Abstract
Numerous pathogens, including Mycobacterium tuberculosis, can activate human γ9δ2 T cells to proliferate and express effector mechanisms. γ9δ2 T cells can directly inhibit the growth of intracellular mycobacteria and may also act as antigen-presenting cells (APC). Despite evidence for γδ T cells having the capacity to function as APC, the mechanisms involved and importance of these effects on overall tuberculosis (TB) immunity are unknown. We prepared M. tuberculosis-specific γ9δ2 T cell lines to study their direct protective effects and APC functions for M. tuberculosis-specific αβ T cells. The direct inhibitory effects on intracellular mycobacteria were measured, and the enhancing effects on proliferative and effector responses of αβ T cells assessed. Furthermore, the importance of cell-to-cell contact and soluble products for γ9δ2 T cell effector responses and APC functions were investigated. We demonstrate, in addition to direct inhibitory effects on intracellular mycobacteria, the following: (i) γ9δ2 T cells enhance the expansion of M. tuberculosis-specific αβ T cells and increase the ability of αβ T cells to inhibit intracellular mycobacteria; (ii) although soluble mediators are critical for the direct inhibitory effects of γ9δ2 T cells, their APC functions do not require soluble mediators; (iii) the APC functions of γ9δ2 T cells involve cell-to-cell contact that is dependent on CD40-CD40 ligand (CD40L) interactions; and (iv) fully activated CD4+ αβ T cells and γ9δ2 T cells provide similar immune enhancing/APC functions for M. tuberculosis-specific T cells. These effector and helper effects of γ9δ2 T cells further indicate that these T cells should be considered important new targets for new TB vaccines.
INTRODUCTION
Tuberculosis (TB) is a major health problem worldwide, killing about 1.5 million people every year (1). Despite widespread use of a TB vaccine (Mycobacterium bovis BCG [bacillus Calmette-Guérin]) for more than 60 years, there has been minimal impact on the overall prevalence of TB infection and disease. Vaccination with BCG given at birth prevents the most severe complications of infection with Mycobacterium tuberculosis, the causative agent of TB (2). However, the estimated overall protective efficacy of BCG against adult pulmonary TB has varied from 0% to 80% (3). The reasons for such wide variations in estimated BCG efficacy are not fully understood, but environmental and genetic factors are likely involved (4). These factors may also affect the efficacy of new TB vaccines. The development of new and more effective TB vaccines will require a more detailed understanding of mycobacterial immunity (4, 5).
M. tuberculosis is an intracellular organism residing mainly in monocytes/macrophages (6) and requiring cellular immune responses for control. Cytokines produced by CD4+ Th1 cells (e.g., tumor necrosis factor alpha [TNF-α] and gamma interferon [IFN-γ]) activate macrophages to inhibit the replication of intracellular bacilli (7). In addition, memory CD8+ T cells can recognize and destroy M. tuberculosis-infected macrophages by the secretion of perforin, granzyme, and granulysin (8). γ9δ2 T cells also have been shown to produce Th1 cytokines and exhibit cytolytic activity, as well as inhibit intracellular mycobacteria (9–12). γ9δ2 T cells comprise only 3% to 5% of total human peripheral blood lymphocytes (13, 14), and yet, they expand to large numbers after exposure to antigen (15). γ9δ2 T cells proliferate and develop relevant effector functions in response to M. tuberculosis (15), HIV (16), Plasmodium spp. (17), and many other human pathogens (18–22). Furthermore, depletion of circulating γ9δ2 T cells during infections with different pathogens has been associated with increased susceptibility to more severe disease (23–25). In addition, γ9δ2 T cells can be stimulated by naturally occurring nonpeptidic antigens, such as prenyl pyrophosphates (also known as phosphoantigens), potentially broadening the host immune recognition of invading mycobacterial pathogens (26, 27). However, although γ9δ2 T cells can be expanded by stimulation with phosphoantigens, we have previously demonstrated that these phosphoantigen-expanded γ9δ2 T cells do not provide optimal protective effects capable of inhibiting intracellular mycobacterial growth (28, 29). Therefore, the specific M. tuberculosis antigens capable of inducing γ9δ2 T cells relevant for TB protective immunity remain to be identified. In addition, the interactions of γ9δ2 T cells with other immune cells are not fully known.
Protective TB immunity will likely depend upon the interplay of multiple different immune cell subsets which must act in concert to prevail over the immune-evading mechanisms of virulent tubercle bacilli. We have investigated the effects of γ9δ2 T cells expanded by different subsets of antigen-presenting cells (APC) on the inhibition of intracellular mycobacteria and on the development of αβ T cell responses directed against mycobacteria. We find that mycobacterium-infected dendritic cells (DC) induce γ9δ2 T cells with potent protective effects against intracellular mycobacterial growth. These γ9δ2 T cells that expanded with infected DC also enhanced the proliferation, effector functions, and inhibitory activities of mycobacterium-specific CD4+ and CD8+ αβ T cells. Mechanistically, the enhancing effects of γ9δ2 T cells for αβ T cell responses were dependent upon antigen processing, antigen presentation, and CD40-CD40 ligand (CD40L) interactions. We further demonstrate that, in contrast to previous reports, γ9δ2 T cells and αβ T cells displayed similar overall antigen presentation capacity after comparable activation.
