NspA MAb, 14C7 (22), as previously described with meningococcal bacteria (23). To measure FH binding, we adapted to E. coli a protocol described previously for meningococci (11). Briefly, 200 μl of an overnight E. coli culture was pelleted and incubated with 100 μl of a 15-μg/ml dilution of human FH for 1 h at room temperature. Following incubation, bound FH was detected using polyclonal sheep anti-human FH (Abcam), followed by washing and incubation with donkey anti-sheep IgG antibody (Sigma) conjugated with Alexa Fluor 488.
Mouse immunogenicity.
The protocol was approved by the Institutional Animal Care and Use Committees at the Children's Hospital Oakland Research Institute (CHORI). The human FH BALB/c mouse line has been described previously (11). At approximately 5 weeks of age, the transgenic mice were bled via the submandibular vein to quantify serum human FH concentrations using an FHbp capture enzyme-linked immunosorbent assay (ELISA), which was performed as previously described (11). In a previous FHbp immunogenicity study in human FH transgenic mice, only mice with serum human FH concentrations of ≥250 μg/ml had impaired anti-FHbp bactericidal antibody responses (11). Therefore, in the present NspA immunogenicity study, we selected transgenic mice with serum concentrations of human FH of ≥250 μg/ml, which are similar to the FH concentrations in normal human serum (11). Wild-type BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA) and housed at the CHORI animal facility for 3 weeks before beginning the immunogenicity study.
At the time of the first dose of vaccine, the ages of the wild-type and human FH transgenic mice ranged from 2 to 4 months, and the respective groups of mice were assigned to the different vaccines to achieve similar representations of male and female mice and of mouse age groups. The mice were immunized with 5 μg of either the E. coli microvesicle vaccine containing recombinant NspA or the control meningococcal NOMV vaccine, each of which was adsorbed with 0.5 mg/dose of aluminum hydroxide (alhydrogel; Brenntag Biosector) with 10 mM histidine and 150 mM NaCl. Three intraperitoneal (i.p.) injections, each separated by 3 weeks, were given, and terminal blood samples were obtained 3 weeks after the last dose. Negative-control mice received either i.p. injections of aluminum hydroxide (0.5 mg/dose) without an antigen or with microvesicles from E. coli cells that were transformed with the control pUC19 vector without the NspA gene and that were adsorbed with aluminum hydroxide.
Serum bactericidal antibody activity.
The serum bactericidal assay has been described previously (23). The assay used bacteria that had been grown to mid-exponential phase in Frantz medium supplemented with 4 mM d,l-lactate (Sigma), 2 mM CMP–N-acetylneuraminic acid (Carbosynth), and exogenous human complement from adult serum that had been depleted of IgG using a protein G column as described previously (11). The bactericidal titer was the dilution of serum that resulted in a 50% decrease in the number of CFU per milliliter compared to the number of CFU per milliliter in the negative-control wells after 60 min of incubation at 37°C.
Bacterial strains.
We tested two meningococcal strains. Group B strain BZ198 (B:P1.7-2,4; ST-41 [CC41/44]) was used to assess the anti-NspA bactericidal activity elicited by the NspA vaccine and anti-FHbp bactericidal activity elicited by the NOMV vaccine. This strain naturally expresses high levels of NspA, is susceptible to anti-NspA bactericidal activity (15), and has a subfamily B FHbp (ID 14) that matches the subfamily of the FHbp (ID 9) overexpressed in the NOMV vaccine. To evaluate anti-PorA serum bactericidal antibody responses to the control meningococcal NOMV vaccine, we used a group W strain, Burkina Faso 2/03 (BuFa 2/03) (W:P1.5,2; ST-11 [CC11]) (16). This strain has the PorA VR sequence type P1.5,2, which matches that of the PorA of the meningococcal strain used to prepare the NOMV vaccine, and expresses a subfamily A FHbp (ID 23) which is mismatched to the overexpressed subfamily B FHbp in the NOMV vaccine. This test strain was also resistant to anti-NspA bactericidal activity (see Results).
Depletion of anti-FHbp serum antibodies.
To determine the role of anti-FHbp antibodies in the serum bactericidal activity elicited by the control meningococcal NOMV vaccine, we depleted anti-FHbp antibodies from the postimmunization serum as previously described (24). In brief, we utilized cyanogen bromide-activated Sepharose coupled with recombinant FHbp ID 1, which contained a single amino acid substitution, R41S, that abrogated the binding of human serum FH without affecting the ability of the mutant antigen to deplete the anti-FHbp antibodies from the serum (25). In the present study, we created serum pools from each vaccine group by combining equal volumes of serum from each mouse. The adequacy of depletion of serum anti-FHbp antibody was quantified by an ELISA, which was performed as previously described (24, 25).
