Abstract
Strongyloides stercoralis is a soil-transmitted helminth organism that infects ∼50 to 100 million people worldwide. Despite its widespread prevalence, very little is known about the immune response that characterizes human S. stercoralis infection. To study the systemic cytokine profile characteristic of Strongyloides infection, we measured the circulating levels of a large panel of pro- and anti-inflammatory cytokines in asymptomatic, infected individuals (n = 32) and compared them to those in uninfected, controls (n = 24). Infected individuals exhibited significantly lower circulating levels of proinflammatory cytokines (gamma interferon [IFN-γ], tumor necrosis factor alpha [TNF-α], and interleukin-1β [IL-1β]) and significantly higher levels of anti-inflammatory cytokines (IL-4, IL-5, IL-9, IL-10, IL-13, IL-27, IL-37, and transforming growth factor β [TGF-β]). Moreover, treatment of Strongyloides infection resulted in a significant reversal of the cytokine profile, with increased levels of proinflammatory (IFN-γ, TNF-α, IL-2, IL-17A, IL-17F, IL-22, IL-23, and IL-1β) and decreased levels of anti-inflammatory (IL-4, IL-5, IL-9, IL-10, IL-13, IL-27, IL-37, and TGF-β) cytokines following treatment. Thus, S. stercoralis infection is characterized by alterations in the levels of systemic cytokines, reflecting major alterations in the underlying immune response to this chronic helminth infection.
INTRODUCTION
Helminths are multicellular eukaryotic worms that reside for long periods of time in their hosts, eliciting type 2 and regulatory T cell immune responses. Among the common helminth parasites known to establish chronic infections in humans, Strongyloides stercoralis, the causative agent of strongyloidiasis, infects over 50 million people worldwide (1). S. stercoralis is unique in its ability to exist in a free-living and auto-infective cycle (2, 3). Strongyloides infection is often clinically asymptomatic and long lasting due, in large part, to the parasites' auto-infective life cycle and their ability to modulate or evade the host immune system (2, 3). Chronic Strongyloides infection can also cause cutaneous, gastrointestinal (GI), and/or pulmonary symptoms and, in the face of immune suppression, may present as hyperinfection syndrome or disseminated strongyloidiasis, conditions that are potentially fatal (4).
Animal models have suggested a role for both innate and adaptive immune mechanisms in mediating resistance to infection (5). The innate response is primarily mediated by eosinophils and interleukin-5 (IL-5), with neutrophils and macrophages playing accessory roles (6, 7). The adaptive immune system specifically involves type 2 responses, with Th2 cells secreting IL-4, IL-5, and IL-13, B cells producing IgG and IgE, and innate lymphoid cells secreting IL-5 and IL-13 (5). In contrast, regulatory T cells help blunt exuberant Th2 responses (8), and the interplay among Th1, Th2, and regulatory T cell responses appears to be crucial in the defense against this infection (4). Very little data are available on the role of these responses in human infection. It has been shown that Th2 responses are essential to protect against hyperinfection (9, 10) and that individuals with strongyloidiasis develop specific antibodies of the IgG, IgA, IgM, and IgE isotypes (11, 12).
To study the association of systemic cytokines (both pro- and anti-inflammatory) with asymptomatic infection, we compared circulating levels of these cytokines in Strongyloides-infected and -uninfected individuals. We also examined the effect of antihelminth treatment by comparing circulating levels of these cytokines before and after treatment. Our study shows that Strongyloides infection is associated with elevated anti-inflammatory and depressed proinflammatory plasma cytokines, a pattern that is reversible following treatment of infection.
MATERIALS AND METHODS
Ethics statement.
All individuals were examined as part of a natural history study protocol approved by Institutional Review Boards of the National Institute of Allergy and Infectious Diseases (USA) and the National Institute for Research in Tuberculosis (India), and informed written consent was obtained from all participants.
Study population.
We studied a total of 58 individuals comprising 34 clinically asymptomatic, Strongyloides-infected (here, infected) individuals and 24 uninfected, healthy (here, uninfected) individuals in Tamil Nadu, South India (Tables 1 and 2). These individuals were all recruited from a rural population by screening of individuals for helminth infection by stool microscopy and serology. Inclusion criteria were age of 18 to 65 years and willingness to give blood and stool samples for examination; exclusion criteria were past antihelminth treatment, other helminth infections, or HIV infection. Follow-up was performed at 6 months following recruitment and treatment. Strongyloides infection was diagnosed by the presence of IgG antibodies to the recombinant antigen, NIE, as described previously (13, 14). This was further confirmed by specialized stool examination with nutrient agar plate cultures (15). None of the study population had lymphatic filariasis or other intestinal helminths (based on stool microscopy). All infected individuals were treated with single doses of ivermectin and albendazole, and follow-up blood draws were obtained 6 months later (Table 3). All uninfected individuals were anti-Strongyloides NIE negative and negative for filarial and other intestinal helminths.
TABLE 1.