MATERIALS AND METHODS
Samples.
Peripheral blood mononuclear cells (PBMC) were obtained by Ficoll-Paque (GE Healthcare, Piscataway, NJ) centrifugation of leukapheresis samples obtained from healthy purified protein derivative (PPD)-positive volunteers. All PPD-positive volunteers had a history of either latent TB infection or BCG vaccination. The protocol for leukapheresis was approved by the Saint Louis University Institutional Review Board (IRB), and informed consent was obtained from each volunteer. Portions of these PBMC were used for the generation of dendritic cells (DC) with cocktails of cytokines, including granulocyte-macrophage colony-stimulating factor (GM-CSF) (Immunex, Seattle, WA), interleukin 4 (IL-4) (R&D, Minneapolis, MN), IL-6 (BD Biosciences, San Jose, CA), IL-1β (BD Biosciences), TNF-α (Roche, Indianapolis, IN), and prostaglandin E2 (ICN Biomedicals, Inc., Aurora, OH), as previously described (30).
Reagents.
IL-2 (Hoffmann-LaRoche, Inc., Basel, Switzerland) was used for expansion of γ9δ2 T cell lines. Connaught BCG at a multiplicity of infection (MOI) of 0.02 was used for in vitro expansion of mycobacterium-specific T cells. The following antibodies from BD Bioscience were used for flow cytometric analyses: anti-γδ T cell receptor (TCR) antibody-phycoerythrin (PE) (clone 11F2), anti-αβ TCR antibody-fluorescein isothiocyanate (FITC) (clone B3), anti-CD3 antibody-peridinin chlorophyll protein (PerCP) (clone SK7), anti-CD4 Pacific Blue (clone RPA-T4), anti-CD8 antibody–PE-Cy7 (clone RPA-T8), anti-δ2 TCR antibody-PE (clone B6), anti-γ9 TCR antibody-FITC (clone B1), anti-IFN-γ APC antibody-Alexa Fluor 700 (clone B27), anti-granzyme A antibody-FITC (clone CB9), and anti-granzyme B antibody-PE (clone GB11). Anti-CD40L antibody (clone TRAP1) from BD Bioscience was used in blocking experiments. Carboxyfluorescein succinimidyl ester (CFSE) was obtained from Molecular Probes (Eugene, OR). Phorbol myristate acetate (PMA; Sigma-Aldrich), ionomycin (Sigma-Aldrich), and the Cytofix/Cytoperm kit (BD Biosciences) were used in the preparation of cells for intracellular staining. 4-Hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP; Echelon Bioscience) was used to stimulate γ9δ2 T cells in some experiments.
Generation of long-term γδ T cell lines and clones.
γ9δ2 T cells were purified from BCG-expanded PBMC using γδ TCR-specific magnetic microbeads (Miltenyi Biotech, Auburn, CA). The purity of these positively selected γ9δ2 T cells was >97%. Purified γ9δ2 T cells were restimulated every 2 weeks with either live BCG-pulsed (MOI of 5) and irradiated PBMC or BCG-infected (MOI of 5) and irradiated mature DC, as APC. Extracellular BCG were removed by washing. IL-2 (20 U/ml) in fresh RPMI 1640 medium with 10% human serum, 2 mM l-glutamine, and 1% penicillin-streptomycin was added every 3 or 4 days as needed to maintain the lines (28, 29). γ9δ2 T cell clones were generated from BCG-specific γ9δ2 T cell lines as described previously (29).
Assay of γ9δ2 T cell-mediated inhibition of intracellular mycobacterial growth.
The assay was performed as previously described (28). Briefly, adherent monocytes were infected overnight with BCG at an MOI of 3 and extracellular BCG washed away. γ9δ2 T cell lines were added to achieve an effector-to-target ratio of 10:1. Cocultures were incubated at 37°C with 5% CO2 for 72 h. The monocytes were lysed with 0.2% saponin in RPMI 1640 medium, and the viable BCG bacilli released were quantified by CFU plating and/or [3H]uridine (GE Healthcare) incorporation. The percentages of BCG growth inhibition were determined using the following formula: % inhibition = 100 − [100 × (CFU or DPM in the presence of γ9δ2 T cells lines/CFU or DPM in the absence of γ9δ2 T cells)].
Antigen specificity assays.
To study antigen-specific lymphoproliferation, APC (PBMC or DC) were infected with BCG or vaccinia virus or cultured in medium alone, irradiated with 3,000 rad, and then used to stimulate purified γ9δ2 T cells (2 × 104/well). These cultures were incubated for 4 to 7 days and pulsed with [3H]thymidine for the last 18 h of incubation. [3H]thymidine incorporation was measured in a Microbeta scintillation counter. Stimulation indices (SI) were calculated by dividing counts per minute (cpm) for wells with infected APC by cpm for wells with uninfected APC.