Statistical methods.
The proportions of mice responding to the vaccine with bactericidal titers of ≥1:10 in postimmunization sera were compared by the Fisher exact test. For calculation of geometric mean titers, titers below the lower limit of detection were assigned a value that was half of the lower limit (i.e., a titer of <1:10 was assigned a titer of 1:5), and the reciprocal titers were log10 transformed. To determine whether the geometric mean serum antibody titers between two independent groups of mice were different, we used a two-tailed Student's t test and the log-transformed reciprocal titers. Differences with a probability of <0.05 (two-tailed) were considered significant.
RESULTS
Expression of recombinant NspA on the surface of E. coli.
Cells transformed with the pEHNspA vector expressed NspA on their cell surface, as measured by flow cytometry, whereas E. coli cells transformed with pUC19 without the NspA gene did not (Fig. 1A, black solid line). Cells expressing NspA on their surface also bound human FH (Fig. 1B, black solid line). These results indicate that NspA was expressed, properly folded, and accessible on the outer membrane.
FIG 1.
Binding of anti-NspA MAb and human FH to E. coli cells expressing NspA. (A) Binding of an anti-NspA MAb, 14C7 (21), to the surface of E. coli cells measured by flow cytometry. (B) Binding of human FH (15 μg/ml) to the surface of live E. coli cells detected with polyclonal sheep anti-human FH antibodies. Black lines, E. coli cells transformed with an NspA expression vector (pEHNspA); black histograms, E. coli cells transformed with a control vector (pUC19) that lacked an NspA gene.
E. coli microvesicles containing recombinant NspA.
We grew the E. coli cells and isolated microvesicles from the culture supernatant as described in Materials and Methods. We visualized the major proteins by PAGE with Coomassie staining (Fig. 2, lanes 1 and 2). The presence of recombinant NspA in microvesicles from E. coli cells transformed with the NspA gene is evident by the appearance in lane 2 of two proteins which are not present in lane 1 (negative-control E. coli microvesicles from cells transformed with the vector without an NspA gene). NspA was reported to be a heat-modifiable protein (15). The two bands in lane 2 with apparent molecular masses of 18.6 and 15 kDa are consistent with the two NspA isoforms previously described to exist after heating of NspA (15). By Western blotting (lanes 3 and 4), NspA was present in the microvesicles from E. coli cells expressing the NspA gene (lane 4) but not in the negative-control E. coli microvesicles (lane 3).
FIG 2.

Characterization of E. coli microvesicle vaccines containing recombinant NspA by SDS-PAGE and Western blotting. Lanes 1 and 2 show Coomassie blue-stained SDS-polyacrylamide gels of E. coli microvesicles that had been heated at 100°C for 10 min. Lane 1, 10 μg of negative-control microvesicles from E. coli cells transformed with a control vector that lacked an NspA gene; lane 2, 10 μg of microvesicles from E. coli cells transformed with pEHNspA expressing NspA. The two bands in lane 2 with apparent molecular masses of 18.6 and 15 kDa (marked by arrows to the right of lanes 3 and 4) are consistent with the two NspA isoforms previously described to appear after heating of NspA (15). Lanes 3 and 4 show a Western blot of the proteins separated by SDS-PAGE, transferred to a nitrocellulose membrane, and detected with an anti-NspA MAb (AL12) (22). The samples in lanes 3 and 4 are identical to those in lanes 1 and 2, respectively.
As a second measure of NspA in the microvesicles and its ability to bind human FH, we performed an ELISA in which E. coli microvesicles were adsorbed to the wells of a microtiter plate. The microvesicles containing NspA but not the control vesicles lacking NspA were detected with an anti-NspA MAb (Fig. 3A). The wells with the E. coli microvesicles with NspA also specifically bound human factor H (Fig. 3B). Both vesicle preparations showed similar levels of binding with a control polyclonal anti-E. coli antiserum (Fig. 3C).
FIG 3.