Demographic profile of infected and uninfected individuals
| Parameter | Value for the groupa |
|
|---|---|---|
| Infected (n = 32) | Uninfected (n = 24) | |
| No. of male subjects | 19 | 16 |
| No. of female subjects | 13 | 8 |
| Mean age (range [yr]) | 36 (20–61) | 40 (20–60) |
| NIE ELISA result | Positive | Negative |
| Clinical status | Healthy | Healthy |
| Symptom(s) | None | None |
| Socioeconomic status | Rural workers | Rural workers |
| Result of stool examination for S. stercoralis | Positive (negative following treatment) | Negative |
| Presence of other helminth infections | Negative | Negative |
Differences in the values for gender and age between infected and uninfected groups were not significant.
TABLE 2.
Hematological profile of infected and uninfected individuals
| Factora | GM (range) for the group |
P valueb | |
|---|---|---|---|
| Infected (n = 32) | Uninfected (n = 24) | ||
| Hb (gm/dl) | 12.48 (4.9–18.6) | 11.14 (4.9–16.3) | 0.0378 |
| RBC (106/ml) | 4.5 (3.5–6.06) | 4.067 (2.11–5.84) | 0.0391 |
| WBC (103/ml) | 8.83 (5.8–16.9) | 8.81 (5.8–13.7) | NS |
| HCT (%) | 36.85 (19.5–53) | 33.55 (15–47.4) | NS |
| PLT (103/ml) | 261.91 (140–417) | 258.18 (198–363) | NS |
| Neutrophils (103/ml) | 5.3 (3.3–7.2) | 5.5 (4.2–6.89) | NS |
| Lymphocytes (103/ml) | 2.63 (1.45–3.71) | 3.04 (2.02–4.27) | 0.0248 |
| Monocytes (103/ml) | 0.67 (0.38–1.2) | 0.71 (0.43–1.09) | NS |
| Eosinophils (103/ml) | 0.68 (0.11–3.45) | 0.43 (0.18–1.1) | 0.0354 |
| Basophils (103/ml) | 0.09 (0.02–0.33) | 0.09 (0.05–0.35) | 0.0028 |
Hb, hemoglobin; RBC, red blood cells; WBC, white blood cells; HCT, hematocrit; PLT, platelets.
NS, not significant.
TABLE 3.
Hematological profile of infected individuals before and after treatment
| Factora | GM (range) for the groupb |
|
|---|---|---|
| Pretreatment (n = 32) | Posttreatment (n = 32) | |
| Hb (gm/dl) | 12.48 (4.9–18.6) | 12.59 (4.6–18.5) |
| RBC (106/ml) | 4.5 (3.5–6.06) | 4.54 (3.49–6.06) |
| WBC (103/ml) | 8.83 (5.8–16.9) | 8.03 (5.6–11.2) |
| HCT (%) | 36.85 (19.5–53) | 37.47 (15.9–53) |
| PLT (103/ml) | 261.91 (140–417) | 262.77 (134–417) |
| Neutrophils (103/ml) | 5.3 (3.3–7.2) | 5.1 (3.31–6.89) |
| Lymphocytes (103/ml) | 2.63 (1.45–3.71) | 2.84 (1.52–4.29) |
| Monocytes (103/ml) | 0.67 (0.38–1.2) | 0.67 (0.38–1.24) |
| Eosinophils (103/ml) | 0.68 (0.11–3.45) | 0.66 (0.11–3.45) |
| Basophils (103/ml) | 0.09 (0.02–0.33) | 0.08 (0.02–0.33) |
Hb, hemoglobin; RBC, red blood cells; WBC, white blood cells; HCT, hematocrit; PLT, platelets.
For all parameters, differences between pretreatment and posttreatment values were not significant.
Hematological parameters.
Leukocyte counts and differentials were performed on all individuals using an AcT 5Diff hematology analyzer (Beckman Coulter).
ELISA.
Plasma levels of gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), IL-2, IL-4, IL-5, IL-10, IL-13, IL-1β, IL-17A, IL-17F, IL-18, IL-22, IL-23, IL-27, IL-37, transforming growth factor β (TGF-β) (all, R&D Systems) and IL-9 (eBioscience) were measured by enzyme-linked immunosorbent assay (ELISA), according to the manufacturer's instructions. All samples were run in duplicates.
Statistical analysis.
Data analyses were performed using GraphPad Prism (GraphPad Software, Inc., San Diego, CA, USA). Geometric means (GM) were used for measurements of central tendencies. Comparisons were made using either a Mann-Whitney U test for comparisons between two groups or a Wilcoxon signed-rank test for comparisons within groups. Corrections for multiple comparisons were performed by Holm's correction. Multidimensional scaling (MDS) analysis was performed on log2-transformed plasma cytokine levels for infected versus uninfected individuals using the R language.
RESULTS
Study population characteristics.
The baseline characteristics, including demographic and hematological features of the study population, are shown in Tables 1 and 2. As can be seen, infected individuals exhibited significantly higher levels of red blood cells, hemoglobin, and absolute eosinophil counts than uninfected individuals but significantly lower levels of absolute lymphocyte and basophil counts. In contrast, no significant differences in age, gender, socioeconomic status, or other hematological parameters were observed between the two groups. There were no significant differences in any of the hematological parameters following antihelminth treatment of infected individuals compared to pretreatment values (Table 3). However, NIE ELISA levels decreased significantly in all of the individuals following treatment (Fig. 1).