To study antigen-specific effector function, APC infected with optimal doses of BCG or vaccinia virus were used to stimulate γ9δ2 T cells for 18 h in the presence of GolgiStop and the costimulants anti-CD28 antibody and anti-CD49d antibody, as directed by the manufacturer (BD Biosciences). Then, cells were harvested, stained for the T cell surface markers CD3 and γδ TCR, permeabilized with Cytofix/Cytoperm solutions (BD Biosciences), and stained for intracellular IFN-γ expression. Data were collected with a multicolor BD FACSCanto II instrument, and analyses were done using FlowJo (Tree Star) software. A minimum of 10,000 events were acquired.
MHC restriction.
PBMC were allotyped for major histocompatibility complex class I (MHC-I) and MHC-II allele expression. Samples without shared MHC were selected for use as allogeneic APC in MHC restriction studies. BCG-specific long-term γ9δ2 T cell lines were stimulated with autologous and allogeneic BCG-infected DC and studied for intracellular IFN-γ responses by flow cytometry as described above. γ9δ2 T cell lines were also stimulated with BCG-infected autologous DC in the presence or absence of anti-MHC-I (clone W6/32; BioLegend, San Diego, CA) and anti-MHC-II (clone L243; BioLegend) antibodies.
CFSE-based flow cytometric assay to study effects of γ9δ2 T cell lines on BCG-specific αβ T cell memory responses.
γδ T cells were depleted from PBMC using magnetic microbeads (Miltenyi Biotech, Auburn, CA), and γδ T cell-depleted PBMC were labeled with CFSE (Molecular Probes) as recommended by the manufacturer. CFSE-labeled, γδ T cell-depleted PBMC (1 × 106/ml) were incubated in the presence or absence of autologous BCG-specific long-term γ9δ2 T cell lines (1 × 103 to 1 × 104/ml), and these cultures were stimulated with live BCG or rested in medium for 7 days at 37°C. On day 7, the cells were restimulated with PMA (50 ng/ml) and ionomycin (750 ng/ml) in the presence of GolgiStop (0.7 μl/ml) for 2 h and studied for intracellular IFN-γ or granzyme expression as described above. Flow cytometric acquisition was performed on a multicolor BD FACSCanto II instrument, and analyses were done using FlowJo (Tree Star) software. A minimum of 10,000 CD4+ or CD8+ events were acquired. CD3+ CD4+ and CD3+ CD8+ T cells were regated from the lymphocyte population gate based on forward and side scatter properties. The proliferating cells were identified as populations with decreased mean FITC fluorescence intensity (CFSElo). The absolute numbers of CFSElo populations were calculated by multiplying the percentage of each subset obtained with flow cytometry by the trypan blue-determined total viable cell count. SI were calculated by dividing the absolute number of each CFSElo subset of CD4+ or CD8+ T cells after expansion with BCG by the matching absolute number of that CFSElo subset after resting in medium. The results are presented as the means, standard errors (SE), and ranges of the data.
Studies of γ9δ2 T cell APC/helper effects on the development of αβ T cell effector functions capable of inhibiting intracellular mycobacteria.
Endogenous γδ T cells were depleted from freshly thawed PBMC samples, and the depleted PBMC (1 × 106/ml) were stimulated with an optimal concentration of BCG in the presence or absence of autologous BCG-specific long-term γ9δ2 T cell lines (1 × 103 to 1 × 104/ml). On day 7, γ9δ2 T cells again were depleted and the remaining αβ T cell preparations were cocultured with BCG-infected monocytes at an effector-to-target ratio of 10:1. Mycobacterial growth was measured after 3 days as described above. γδ T cell depletions routinely resulted in <1% residual γδ T cells.
Cell-to-cell contact requirements for γ9δ2 T cell APC/helper effects.
γ9δ2 T cell clones were prepared by limiting dilution from BCG-expanded γ9δ2 T cell lines as previously described (29). γδ T cells were depleted from an aliquot of autologous PBMC prior to stimulation with BCG. These γδ T cell-depleted PBMC were labeled with CFSE and stimulated with BCG for 7 days in the presence or absence of autologous γ9δ2 T cell clones. Additional experiments separated PBMC from γδ T cells across semipermeable transwell membranes (0.4-μm pores). Furthermore, prior to being added to γδ T cell-depleted PBMC, some aliquots of γ9δ2 T cells were fixed with 0.05% glutaraldehyde for 30 s, followed by 0.2 M glycine neutralization and extensive washing. Stimulation indices were calculated as described above.
Comparing APC functions of γ9δ2 T cells and CD4+ αβ T cells.
γ9δ2 T cells were preincubated with HMB-PP and IL-2, with or without the M. tuberculosis whole-lysate (WL) antigen. In parallel, purified CD4+ αβ T cells from the same individuals were preincubated with anti-CD3 and anti-CD28 antibodies, with or without M. tuberculosis WL antigen. After 24 h of optimal stimulation, cells were washed, irradiated with 3,000 rad, and cocultured with autologous CFSE-labeled effector CD4+ T cells for 6 days. The importance of CD40-CD40L interactions for the APC/helper functions of γ9δ2 and CD4+ T cells was studied by adding anti-CD40L blocking antibody. Stimulation indices were calculated by dividing the absolute numbers of CD4+ T cells that had proliferated and produced IFN-γ in cocultures incubated with APC by the same results detected in control cultures with CFSE-labeled CD4+ T cells alone.
Statistical analysis.