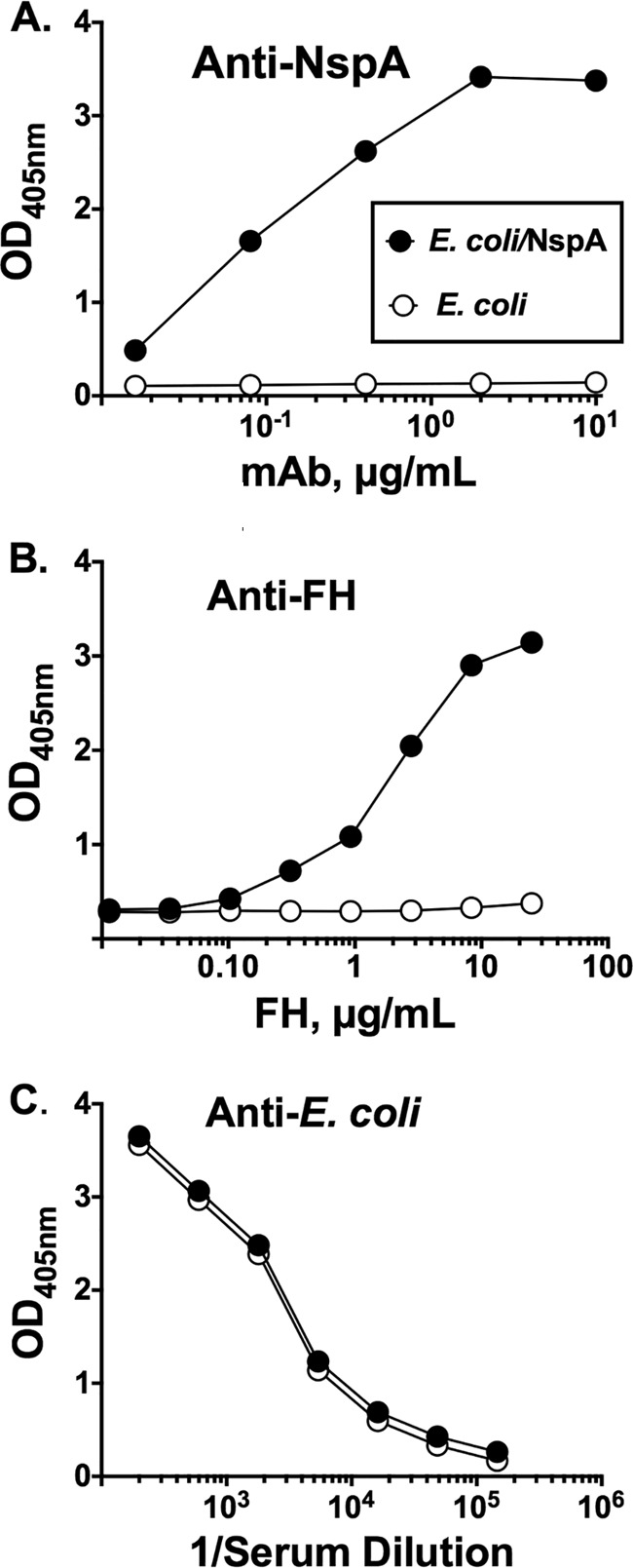
E. coli microvesicles containing recombinant NspA bind human FH, as determined by ELISA. (A) Binding of an anti-NspA MAb (AL12) to E. coli microvesicles; (B) binding of human FH to E. coli microvesicles; (C) binding of polyclonal mouse anti-E. coli antisera. Black circles with solid line, microvesicles from E. coli expressing NspA; open circles with solid line, microvesicles from E. coli lacking an NspA gene.
Effect of human FH on NspA immunogenicity.
We immunized human FH transgenic or wild-type mice with E. coli vesicles containing recombinant NspA (referred to here as the “NspA vaccine”). Figure 4A shows the serum bactericidal titers of antibodies against serogroup B strain BZ198, which naturally expresses high levels of NspA and is susceptible to anti-NspA bactericidal activity (15), measured in individual mice immunized with the NspA vaccine. Three of 12 (25%) transgenic mice and 13 of 14 wild-type mice responded with bactericidal titers of ≥1:10 in postimmunization sera (P = 0.0008, Fisher's exact test). The sera from individual wild-type and transgenic mice immunized with the E. coli vesicles that lacked NspA, which were used as a control, had no detectable serum bactericidal activity (titers, <1:10) (Fig. 4A).
FIG 4.