FIG 1.
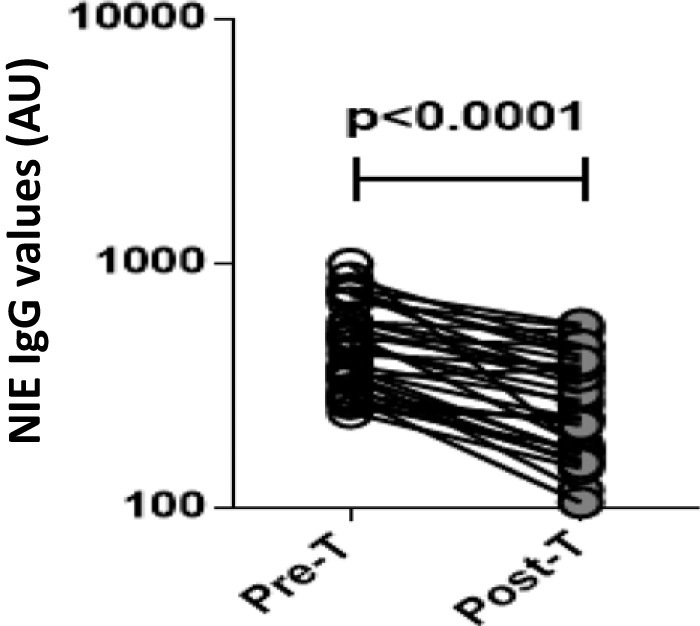
Treatment of Strongyloides infection is associated with decreased NIE-IgG ELISA values. The NIE-IgG levels (in arbitrary units, AU) were measured by ELISA in infected (n = 32) individuals before (Pre-T) and 6 months after (Post-T) antihelminth treatment. The results are shown as line diagrams with each line representing a single individual. The P value was calculated using a Wilcoxon signed-rank test.
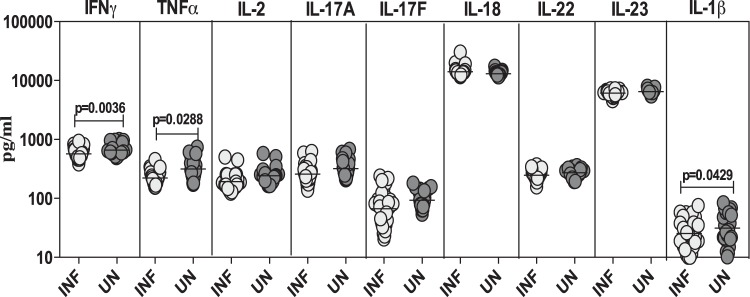
Strongyloides infection is associated with diminished systemic levels of proinflammatory cytokines.
To determine the systemic proinflammatory cytokine profile in Strongyloides infection, we measured the circulating levels of IFN-γ, TNF-α, IL-2, IL-17A, IL-17F, IL-18, IL-22, IL-23, and IL-1β in asymptomatic, infected and in control, uninfected individuals (Fig. 2). As shown in Fig. 2, the systemic levels of the proinflammatory cytokines IFN-γ (GM of 568.9 pg/ml in infected versus 656.1 pg/ml in uninfected subjects; P = 0.0036), TNF-α (GM of 222.1 pg/ml versus 315.2 pg/ml; P = 0.0288), and IL-1β (GM of 25.5 pg/ml versus 31.4 pg/ml; P = 0.0429) were significantly lower in infected than in uninfected individuals.
FIG 2.
Strongyloides infections are associated with diminished plasma levels of proinflammatory cytokines at homeostasis. The plasma levels of proinflammatory cytokines (IFN-γ, TNF-α, IL-2, IL-17A, IL-17F, IL-18, IL-22, IL-23, and IL-1β) were measured by ELISA in infected (INF; n = 32) and uninfected (UN; n = 24) individuals. The results are shown as scatter plots with each circle representing a single individual and the bar representing the GM. P values were calculated using a Mann-Whitney test with Holm's correction for multiple comparisons.
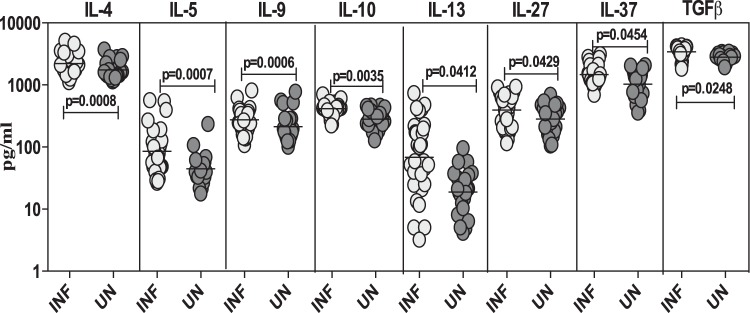
Strongyloides infection is associated with elevated levels of anti-inflammatory cytokines.