Graphics and statistical results were generated using Microsoft Excel or Statistica. Percent inhibition is displayed as the mean result ± the standard error. Effector functions of BCG-specific γ9δ2 T cell lines were analyzed using Mann-Whitney U tests. Differences in BCG-specific αβ T cell responses in the presence and absence of BCG-specific γ9δ2 T cell lines were analyzed using the Wilcoxon matched-pairs test.
RESULTS
Generation and characterization of long-term γ9δ2 T cell lines capable of inhibiting intracellular mycobacteria.
In order to study the role of memory γ9δ2 T cells in mycobacterial immunity, we generated long-term γ9δ2 T cell lines by repeated stimulation of immunomagnetically purified γ9δ2 T cells with BCG-infected and irradiated APC. These γ9δ2 T cells were stimulated with BCG-infected APC every 2 weeks and were supplemented with fresh medium plus 20 units/ml IL-2 every 3 to 4 days as needed. Cultures were routinely monitored for outgrowth of αβ T cells and immunomagnetically purified in order to maintain pure populations of γ9δ2 T cells. It was possible to maintain pure cultures of γ9δ2 T cells for ≥6 months.
During the generation of these long-term γ9δ2 T cell lines, we compared total PBMC and DC as APC to generate optimal expansions of γ9δ2 T cells. In addition, the ability of these γ9δ2 T cell lines to inhibit intracellular mycobacterial growth was tested using a previously developed in vitro mycobacterial growth inhibition assay (28, 31, 32). The results shown in Fig. 1A show that γ9δ2 T cell lines generated by repeated stimulation with BCG-infected DC could directly inhibit the growth of intracellular bacilli by ≥60% compared with the growth of control cocultures incubated with medium-rested PBMC (P < 0.01 by Mann-Whitney U test; n = 5). In contrast, γ9δ2 T cell lines repeatedly stimulated with BCG-infected PBMC did not inhibit intracellular mycobacterial growth. These results demonstrated that only the use of BCG-infected DCs as APC for repeated stimulation of γ9δ2 T cells induced antigen-specific expansions of γ9δ2 T cells relevant for protective TB immunity. Further experiments reported here were conducted with γ9δ2 T cells expanded with BCG-infected DC.
FIG 1.
Generation and characterization of long-term γ9δ2 T cell lines. (A) Only long-term γ9δ2 T cell lines generated by serial stimulation with BCG-infected DC inhibit intracellular mycobacterial growth. Long-term γ9δ2 T cell lines were generated with BCG-infected APC as indicated and cocultured with BCG-infected macrophages, as described in Materials and Methods. Only γ9δ2 T cell lines generated with BCG-infected DC significantly inhibited the growth of intracellular bacilli (*, P < 0.01 by Mann-Whitney U test comparing γ9δ2 T cells generated with BCG-infected DC to PBMC). (B) Serially stimulated γ9δ2 T cell lines proliferate in an antigen-specific manner. DC were infected with BCG (MOI of 0.2) or vaccinia virus (MOI of 0.2) for 2 h and irradiated with 3,000 rad. Aliquots of these DC (2 × 103) were cocultured with BCG-expanded γ9δ2 T cell lines (2 × 104), and proliferation was assessed on day 4, after pulsing with [3H]thymidine for the last 18 h. Long-term BCG-expanded γ9δ2 T cells proliferated in response to BCG-infected DC but not to vaccinia virus-infected DC or uninfected DC (*, P < 0.05 by Mann-Whitney U test; n = 3). (C) Long-term γ9δ2 T cell lines produce IFN-γ specifically in response to BCG-infected DC stimulation but not heterologous stimuli (vaccinia virus-infected DC). γ9δ2 T cells were stimulated with BCG- or vaccinia virus-infected DC in the presence of costimulants (anti-CD28 and anti-CD49d antibodies) and GolgiStop for 18 h and stained for flow cytometric detection of IFN-γ-producing cells. The percentage of γ9δ2 T cells producing IFN-γ was markedly higher in γ9δ2 T cells stimulated with BCG-infected DC (*, P < 0.05 by Mann-Whitney U test; n = 3). (D) In parallel experiments, BCG-specific γ9δ2 T cell lines and vaccinia virus-specific γ9δ2 T cell lines were stimulated with both BCG- and vaccinia virus-infected DC in the presence of costimulants (anti-CD28 and anti-CD49d antibodies) and GolgiStop for 18 h and stained for flow cytometric detection of IFN-γ-producing cells. The percentage of γ9δ2 T cells producing IFN-γ was markedly higher when γ9δ2 T cell lines were stimulated with the model pathogen used for initial expansion of the γ9δ2 T cells (*, P < 0.05). (E and F) Long-term γ9δ2 T cell lines are not restricted by MHC allotypes. Long-term γ9δ2 T cell lines were stimulated for 18 h with BCG-infected (MOI of 10) or uninfected autologous or allogeneic DC in the presence of costimulants (anti-CD28 and anti-CD49d antibodies) and stained for flow cytometric detection of IFN-γ production. (E) The percentages of γ9δ2 T cells producing IFN-γ after stimulation with autologous and allogeneic infected DC were similar. (F) In addition, the expression of IFN-γ following stimulation with BCG-infected DC was not inhibited by the combination of optimal concentrations of anti-MHC-I and anti-MHC-II antibodies (Abs). The results in panels A, B, and D are presented as means ± SE.