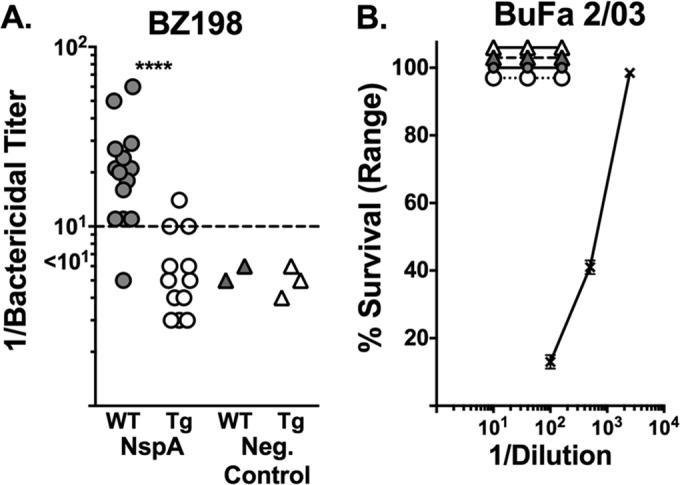
Human FH impairs serum anti-NspA bactericidal antibody responses. (A) Serum titers of antibodies against strain BZ198 measured in individual mice immunized with recombinant NspA expressed in E. coli microvesicles (NspA vaccine). Three of 12 (25%) transgenic (Tg) mice and 13 of 14 wild-type (WT) mice responded with bactericidal titers of ≥1:10 in postimmunization sera (****, P = 0.0008, Fisher's exact test). (B) Percent survival of strain BuFa 2/03 when incubated for 60 min with 20% human complement and different dilutions of serum pools from wild-type or transgenic mice immunized with the NspA vaccine or a control E. coli microvesicle vaccine without NspA. Data for all four pools (one for each mouse strain immunized with one of the two vaccines) overlap each other. The positive-control anti-PorA P1.2 MAb (× with solid line) was bactericidal (1:100 dilution = 1 μg/ml).
As described below, to determine whether the impaired anti-NspA bactericidal antibody responses of the human FH transgenic mice were specific for NspA or reflected a more global impairment of immune responses to other antigens with or low or absent FH binding, we tested the serum bactericidal responses of wild-type and human FH transgenic mice immunized with a control meningococcal NOMV vaccine. In addition to testing the bactericidal activity against strain BZ198, we tested the bactericidal activity against a second serogroup W strain, BuFa 2/03 (16), which has a PorA variable region sequence type, P1.5,2, that matched that of the PorA in the NOMV vaccine. PorA is not known to bind to FH, and strain BuFa 2/03 was expected to be susceptible to the anti-PorA antibody induced by the NOMV vaccine. Serum pools from mice immunized with the NspA vaccine had no detectable bactericidal activity against BuFa 2/03 (100% survival when tested with serum pools that were diluted 1:10, 1:40, or 1:160; Fig. 4B). As expected, the strain was killed by complement and a control anti-PorA P1.2 MAb (Fig. 4B).
Wild-type and human FH transgenic mice have similar serum bactericidal antibody responses to two antigens with low or no known binding with human FH.
As described above, to determine whether the impaired anti-NspA bactericidal antibody responses of the human FH transgenic mice were specific for NspA, we immunized groups of wild-type and human FH transgenic mice with a control meningococcal NOMV vaccine with overexpressed mutant R41S FHbp that had low levels of FH binding (11, 26). Based on previous data, we expected that most of the serum bactericidal antibody would be directed at subfamily B FHbp (16) or PorA P1.5,2 (26, 27).
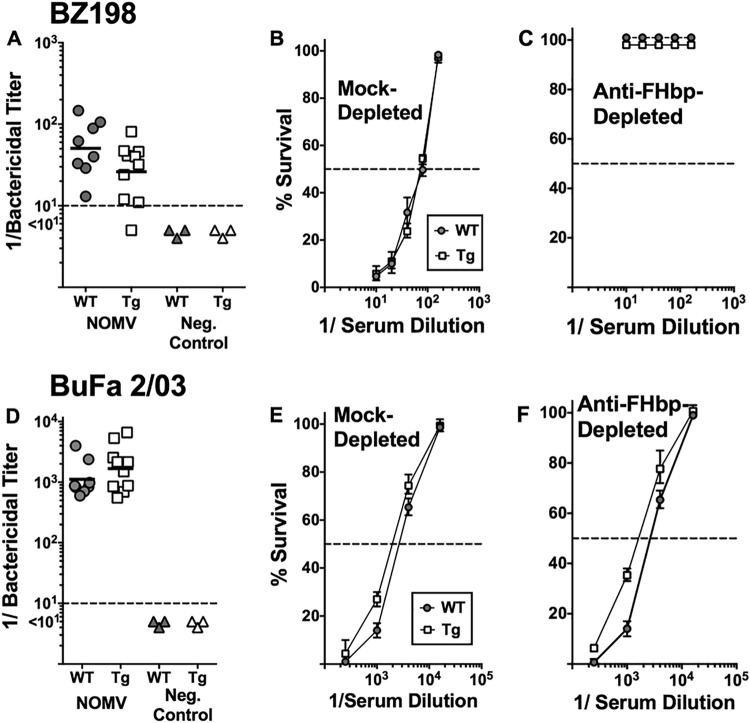
Figure 5 shows the serum bactericidal titers of antibodies against BZ198 measured in individual wild-type and human FH transgenic mice immunized with the meningococcal NOMV vaccine (Fig. 5A) and BuFa 2/03 (Fig. 5D). Strain BZ198 expresses a subfamily B FHbp (ID 14) that is matched with the subfamily B FHbp (ID 9) in the NOMV vaccine and has a PorA variable region sequence type (P1.7-2,4) that is mismatched for the PorA P1.5,2 in the NOMV vaccine. In contrast, the PorA of strain BuFa 2/03 is matched with the PorA in the NOMV vaccine, but strain BuFa 2/03 has a subfamily A FHbp (ID 22) that is mismatched to the overexpressed subfamily B FHbp antigen in the vaccine. In both the wild-type and transgenic mice, the meningococcal NOMV vaccine elicited serum bactericidal antibodies against both test strains. The differences in the reciprocal geometric mean bactericidal titers of the two groups of mice were not significantly different (P = 0.11 for strain BZ198 and P = 0.28 for strain BuFa 2/03).