To determine the systemic anti-inflammatory cytokine profiles in Strongyloides infection, we measured the circulating levels of IL-4, IL-5, IL-9, IL-10, IL-13, IL-27, IL-37, and TGF-β in infected and uninfected individuals (Fig. 3). As shown in Fig. 3, the systemic levels of the cytokines IL-4 (GM of 2,219.8 pg/ml in infected versus 1,746.1 pg/ml in uninfected; P = 0.0008), IL-5 (GM of 85.4 pg/ml versus 44.4 pg/ml; P = 0.0007), IL-9 (GM of 274.2 pg/ml versus 212.6 pg/ml; P = 0.0006), IL-10 (GM of 415.2 pg/ml versus 280.7 pg/ml; P = 0.0035), IL-13 (GM of 68.6 pg/ml versus 18.9 pg/ml; P = 0.0412), IL-27 (GM of 393.1 pg/ml versus 282.9 pg/ml; P = 0.0429), IL-37 (GM of 1,472.2 pg/ml versus 1,025.8 pg/ml; P = 0.0454), and TGF-β (GM of 3,416.9 pg/ml versus 2,809.2 pg/ml; P = 0.0248) were all significantly higher in infected than in uninfected individuals.
FIG 3.
Strongyloides infections are associated with heightened plasma levels of anti-inflammatory cytokines at homeostasis. The plasma levels of anti-inflammatory cytokines (IL-4, IL-5, IL-9, IL-10, IL-13, IL-27, IL-37, and TGF-β) were measured by ELISA in infected (INF; n = 32) and uninfected (UN; n = 24) individuals. The results are shown as scatter plots with each circle representing a single individual and the bar representing the GM. P values were calculated using a Mann-Whitney test with Holm's correction for multiple comparisons.
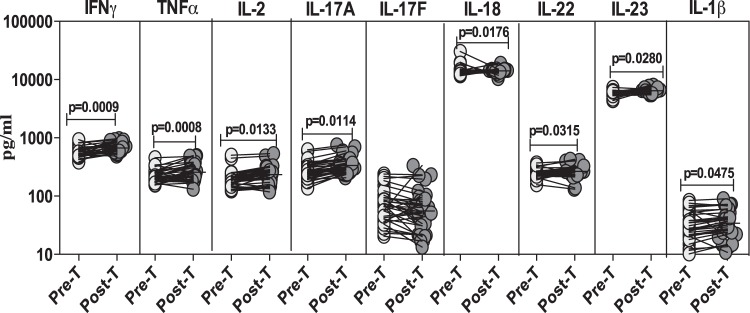
Alterations in systemic levels of pro- and anti-inflammatory cytokines following treatment of Strongyloides infection.
To determine the effect of treatment on the systemic proinflammatory cytokine profile in Strongyloides infection, we measured the circulating levels of IFN-γ, TNF-α, IL-2, IL-17A, IL-17F, IL-18, IL-22, IL-23, and IL-1β in infected individuals before and after treatment (Fig. 4). As shown in Fig. 4, the systemic levels of the proinflammatory cytokines IFN-γ (GM of 568.9 pg/ml pretreatment versus 670.7 pg/ml posttreatment; P = 0.0009), TNF-α (GM of 222.1 pg/ml versus 256.3 pg/ml; P = 0.0008), IL-2 (GM of 192.7 pg/ml versus 234.3 pg/ml; P = 0.0133), IL-17A (GM of 258.8 pg/ml versus 335.5 pg/ml; P = 0.0114), IL-18 (GM of 14,031.3 pg/ml versus 14,052.2 pg/ml; P = 0.0176), IL-22 (GM of 247.3 pg/ml versus 266.4 pg/ml; P = 0.0315), IL-23 (GM of 6,092.3 pg/ml versus 6,424.3 pg/ml; P = 0.0280), and IL-1β (GM of 25.5 pg/ml versus 34.1 pg/ml; P = 0.0475) were significantly increased from pretreatment levels 6 months following treatment.
FIG 4.
Treatment of Strongyloides infection is associated with heightened plasma levels of proinflammatory cytokines. The plasma levels of proinflammatory cytokines (IFN-γ, TNF-α, IL-2, IL-17A, IL-17F, IL-18, IL-22, IL-23, and IL-1β) were measured by ELISA in infected (n = 32) individuals before (Pre-T) and 6 months after (Post-T) antihelminth treatment. The results are shown as line diagrams with each line representing a single individual. P values were calculated using a Wilcoxon signed-rank test with Holm's correction for multiple comparisons.