We next investigated whether these long-term γ9δ2 T cell lines developed antigen-specificity for mycobacteria. The results shown in Fig. 1B demonstrate that BCG-expanded γ9δ2 T cells proliferated only in response to BCG-infected APC and not uninfected APC or APC infected with an irrelevant pathogen (vaccinia virus). The mean proliferative stimulation indices (SI) for γ9δ2 T cells incubated with BCG- and vaccinia virus-infected DC were 6 and 0.7, respectively. Furthermore, these γ9δ2 T cell lines produced IFN-γ solely in response to BCG-infected DC and not to DC infected with heterologous antigen (vaccinia virus). Figure 1C shows the results of flow cytometric analysis of one of three similar experiments. Intracellular IFN-γ expression by γ9δ2 T cell lines was measured after overnight stimulation with infected APC (Fig. 1C, top). IFN-γ expression in BCG-specific γ9δ2 T cell lines was significantly higher when these T cell lines were stimulated with BCG-infected DC than after stimulation with uninfected DC or vaccinia virus-infected DC (P < 0.05 by Mann-Whitney U test; n = 3) (Fig. 1D). In order to control for suboptimal antigen processing or antigen presentation in vaccinia virus-infected DC, we generated vaccinia virus-specific γ9δ2 T cells by repeated stimulation with vaccinia virus-infected DC. Vaccinia virus-specific γ9δ2 T cell lines produced IFN-γ only in response to stimulation with vaccinia virus-infected DC and not after stimulation with BCG-infected DC (Fig. 1C, bottom). These results demonstrate that BCG- and vaccinia virus-expanded γδ T cell lines maintain antigen-specific proliferative and effector responses, indicating that γ9δ2 T cells can develop differential pathogen recognition capabilities.
We next confirmed that our long-term γ9δ2 T cell lines were not restricted by MHC. After coculturing γ9δ2 T cells with autologous or allogeneic DC for 16 h, the expression of intracellular IFN-γ was measured by flow cytometry (Fig. 1E). Stimulation with uninfected autologous and allogeneic DC did not induce IFN-γ expression in these γ9δ2 T cells (0.9% and 1% IFN-γ+, respectively), demonstrating the absence of alloreactivity. In contrast, similar 10-fold increases in the percentages of γ9δ2 T cells that produced IFN-γ were induced by autologous and allogeneic BCG-infected DC, indicating that γ9δ2 T cell responses were not MHC restricted. We also blocked MHC-TCR interactions with anti-MHC-I (20 μg/ml) and anti-MHC-II (20 μg/ml) antibodies, using concentrations that optimally blocked IFN-γ expression by BCG-stimulated CD8+ and CD4+ T cells, respectively (data not shown). The percentages of γ9δ2 T cells expressing IFN-γ were unchanged by the presence of both anti-MHC-I and anti-MHC-II blocking antibodies, confirming that these γ9δ2 T cell lines were not MHC restricted (Fig. 1F).
Mycobacterium-specific memory γ9δ2 T cells enhance the development of CD4+ and CD8+ αβ T cell responses.
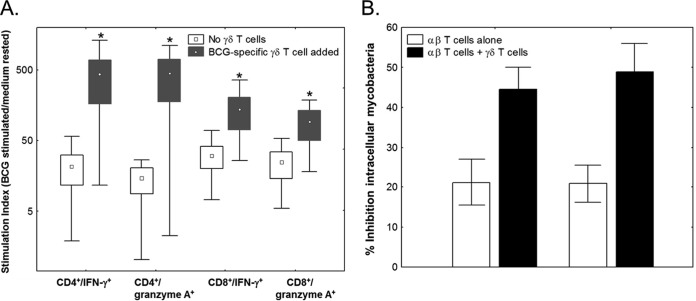
To further study the effects of γ9δ2 T cells on mycobacterium-specific αβ T cells, we performed a CFSE-based flow cytometric assay measuring the effects of autologous, in vitro-expanded, BCG-specific γδ Τ cell lines on CFSE-labeled αβ T cells. This enables the identification of T cells that proliferate (become CFSE low) and produce effector molecules in response to antigen-specific stimuli (33). Briefly, γδ Τ cell-depleted autologous PBMC were labeled with CFSE and stimulated with BCG in the presence or absence of BCG-specific autologous γ9δ2 T cell lines (reconstituted to 0.1% of total PBMC). The proportions of CD4+ and CD8+ T cells that proliferated and produced either IFN-γ or granzyme A intracellularly were determined by flow cytometry on day 7 of the expansion. The results shown in Fig. 2A demonstrate that γ9δ2 T cell lines significantly enhanced the ability of BCG-specific CD4+ and CD8+ T cells to proliferate and express IFN-γ, as well as granzyme A (P < 0.05 by Wilcoxon matched-pairs test; n = 5). The SI (mean ± SE) for CD4+ CFSElo IFN-γ+ αβ T cell responses were 429.3 ± 263 and 21.4 ± 9.8 in the presence and absence of γ9δ2 T cells, respectively. In addition, the SI for CD4+ CFSElo granzyme A+ responses were 445 ± 264 and 14.6 ± 5.9 in the presence and absence of γ9δ2 T cells, respectively. The SI (mean ± SE) for CD8+ CFSElo IFN-γ+ αβ T cell responses were 136.6 ± 66 and 30.5 ± 10.5 in the presence and absence of γ9δ2 T cells, respectively. The SI for CD8+ CFSElo granzyme A+ responses were 91.3 ± 41.6 and 24.3 ± 10.1 in the presence and absence of γ9δ2 T cells, respectively. All of these αβ T cell effector responses were significantly higher in the presence of γ9δ2 T cells (P < 0.05 by Wilcoxon matched-pairs test; n = 5). These helper effects could be either because of direct interaction of γ9δ2 T cells with αβ T cells or indirect through effects of γ9δ2 on other APC.