FIG 5.
Serum bactericidal antibody responses of wild-type and human FH transgenic mice immunized with a control meningococcal NOMV vaccine. The NOMV vaccine contained PorA P1.5,2 and overexpressed subfamily B R41S mutant FHbp with a low level of binding to human FH. (A and D) Serum bactericidal antibody responses measured against strains BZ198 (anti-FHbp) (A) and BuFa 2/03 (anti-PorA) (D) (see the text). Each data point represents the titer in serum from an individual mouse. Horizontal bars represent 1/geometric mean titers. There were no significant differences in the respective geometric mean antibody responses of human FH transgenic (Tg) and wild-type (WT) mice (P = 0.11 for strain BZ198, P = 0.28 for BuFa 2/03). (B, C, E, and F) Effect of adsorption of serum anti-FHbp on percent survival of meningococci incubated for 60 min with 20% human complement and different dilutions of serum pools from wild-type or transgenic mice immunized with the meningococcal NOMV vaccine. Filled circles and solid line, serum pool from wild-type mice immunized with the meningococcal NOMV vaccine; open squares and solid line, serum pool from transgenic mice immunized with the meningococcal NOMV vaccine. (B and E) Serum pools from immunized wild-type or transgenic mice that had been mock adsorbed with Sepharose without an antigen; (C and F) the corresponding serum pools that had been adsorbed with FHbp-Sepharose to deplete anti-FHbp antibodies. Each data point represents the average percent survival from values obtained with three replicate dilution curves (two assays). Error bars represent ranges.
To confirm that the serum bactericidal activity against strain BZ198 required anti-FHbp antibody, we depleted the anti-FHbp antibodies from pools of sera from the NOMV-vaccinated human FH transgenic and wild-type mice using a FHbp-Sepharose column (see Materials and Methods). As a control, the serum pools were mock adsorbed with Sepharose that had not been coupled to FHbp. By ELISA, the depletion removed >90% of the serum anti-FHbp antibody but had no effect on the serum anti-NOMV antibody titers (data not shown). The depletion completely eliminated the bactericidal activity of both serum pools against strain BZ198 (Fig. 5D) but had no effect on the bactericidal activity against strain BuFa 2/03 (Fig. 5F), which was expected to be killed by anti-PorA antibody. The mock serum adsorption had no effect on bactericidal activity against either strain. Collectively, the data indicate that the human and transgenic mice had similar anti-FHbp and anti-PorA bactericidal antibody responses to the meningococcal NOMV vaccine but the transgenic mice had impaired serum bactericidal antibody responses to NspA, which bound human FH.
DISCUSSION
Following its discovery in 1997 by Martin et al., NspA was considered a highly promising vaccine candidate for the prevention of invasive meningococcal disease because NspA was surface exposed and highly conserved among diverse strains of N. meningitidis (1). Despite the preclinical potential of NspA in animal models, a recombinant NspA vaccine failed to elicit serum bactericidal antibodies in humans (8). The lack of serum bactericidal antibody responses was not anticipated and at the time was attributed to either improper folding of the recombinant protein or, possibly, to a human-specific host response.