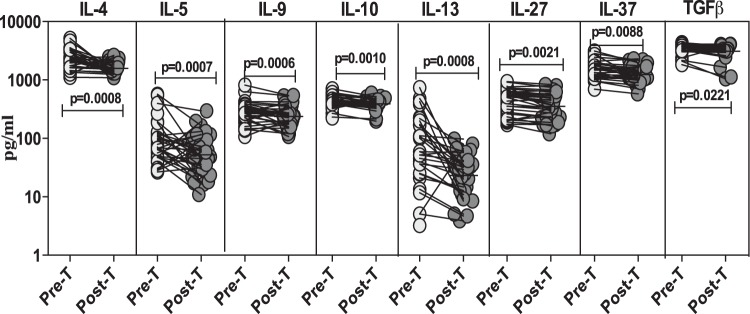
To determine the effect of treatment on the systemic anti-inflammatory cytokine profile in Strongyloides infection, we measured the circulating levels of IL-4, IL-5, IL-9, IL-10, IL-13, IL-27, IL-37, and TGF-β in infected individuals before and after treatment (Fig. 5). As shown in Fig. 5, the systemic levels of the anti-inflammatory cytokines IL-4 (GM of 2,219.8 pg/ml pretreatment versus 1,581.7 pg/ml posttreatment; P = 0.0008), IL-5 (GM of 85.5 pg/ml versus 52.4 pg/ml; P = 0.0007), IL-9 (GM of 274.2 pg/ml versus 237.7 pg/ml; P = 0.0006), IL-10 (GM of 415.2 pg/ml versus 389.8 pg/ml; P = 0.0010), IL-13 (GM of 68.6 pg/ml versus 23.3 pg/ml; P = 0.0008), IL-27 (GM of 393.1 pg/ml versus 353.4 pg/ml; P = 0.0021), IL-37 (GM of 1,472.2 pg/ml versus 1,236.9 pg/ml; P = 0.0088), and TGF-β (GM of 3,416.9 pg/ml versus 3,103.1 pg/ml; P = 0.0221) were markedly lower than pretreatment levels 6 months following treatment.
FIG 5.
Treatment of Strongyloides infection is associated with diminished plasma levels of anti-inflammatory cytokines. The plasma levels of anti-inflammatory cytokines (IL-4, IL-5, IL-9, IL-10, IL-13, IL-27, IL-37, and TGF-β) were measured by ELISA in infected (n = 32) individuals before (Pre-T) and 6 months after (Post-T) antihelminth treatment. The results are shown as line diagrams with each line representing a single individual. P values were calculated using a Wilcoxon signed-rank test with Holm's correction for multiple comparisons.
MDS analysis reveals trends of cytokine modulation in Strongyloides infection.
Because many of these cytokines are coordinately regulated, we sought to visualize the trends in the modulation of cytokines in Strongyloides infection by treating the cytokines as interdependent variables using MDS analysis on values for infected versus uninfected individuals (Fig. 6). MDS analysis enables the discrimination and visual clustering of two or more groups wherein individuals with similar patterns of cytokine expression are placed next to each other. As shown in Fig. 6, infected and uninfected individuals visually segregate on an MDS plot, based on their baseline plasma cytokine profiles. Thus, MDS analysis reveals clear trends in the modulation of systemic cytokines in Strongyloides infection.
FIG 6.
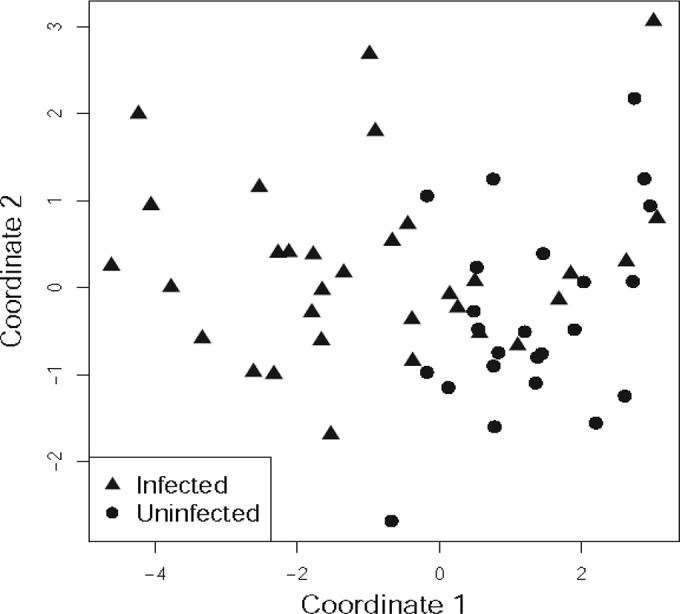
MDS analysis reveals clear trends in the systemic cytokine levels in Strongyloides infection. MDS plots for log2-transformed plasma cytokine levels were constructed to analyze the trends in the differences of systemic cytokine levels between infected and uninfected individuals. Each symbol represents one individual based on values of all of the cytokines studied. The percentage of variation is depicted on the two axes. The distance between each symbol represents the relatedness between each individual.
DISCUSSION
Helminth infections are typically associated with a profound modulation of the immune response such that many arms of the immune system, including innate and adaptive immunity, as well as humoral and cellular responses, are affected (16, 17). This is classically exemplified in animal models of helminth infection, wherein proinflammatory responses, including type 1 and type 17 responses, are down-modulated and type 2 and anti-inflammatory responses are typically upregulated (16, 17). This has been shown to be true in human helminth (soil-transmitted and vector-borne) infections as well (18–20). However, scant data exist on the immune response engendered by the relatively common helminth parasite S. stercoralis. In this study, we sought to examine the systemic cytokine profile of Strongyloides-infected individuals and the effect of treatment on this cytokine profile.