FIG 2.
(A) γ9δ2 T cells enhance the proliferation and intracellular production of effector function by memory αβ T cells. PBMC depleted of γδ T cells were labeled with CFSE and stimulated with BCG or rested in medium for 7 days in the presence or absence of autologous γ9δ2 T cell lines (0.1% of total T cells). Stimulation indices were calculated by dividing the percentages of CD4+ and CD8+ T cells that were CFSElo IFN-γ+ or CFSElo granzyme A+ by the corresponding percentages in medium-rested PBMC. Greater numbers of BCG-specific CD4+ and CD8+ αβ T cells proliferated and produced IFN-γ and granzyme A in the presence of BCG-specific γ9δ2 T cells (*, P < 0.05 for comparison to the absence of γ9δ2 T cells by Wilcoxon matched-pairs tests; n = 5). The results are presented as the medians (points), mid-50% ranges (boxes), and extreme quartiles (whiskers) of the data. (B) BCG-specific γ9δ2 T cells enhance the antimycobacterial effects of αβ T cells. γ9δ2 T cells were depleted from freshly thawed PBMC, and these depleted cells were stimulated with live BCG in the presence or absence of autologous long-term, BCG-specific γ9δ2 T cells. On day 7, γ9δ2 T cells were removed and the αβ T cells alone were cocultured with BCG-infected monocytes at a 10:1 ratio to assess antimycobacterial activity. γ9δ2 T cell lines enhanced the ability of αβ T cells to inhibit the growth of intracellular mycobacteria. The results are presented as means ± SE.
The experiments described above demonstrated that γ9δ2 T cells helped mycobacterium-specific αβ T cells develop significantly increased proliferation and cytokine and cytolytic molecule production. However, a more important measurement of T cell function essential for mycobacterial immunity is the ability to inhibit intracellular mycobacterial growth. Therefore, we studied the mycobacterial inhibitory activities of αβ T cells stimulated with live BCG in the presence and absence of γ9δ2 T cells. PBMC from PPD-positive individuals were depleted of γδ T cells and expanded with live BCG in the presence or absence of autologous γ9δ2 T cell lines for 7 days. The γ9δ2 T cells were then removed by immunomagnetic depletion to allow direct assessment of the ability of the expanded αβ T cells to inhibit intracellular mycobacteria. The inhibitory effects of αβ T cells on mycobacterial growth were markedly increased when γ9δ2 T cells were present during the expansion of BCG-specific αβ T cells (Fig. 2B). The percentages of intracellular mycobacterial growth inhibition increased from 21% to 44% in PBMC from volunteer number 1 and from 21% to 48% in PBMC from volunteer number 2 due to the helper effects provided by BCG-specific γ9δ2 T cells.
Enhancing effects of γ9δ2 T cells for M. tuberculosis-specific αβ T cell responses require cell-to-cell contact.
We next probed the mechanism responsible for γ9δ2 T cell-mediated enhancing effects for αβ T cells. We initially investigated the role of soluble mediators in the enhancement of αβ T cell effector functions by fixing γ9δ2 T cells with glutaraldehyde. Cellular fixation prevents the secretion of soluble mediators but maintains cellular structure. Glutaraldehyde fixation of activated γ9δ2 T cells prior to addition to αβ T cell cultures did not significantly reduce these helper effects (P > 0.05; n = 5) (Fig. 3A). These results indicate that soluble mediators are not important for the γ9δ2 T cell helper effects on αβ T cell effector functions.
FIG 3.
Immune-enhancing effects of γ9δ2 T cells require cell-to-cell contact. CFSE-labeled αβ T cells were activated by live BCG for 7 days in the presence or absence of γ9δ2 T cells. (A) Fixation of γ9δ2 T cells with glutaraldehyde does not significantly affect their immune-enhancing effects. Prior to addition to γδ T cell-depleted PBMC, γ9δ2 T cells were fixed with 0.05% glutaraldehyde for 30 s, followed by neutralization with 0.2 M glycine and extensive washing. Glutaraldehyde fixation did not significantly reduce the immune-enhancing effects of γ9δ2 T cells (*, P < 0.05 by Wilcoxon matched-pairs test; n = 5). (B) The molecular mechanisms involved in γ9δ2 T cell immune-enhancing effects were further analyzed by separation of memory γ9δ2 T cells and γδ T cell-depleted PBMC across semipermeable transwell membranes (0.4-μm pores) during stimulation with BCG. Stimulation indices were calculated by dividing the percentages of αβ Τ cells that were CFSElo IFN-γ+ or CFSElo granzyme+ when cultured with γ9δ2 T cells by the corresponding percentages in cultures containing no γ9δ2 T cells. Separation of γ9δ2 T cells and αβ T cells by the semipermeable membrane significantly decreased the immune-enhancing activity of γ9δ2 T cells (**, P < 0.01 by Wilcoxon matched-pairs tests; n = 5). The results are presented as the medians (points), mid-50% ranges (boxes), and extreme quartiles (whiskers) of the data.