Subsequently, NspA was shown by Lewis and colleagues to bind specifically to human FH (9). Since binding of human FH to another meningococcal vaccine antigen, FHbp, impaired protective anti-FHbp antibody responses (11, 13, 26, 28, 29), we considered whether the binding of human FH to the NspA vaccine might also impair anti-NspA bactericidal antibody responses. In the present study, we immunized wild-type and human FH transgenic mice with a recombinant NspA vaccine expressed in E. coli vesicles. Our most important result was lower serum anti-NspA bactericidal antibody responses in the human FH transgenic mice than in wild-type mice (P = 0.0008). In contrast, the two groups of mice had similar bactericidal antibody responses to a control meningococcal NOMV vaccine. The results obtained with the control vaccine were consistent with those obtained in previous studies demonstrating that the human FH transgenic mouse line had normal antibody responses to other antigens that did not bind human FH, including a serogroup C meningococcal polysaccharide conjugate vaccine (11) and recombinant meningococcal NadA and outer membrane vesicle PorA (13). Collectively, the data indicate that the impaired serum bactericidal antibody responses in the human FH transgenic mice are specific for FHbp and NspA, both of which strongly bind human FH.
One limitation of the present study is that we used a prototype recombinant NspA vaccine presented in E. coli microvesicles, but such a vaccine is unsuitable for use in humans. However, in our previous study, serum from mice immunized with purified unfolded NspA failed to elicit complement-mediated bactericidal activity (15), even after attempts to reconstitute the protein in liposomes (14). The E. coli microvesicle/recombinant NspA vaccine tested in the present study contained a fully functional NspA that bound human FH and, therefore, provided a convenient means to investigate the possible effect of human FH binding on NspA immunogenicity. Further, in future studies this approach could also be used to investigate the immunogenicity of low-FH-binding mutant recombinant NspA vaccines in human FH transgenic mice (as was done for FHbp antigens [11, 26, 30, 31]). For a vaccine intended for use in humans, the expression of NspA in N. meningitidis is more likely to retain native folding and important epitopes for eliciting bactericidal antibodies than the expression of a purified recombinant NspA in E. coli (14). Thus, it should be possible to prepare a meningococcal NOMV-NspA vaccine from a mutant N. meningitidis strain with a genetically attenuated endotoxin and overexpressed NspA by methods similar to those used to prepare meningococcal NOMV vaccines with overexpressed FHbp (27, 32, 33) which are intended for use in humans.
In summary, our results support the conclusion that human FH impairs NspA immunogenicity and thus provide one explanation for why a recombinant NspA vaccine that elicited serum bactericidal antibodies in animal models paradoxically failed to do so in humans. The lack of anti-NspA serum bactericidal antibody responses in immunized humans essentially halted further efforts to include NspA in serogroup B vaccines (8). With the information suggesting that binding of human FH to NspA impairs protective anti-NspA antibody responses, it may be possible to revisit NspA as a serogroup B vaccine candidate by investigating the vaccine potential of mutants of NspA that abrogate FH binding but retain important epitopes for eliciting serum bactericidal activity.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants R01 AI046464 and R01 AI114701 (to D.M.G.) from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH). E.L. was supported in part by a San Francisco State University MBRS-RISE fellowship funded by grant R25-GM059298 from NIH. The work was performed in a facility funded by grant C06 RR016226 from the Research Facilities Improvement Program, National Center for Research Resources, NIH.
We declare no conflicts of interest.
We are grateful to Sanjay Ram, University of Massachusetts School of Medicine, Worcester, MA, for reviewing the manuscript and providing critical comments.
Funding Statement
E.L. was supported in part by a San Francisco State University MBRS-RISE fellowship funded by the R25-GM059298 grant from the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01267-15.