Helminth infections have the propensity to control harmful inflammatory responses and promote homeostasis through systemic immune responses (21, 22). The induction of both type 2 and regulatory cytokine responses is postulated to contribute to the modulation of proinflammatory, type 1, and type 17 cytokine responses (21, 22). In this study, we examined the systemic cytokine levels of type 1, type 17, and other proinflammatory cytokines in Strongyloides infection and demonstrated that Strongyloides infection is associated with markedly diminished levels of some of these proinflammatory cytokines. Of greater interest perhaps are our data on the posttreatment responses that suggest that the presence of Strongyloides infection has a major influence on the systemic levels of other proinflammatory cytokines as well since the majority of proinflammatory cytokines examined exhibited enhanced levels posttreatment compared to their pretreatment levels. Type 1 cytokines, including IFN-γ, TNF-α, and IL-2, are typically associated with immunity to intracellular pathogens but could also contribute to the development of inflammatory and autoimmune disorders in a variety of settings (23, 24). On a similar note, type 17 cytokines, including IL-17A, IL-17F, IL-22, and IL-23, are associated with protective immunity against fungal pathogens but also contribute heavily to the pathogenesis of inflammatory and autoimmune disorders (25, 26). Finally, other proinflammatory cytokines, including IL-1β and IL-18, are major components driving harmful host inflammation (27). Thus, the fact that Strongyloides infection is associated with diminished levels (from normal) of most of the above-mentioned cytokines provides evidence that Strongyloides infection has the potential benefit of modulating harmful inflammation and autoimmunity in the host.
Helminth infections are typically characterized by increased production of type 2 cytokines, including IL-4, IL-5, IL-9, and IL-13 (16). In addition, helminth infections are also intricately associated with the increased expression of the regulatory cytokines IL-10 and TGF-β (17). Thus, the immune response induced by most helminth infections is composed of two compartments, a type 2 cytokine response and a regulatory cytokine response, both of which may contribute to the overall protective immune response to these infections (21). In addition, type 2 cytokine responses (particularly IL-13) also play an important role in fibrosis and wound healing, postulated to help heal the tissue damage induced by many tissue-invasive helminth parasites (28). Our study confirms data from both animal models and human infections for which predominant type 2 and regulatory cytokine responses in the setting of chronic, asymptomatic helminth infection have been reported. Both type 2 (IL-4, IL-5, IL-9, and IL-13) and regulatory (IL-10 and TGF-β) cytokines were present at markedly higher levels in infected individuals, and this was reversed following treatment. Of additional interest, we have also explored the role of two other anti-inflammatory cytokines in this infection and demonstrated that IL-27 and IL-37 are both present at elevated levels in infected individuals and that these levels are significantly diminished following therapy. IL-27 was initially described as a Th1-promoting factor, but subsequent studies have demonstrated its anti-inflammatory role (29–31). IL-27 has been shown to convert activated CD4+ T cells into IL-10-producing Th1 cells or Tr1 cells, to suppress the production of IL-2, and to downregulate Th17 responses (32). IL-37 belongs to the IL-1 family of cytokines, but, unlike the other members of this family, its main role is the downregulation of inflammation (33–35). The anti-inflammatory activity of IL-37 appears to be IL-10 independent (35). Our study is the first to our knowledge to examine IL-27 and IL-37 in helminth infections and suggests that these cytokines could have immunoregulatory effects in Strongyloides infection.
Although Strongyloides is an intestinal helminth infection, it is clearly associated with profound alterations in the systemic cytokine response, a finding that may relate to the auto-infective cycle seen in Strongyloides. It has been postulated that this parasite can trigger a potent immune response in the gut that stimulates activated GI-associated dendritic cells to migrate through the lymphatic ducts to stimulate Th2 cell and regulatory T and B cell responses in the draining lymph nodes (21). In addition, excretory/secretory products might also disseminate and contribute to the development of systemic cytokine responses (36). Finally, since S. stercoralis migrates from the skin to the lungs through the circulation and then subsequently enters the intestine, it can also stimulate local immune responses at these various sites that can contribute to systemic cytokine responses, as has been reported for other intestinal helminths in animal models (37, 38). Thus, there are multiple mechanisms by which Strongyloides infection can exert its effects. Our study depends on both serological and microscopic confirmation of Strongyloides infection and illustrates the reliability of an NIE ELISA in diagnosing infection since ELISA values decreased significantly after treatment. However, a limitation of the study is that while statistically significant changes were observed in the different groups, the biological significance of these changes remains to be elucidated. Moreover, the sample size is another limitation of the study.
Helminths may protect humans against allergic and autoimmune disease, and, indeed, helminth-derived products have been shown to prevent the development of such inflammatory diseases in mouse models and in experimental human trials (39). Here, we show that Strongyloides infection can profoundly modulate the systemic cytokine environment of the host by inducing strong anti-inflammatory responses and suppressing (possibly pathological) proinflammatory responses. In addition, independent analysis using MDS also confirms the trends observed in the modulation of systemic cytokines in Strongyloides infection and the separation of infected from uninfected individuals. Hence, it is possible that such immune modulation could also protect against exaggerated inflammatory responses associated with inflammatory and autoimmune diseases.