In addition, the importance of cell-to-cell contact was studied by separating the BCG-stimulated γ9δ2 T cells from the BCG-stimulated CFSE-labeled αβ T cells using transwell chambers with 0.4-μm pores. Additional BCG-infected DC were incubated as APC in the isolation chambers to ensure maximal activation of γδ Τ cells. Separating γ9δ2 T cells and αβ T cells eliminated the enhancing effects of γ9δ2 T cells on the proliferation of granzyme- or IFN-γ-expressing effector αβ T cells (P = 0.05 by Wilcoxon matched-pairs test; n = 5) (Fig. 3B).
The ability of mycobacterium-specific γ9δ2 T cells to enhance αβ T cells is dependent on CD40-CD40L interactions.
Since cell-to-cell contact was required for γ9δ2 T cell-mediated enhancement of αβ T cell effector function, we further investigated the costimulatory effects of γ9δ2 T cells on αβ T cell stimulation. CD40-CD40L interactions have costimulatory functions for T cells during APC-mediated activation (34). Therefore, we examined the role of CD40-CD40L-mediated costimulatory signals for the γ9δ2 T cell immune-enhancing effects on αβ T cell effector functions. Antibody-mediated blockade of this interaction significantly reduced the immune-enhancing effects of memory γ9δ2 T cells (P = 0.05 by Wilcoxon matched-pairs test; n = 5) (Fig. 4A), whereas the inclusion of an isotype control antibody had no effect (P = 0.38; n = 5).
FIG 4.
BCG-specific γ9δ2 T cells enhance the development of effector functions in BCG-specific αβ T cells through CD40-CD40L-mediated costimulation. (A) The immune-enhancing effects of γ9δ2 T cells require CD40-CD40L interactions with responder T cells. γ9δ2 T cells were cocultured with αβ T cells and stimulated with BCG in the presence or absence of a neutralizing anti-CD40L antibody. Neutralization of CD40L significantly decreased the immune-enhancing effects of γ9δ2 T cells (*, P < 0.05 for comparison to γ9δ2 T cells by Wilcoxon matched-pairs tests; n = 5). (B) To compensate for possible dissociation/consumption of anti-CD40L antibody during the 7-day assay, cells were treated with anti-CD40L antibody and then fixed with glutaraldehyde. Glutaraldehyde fixation after the addition of anti-CD40L completely prevented all immune-enhancing effects (P > 0.05 by Wilcoxon matched-pairs test; n = 5). The results are presented as the medians (points), mid-50% ranges (boxes), and extreme quartiles (whiskers) of the data.
Although CD40-CD40L interactions should be blocked during the initial incubation of γ9δ2 T cells with αβ T cells during mycobacterial stimulation in our assay system, this blockade is not likely to be complete during the 7 days of the assay due to dissociation/consumption of the antibody. Therefore, we attempted to disrupt CD40-CD40L binding during the entire assay by incubating the γ9δ2 T cells with anti-CD40L antibody followed by glutaraldehyde fixation to cross-link the antibody to the cell surface molecule for the entire course of the assay. γ9δ2 T cells bound by anti-CD40L antibody prior to fixation were completely unable to mediate any helper effects for αβ T cell effector functions (P = 0.05 by Wilcoxon matched-pairs test; n = 5) (Fig. 4B). These data have allowed us to conclude that γ9δ2 T cells provide costimulation for the development of antigen-specific αβ T cells through CD40-CD40L signaling.
Comparisons of the APC and immune-enhancing effects of γ9δ2 T cells and optimally stimulated CD4+ T cells.
Because both CD4+ αβ T cells and γδ T cells have been shown to have immune-enhancing effects and γδ T cells were reported to have potent APC activity, we next compared CD4+ αβ T cells and γ9δ2 T cells for both overall T cell immune-enhancing effects and specific APC activities important for inducing mycobacterium-specific immunity. We purified γ9δ2 and CD4+ αβ T cells from the same subjects, activated them with maximal stimuli in the absence of other cells (HMB PP plus IL-2 and anti-CD3+ and anti-CD28 antibodies, respectively), and pulsed them or not with M. tuberculosis WL antigen. These γ9δ2 and CD4+ αβ activated T cells pulsed or not with M. tuberculosis WL antigen were then cocultured with autologous CFSE-labeled CD4+ T cells as responder T cells. Both γ9δ2 and CD4+ T cells preactivated in the presence of mycobacterial antigen (but not cells preactivated in the absence of antigen) enhanced the proliferation and IFN-γ expression of autologous TB-specific CD4+ T cells (Fig. 5A and C). The fact that preactivated T cells without antigen did not enhance TB-specific responder T cell proliferation/effector function indicates that antigen processing and presentation by the γ9δ2 and αβ T cells were required. These immune-enhancing/APC functions of γ9δ2 and CD4+ T cells were blocked by anti-CD40L antibody, indicating that CD40-CD40L interactions were absolutely required for the immune-enhancing/APC functions (Fig. 5B and D). Our results demonstrate for the first time that both γ9δ2 and CD4+ αβ T cells can provide similarly potent immune-enhancing/APC functions for stimulation of TB-specific T cells.