REFERENCES
- 1.Martin D, Cadieux N, Hamel J, Brodeur BR. 1997. Highly conserved Neisseria meningitidis surface protein confers protection against experimental infection. J Exp Med 185:1173–1183. doi: 10.1084/jem.185.7.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cadieux N, Plante M, Rioux CR, Hamel J, Brodeur BR, Martin D. 1999. Bactericidal and cross-protective activities of a monoclonal antibody directed against Neisseria meningitidis NspA outer membrane protein. Infect Immun 67:4955–4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin D, Brodeur BR, Hamel J, Couture F, De Alwis U, Lian Z, Martin S, Andrews D, Ellis RW. 2000. Candidate Neisseria meningitidis NspA vaccine. J Biotechnol 83:27–31. doi: 10.1016/S0168-1656(00)00294-7. [DOI] [PubMed] [Google Scholar]
- 4.Lissolo L, Maitre-Wilmotte G, Dumas P, Mignon M, Danve B, Quentin-Millet MJ. 1995. Evaluation of transferrin-binding protein 2 within the transferrin-binding protein complex as a potential antigen for future meningococcal vaccines. Infect Immun 63:884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ala'Aldeen DA. 1996. Transferrin receptors of Neisseria meningitidis: promising candidates for a broadly cross-protective vaccine. J Med Microbiol 44:237–243. doi: 10.1099/00222615-44-4-237. [DOI] [PubMed] [Google Scholar]
- 6.Danve B, Lissolo L, Mignon M, Dumas P, Colombani S, Schryvers AB, Quentin-Millet MJ. 1993. Transferrin-binding proteins isolated from Neisseria meningitidis elicit protective and bactericidal antibodies in laboratory animals. Vaccine 11:1214–1220. doi: 10.1016/0264-410X(93)90045-Y. [DOI] [PubMed] [Google Scholar]
- 7.Danve B, Cadoz M, Lissolo L, Boutry E, Guinet F, Speck D, Nassif X, Quentin-Millet M-J. 1998. Safety and immunogenicity of a Neisseria meningitidis group B transferrin binding protein vaccine in adults, p 53 Abstr Eleventh Int Pathogenic Neisserial Conf, Nice, France http://neisseria.org/ipnc/1998/Abstracts_Neisseria98.pdf. [Google Scholar]
- 8.Halperin SA, Langley JM, Smith B, Wunderli P, Kaufman L, Kimura A, Martin D. 2007. Phase 1 first-in-human studies of the reactogenicity and immunogenicity of a recombinant meningococcal NspA vaccine in healthy adults. Vaccine 25:450–457. doi: 10.1016/j.vaccine.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Lewis LA, Ngampasutadol J, Wallace R, Reid JE, Vogel U, Ram S. 2010. The meningococcal vaccine candidate neisserial surface protein A (NspA) binds to factor H and enhances meningococcal resistance to complement. PLoS Pathog 6:e1001027. doi: 10.1371/journal.ppat.1001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarantonelli ML, Szatanik M, Giorgini D, Hong E, Huerre M, Guillou F, Alonso JM, Taha MK. 2007. Transgenic mice expressing human transferrin as a model for meningococcal infection. Infect Immun 75:5609–5614. doi: 10.1128/IAI.00781-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beernink PT, Shaughnessy J, Braga EM, Liu Q, Rice PA, Ram S, Granoff DM. 2011. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J Immunol 186:3606–3614. doi: 10.4049/jimmunol.1003470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frandoloso R, Martinez-Martinez S, Calmettes C, Fegan J, Costa E, Curran D, Yu RH, Gutierrez-Martin CB, Rodriguez-Ferri EF, Moraes TF, Schryvers AB. 2015. Nonbinding site-directed mutants of transferrin binding protein B exhibit enhanced immunogenicity and protective capabilities. Infect Immun 83:1030–1038. doi: 10.1128/IAI.02572-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa I, Pajon R, Granoff DM. 2014. Human factor H (FH) impairs protective meningococcal anti-FHbp antibody responses and the antibodies enhance FH binding. mBio 5:e01625-14. doi: 10.1128/mBio.01625-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou VC, Moe GR, Raad Z, Wuorimaa T, Granoff DM. 2003. Conformational epitopes recognized by protective anti-neisserial surface protein A antibodies. Infect Immun 71:6844–6849. doi: 10.1128/IAI.71.12.6844-6849.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moe GR, Tan S, Granoff DM. 1999. Differences in surface expression of NspA among Neisseria meningitidis group B strains. Infect Immun 67:5664–5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pajon R, Fergus AM, Granoff DM. 2013. Mutant native outer membrane vesicles combined with a serogroup A polysaccharide conjugate vaccine for prevention of meningococcal epidemics in Africa. PLoS One 8:e66536. doi: 10.1371/journal.pone.0066536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zarantonelli ML, Lancellotti M, Deghmane AE, Giorgini D, Hong E, Ruckly C, Alonso JM, Taha MK. 2008. Hyperinvasive genotypes of Neisseria meningitidis in France. Clin Microbiol Infect 14:467–472. doi: 10.1111/j.1469-0691.2008.01955.