ACKNOWLEDGMENTS
We thank Satiswaran and Prabbu Balakrishnan for valuable assistance in collecting the clinical data for this study. We thank Kadar Moideen, Jovvian George, and Pavan Kumar for technical assistance. We thank the staff of the Department of Epidemiology, NIRT, for valuable assistance in recruiting the patients for this study.
Funding Statement
This work was funded by the Division of Intramural Research, NIAID, NIH. The funders had no role in study design, data collection or interpretation, or the decision to publish.
REFERENCES
- 1.Puthiyakunnon S, Boddu S, Li Y, Zhou X, Wang C, Li J, Chen X. 2014. Strongyloidiasis—an insight into its global prevalence and management. PLoS Negl Trop Dis 8:e3018. doi: 10.1371/journal.pntd.0003018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montes M, Sawhney C, Barros N. 2010. Strongyloides stercoralis: there but not seen. Curr Opin Infect Dis 23:500–504. doi: 10.1097/QCO.0b013e32833df718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toledo R, Munoz-Antoli C, Esteban JG. 2015. Strongyloidiasis with emphasis on human infections and its different clinical forms. Adv Parasitol 88:165–241. doi: 10.1016/bs.apar.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Keiser PB, Nutman TB. 2004. Strongyloides stercoralis in the Immunocompromised Population. Clin Microbiol Rev 17:208–217. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonne-Annee S, Hess JA, Abraham D. 2011. Innate and adaptive immunity to the nematode Strongyloides stercoralis in a mouse model. Immunol Res 51:205–214. doi: 10.1007/s12026-011-8258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galioto AM, Hess JA, Nolan TJ, Schad GA, Lee JJ, Abraham D. 2006. Role of eosinophils and neutrophils in innate and adaptive protective immunity to larval strongyloides stercoralis in mice. Infect Immun 74:5730–5738. doi: 10.1128/IAI.01958-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe K, Noda K, Hamano S, Koga M, Kishihara K, Nomoto K, Tada I. 2000. The crucial role of granulocytes in the early host defense against Strongyloides ratti infection in mice. Parasitol Res 86:188–193. doi: 10.1007/s004360050030. [DOI] [PubMed] [Google Scholar]
- 8.Montes M, Sanchez C, Verdonck K, Lake JE, Gonzalez E, Lopez G, Terashima A, Nolan T, Lewis DE, Gotuzzo E, and White AC Jr. 2009. Regulatory T cell expansion in HTLV-1 and strongyloidiasis co-infection is associated with reduced IL-5 responses to Strongyloides stercoralis antigen. PLoS Negl Trop Dis 3:e456. doi: 10.1371/journal.pntd.0000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iriemenam NC, Sanyaolu AO, Oyibo WA, Fagbenro-Beyioku AF. 2010. Strongyloides stercoralis and the immune response. Parasitol Int 59:9–14. doi: 10.1016/j.parint.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Porto AF, Neva FA, Bittencourt H, Lisboa W, Thompson R, Alcantara L, Carvalho EM. 2001. HTLV-1 decreases Th2 type of immune response in patients with strongyloidiasis. Parasite Immunol 23:503–507. doi: 10.1046/j.1365-3024.2001.00407.x. [DOI] [PubMed] [Google Scholar]
- 11.Atkins NS, Lindo JF, Lee MG, Conway DJ, Bailey JW, Robinson RD, Bundy DA. 1997. Humoral responses in human strongyloidiasis: correlations with infection chronicity. Trans R Soc Trop Med Hyg 91:609–613. doi: 10.1016/S0035-9203(97)90049-3. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues RM, de Oliveira MC, Sopelete MC, Silva DA, Campos DM, Taketomi EA, Costa-Cruz JM. 2007. IgG1, IgG4, and IgE antibody responses in human strongyloidiasis by ELISA using Strongyloides ratti saline extract as heterologous antigen. Parasitol Res 101:1209–1214. doi: 10.1007/s00436-007-0602-z. [DOI] [PubMed] [Google Scholar]
- 13.Bisoffi Z, Buonfrate D, Sequi M, Mejia R, Cimino RO, Krolewiecki AJ, Albonico M, Gobbo M, Bonafini S, Angheben A, Requena-Mendez A, Munoz J, Nutman TB. 2014. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis 8:e2640. doi: 10.1371/journal.pntd.0002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buonfrate D, Sequi M, Mejia R, Cimino RO, Krolewiecki AJ, Albonico M, Degani M, Tais S, Angheben A, Requena-Mendez A, Munoz J, Nutman TB, Bisoffi Z. 2015. Accuracy of five serologic tests for the follow up of Strongyloides stercoralis infection. PLoS Negl Trop Dis 9:e0003491. doi: 10.1371/journal.pntd.0003491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato Y, Kobayashi J, Toma H, Shiroma Y. 1995. Efficacy of stool examination for detection of Strongyloides infection. Am J Trop Med Hyg 53:248–250. [DOI] [PubMed] [Google Scholar]