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhang MY, Choudhry V, Sidorov IA, Tenev V, Vu BK, Choudhary A, Lu H, Stiegler GM, Katinger HW, Jiang S, Broder CC, Dimitrov DS. 2006. Selection of a novel gp41-specific HIV-1 neutralizing human antibody by competitive antigen panning. J Immunol Methods 317:21–30. doi: 10.1016/j.jim.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pajon R, Beernink PT, Granoff DM. 2012. Design of meningococcal factor H binding protein mutant vaccines that do not bind human complement factor H. Infect Immun 80:2667–2677. doi: 10.1128/IAI.00103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koeberling O, Delany I, Granoff DM. 2011. A critical threshold of meningococcal factor H binding protein expression is required for increased breadth of protective antibodies elicited by native outer membrane vesicle vaccines. Clin Vaccine Immunol 18:736–742. doi: 10.1128/CVI.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moe GR, Zuno-Mitchell P, Lee SS, Lucas AH, Granoff DM. 2001. Functional activity of anti-neisserial surface protein A monoclonal antibodies against strains of Neisseria meningitidis serogroup B. Infect Immun 69:3762–3771. doi: 10.1128/IAI.69.6.3762-3771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moe GR, Zuno-Mitchell P, Hammond SN, Granoff DM. 2002. Sequential immunization with vesicles prepared from heterologous Neisseria meningitidis strains elicits broadly protective serum antibodies to group B strains. Infect Immun 70:6021–6031. doi: 10.1128/IAI.70.11.6021-6031.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giuntini S, Reason DC, Granoff DM. 2011. Complement-mediated bactericidal activity of anti-factor H binding protein monoclonal antibodies against the meningococcus relies upon blocking factor H binding. Infect Immun 79:3751–3759. doi: 10.1128/IAI.05182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vu DM, Wong TT, Granoff DM. 2011. Cooperative serum bactericidal activity between human antibodies to meningococcal factor H binding protein and neisserial heparin binding antigen. Vaccine 29:1968–1973. doi: 10.1016/j.vaccine.2010.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossi R, Beernink PT, Giuntini S, Granoff DM. 2015. Susceptibility of meningococcal strains responsible for two serogroup B outbreaks on U.S. university campuses to serum bactericidal activity elicited by the MenB-4C vaccine. Clin Vaccine Immunol 22:1227–1234. doi: 10.1128/CVI.00474-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beernink PT, Shaughnessy J, Pajon R, Braga EM, Ram S, Granoff DM. 2012. The effect of human factor H on immunogenicity of meningococcal native outer membrane vesicle vaccines with over-expressed factor H binding protein. PLoS Pathog 8:e1002688. doi: 10.1371/journal.ppat.1002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koeberling O, Seubert A, Granoff DM. 2008. Bactericidal antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed factor H-binding protein and genetically attenuated endotoxin. J Infect Dis 198:262–270. doi: 10.1086/589308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Granoff DM, Ram S, Beernink PT. 2013. Does binding of complement factor H to the meningococcal vaccine antigen, factor H binding protein, decrease protective serum antibody responses? Clin Vaccine Immunol 20:1099–1107. doi: 10.1128/CVI.00260-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granoff DM, Costa I, Konar M, Giuntini S, Van Rompay KK, Beernink PT. 2015. Binding of complement factor H (FH) decreases protective anti-FH binding protein antibody responses of infant rhesus macaques immunized with a meningococcal serogroup B vaccine. J Infect Dis 212:784–792. doi: 10.1093/infdis/jiv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konar M, Rossi R, Walter H, Pajon R, Beernink PT. 2015. A mutant library approach to identify improved meningococcal factor H binding protein vaccine antigens. PLoS One 10:e0128185. doi: 10.1371/journal.pone.0128185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossi R, Granoff DM, Beernink PT. 2013. Meningococcal factor H-binding protein vaccines with decreased binding to human complement factor H have enhanced immunogenicity in human factor H transgenic mice. Vaccine 31:5451–5457. doi: 10.1016/j.vaccine.2013.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koeberling O, Ispasanie E, Hauser J, Rossi O, Pluschke G, Caugant DA, Saul A, Maclennan CA. 2014. A broadly-protective vaccine against meningococcal disease in sub-Saharan Africa based on generalized modules for membrane antigens (GMMA). Vaccine 32:2688–2695. doi: 10.1016/j.vaccine.2014.03.068. [DOI] [PubMed] [Google Scholar]
- 33.Koeberling O, Seubert A, Santos G, Colaprico A, Ugozzoli M, Donnelly J, Granoff DM. 2011. Immunogenicity of a meningococcal native outer membrane vesicle vaccine with attenuated endotoxin and over-expressed factor H binding protein in infant rhesus monkeys. Vaccine 29:4728–4734. doi: 10.1016/j.vaccine.2011.04.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.