- 16.Allen JE, Maizels RM. 2011. Diversity and dialogue in immunity to helminths. Nat Rev Immunol 11:375–388. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 17.Maizels RM, Yazdanbakhsh M. 2003. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol 3:733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 18.Babu S, Nutman TB. 2014. Immunology of lymphatic filariasis. Parasite Immunol 36:338–346. doi: 10.1111/pim.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girgis NM, Gundra UM, Loke P. 2013. Immune regulation during helminth infections. PLoS Pathog 9:e1003250. doi: 10.1371/journal.ppat.1003250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grencis RK. 2015. Immunity to helminths: resistance, regulation, and susceptibility to gastrointestinal nematodes. Annu Rev Immunol 33:201–225. doi: 10.1146/annurev-immunol-032713-120218. [DOI] [PubMed] [Google Scholar]
- 21.Mishra PK, Palma M, Bleich D, Loke P, Gause WC. 2014. Systemic impact of intestinal helminth infections. Mucosal Immunol 7:753–762. doi: 10.1038/mi.2014.23. [DOI] [PubMed] [Google Scholar]
- 22.Weinstock JV, Elliott DE. 2014. Helminth infections decrease host susceptibility to immune-mediated diseases. J Immunol 193:3239–3247. doi: 10.4049/jimmunol.1400927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbas AK, Murphy KM, Sher A. 1996. Functional diversity of helper T lymphocytes. Nature 383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 24.Pearson CI, McDevitt HO. 1999. Redirecting Th1 and Th2 responses in autoimmune disease. Curr Top Microbiol Immunol 238:79–122. [DOI] [PubMed] [Google Scholar]
- 25.Baeten DL, Kuchroo VK. 2013. How cytokine networks fuel inflammation: interleukin-17 and a tale of two autoimmune diseases. Nat Med 19:824–825. doi: 10.1038/nm.3268. [DOI] [PubMed] [Google Scholar]
- 26.Zuniga LA, Jain R, Haines C, Cua DJ. 2013. Th17 cell development: from the cradle to the grave. Immunol Rev 252:78–88. doi: 10.1111/imr.12036. [DOI] [PubMed] [Google Scholar]
- 27.Garlanda C, Dinarello CA, Mantovani A. 2013. The interleukin-1 family: back to the future. Immunity 39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen JE, Sutherland TE. 2014. Host protective roles of type 2 immunity: parasite killing and tissue repair, flip sides of the same coin. Semin Immunol 26:329–340. doi: 10.1016/j.smim.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamano S, Himeno K, Miyazaki Y, Ishii K, Yamanaka A, Takeda A, Zhang M, Hisaeda H, Mak TW, Yoshimura A, Yoshida H. 2003. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity 19:657–667. doi: 10.1016/S1074-7613(03)00298-X. [DOI] [PubMed] [Google Scholar]
- 30.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. 2002. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 16:779–790. doi: 10.1016/S1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 31.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. 2003. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity 19:645–655. doi: 10.1016/S1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida H, Hunter CA. 2015. The immunobiology of interleukin-27. Annu Rev Immunol 33:417–443. doi: 10.1146/annurev-immunol-032414-112134. [DOI] [PubMed] [Google Scholar]
- 33.Bulau AM, Fink M, Maucksch C, Kappler R, Mayr D, Wagner K, Bufler P. 2011. In vivo expression of interleukin-37 reduces local and systemic inflammation in concanavalin A-induced hepatitis. ScientificWorldJournal 11:2480–2490. doi: 10.1100/2011/968479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNamee EN, Masterson JC, Jedlicka P, McManus M, Grenz A, Collins CB, Nold MF, Nold-Petry C, Bufler P, Dinarello CA, Rivera-Nieves J. 2011. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci U S A 108:16711–16716. doi: 10.1073/pnas.1111982108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. 2010. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol 11:1014–1022. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McSorley HJ, Hewitson JP, Maizels RM. 2013. Immunomodulation by helminth parasites: defining mechanisms and mediators. Int J Parasitol 43:301–310. doi: 10.1016/j.ijpara.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 37.Harvie M, Camberis M, Tang SC, Delahunt B, Paul W, Le Gros G. 2010. The lung is an important site for priming CD4 T-cell-mediated protective immunity against gastrointestinal helminth parasites. Infect Immun 78:3753–3762. doi: 10.1128/IAI.00502-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obata-Ninomiya K, Ishiwata K, Tsutsui H, Nei Y, Yoshikawa S, Kawano Y, Minegishi Y, Ohta N, Watanabe N, Kanuka H, Karasuyama H. 2013. The skin is an important bulwark of acquired immunity against intestinal helminths. J Exp Med 210:2583–2595. doi: 10.1084/jem.20130761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harnett W, Harnett MM. 2010. Helminth-derived immunomodulators: can understanding the worm produce the pill? Nat Rev Immunol 10:278–284. doi: 10.1038/nri2730. [DOI] [PubMed] [Google Scholar]