FIG 5.
Both γ9δ2 T cells and CD4+ αβ T cells can mediate antigen presentation capable of activating TB-specific T cell responses. (A) γ9δ2 T cells were prestimulated with HMB-PP and IL-2 and antigen pulsed or not for 24 h. Then, cells were washed, irradiated with 3,000 rad, and cocultured with CFSE-labeled autologous CD4+ T cells obtained from PPD-positive healthy individuals. On day 7, proliferating and IFN-γ-expressing CD4+ T cells were analyzed by flow cytometry. Stimulation indices were calculated by dividing the percentages of effector CD4+ Τ cells that were CFSElo IFN-γ+ when cocultured with γ9δ2 T cells by the corresponding percentages in cultures containing no γ9δ2 T cells. γ9δ2 T cells prestimulated with HMB-PP and pulsed with M. tuberculosis WL antigen showed much higher stimulation indices in CD4+ αβ T cells than γ9δ2 T cells treated with HMB-PP alone (*, P < 0.05 for comparison to γδ T cells by Wilcoxon matched-pairs tests; n = 6). (B) The effects of neutralizing anti-CD40L antibody on γ9δ2 T cell APC effects were studied with cells from 2 individuals. Anti-CD40L antibody was added during coculture with CFSE-labeled CD4+ T cells. Anti-CD40L antibody markedly inhibited the enhancing effects of γ9δ2 T cells prestimulated with HMB-PP and pulsed with M. tuberculosis WL antigen. (C) In parallel experiments, CD4+ αβ T cells were prestimulated with anti-CD3 and anti-CD28 antibodies and pulsed or not with M. tuberculosis WL antigen for 24 h. Then, cells were washed, irradiated at 3,000 rad, and cocultured with CFSE-labeled autologous CD4+ T cells from PPD-positive healthy individuals. On day 7, proliferating and IFN-γ-expressing CD4+ T cells were analyzed by flow cytometry. Preactivated and M. tuberculosis antigen-pulsed CD4+ αβ T cells provided APC functions similar to those of γ9δ2 T cells (n = 4). (D) The effects of neutralizing anti-CD40L antibody on APC functions of CD4+ T cells were studied with cells from 2 individuals. Anti-CD40L antibody was added during coculture with CFSE-labeled effector CD4+ T cells. Anti-CD40L antibody markedly inhibited the APC effects of CD4+ T cells prestimulated with anti-CD3 and anti-CD28 antibodies and pulsed with M. tuberculosis WL antigen. The results in panels A and C are presented as means ± SE.
DISCUSSION
γδ T cells are important for protective TB immunity. Their interaction with antigen-presenting cells is not restricted by MHC allotypes, and yet, they are antigen specific. In addition, γδ T cells tend to accumulate in mucosal epithelial tissues, including the lung, where they would be among the first immune cells encountering tubercle bacilli (35, 36). These properties make γδ T cells well suited for early responses to TB infection or reactivation. There is evidence that γδ T cells are important contributors of mycobacterium-specific type I immune responses, including IFN-γ production and cytolytic activity, critical for TB immunity (28, 37–41). Indeed, γδ T cells have been shown to be more potent producers of IFN-γ than CD4+ and CD8+ αβ T cells after restimulation in vitro with M. tuberculosis-infected APC (42, 43). Despite the limitations of in vitro results to recapitulate in vivo phenomena, our results provide additional evidence for the importance of γ9δ2 T cells as protective effector cells in mycobacterial immunity by demonstrating that γ9δ2 T cells can potently inhibit intracellular mycobacterial growth, as well as potentiate the expansion of mycobacterium-specific αβ T effector cells. In fact, this is the first time that BCG-specific γ9δ2 T cell lines have been used to study helping/APC functions or interactions with αβ T cells.
We found that only γ9δ2 T cell lines generated by repeated stimulation with BCG-infected DC and not those generated with BCG-infected PBMC were capable of mediating mycobacterial growth inhibition. These results may be due to differential antigen-specific focusing events (28). Only BCG-infected DC may induce γ9δ2 T cells capable of recognizing antigens expressed by intracellular mycobacteria. Only a minor fraction of PBMC (monocytes/macrophages, <10%) are known to be infected by mycobacteria and capable of supporting their intracellular replication. The bulk of live BCG added to PBMC may remain extracellular and stimulate γ9δ2 T cell responses directed against extracellular mycobacterial components that are different from those presented by infected cells. Alternatively, DC and not PBMC may activate sufficient effector functions that are required for inhibitory effects on intracellular mycobacteria.
The possibility that γ9δ2 T cells may develop discrete antigen specificities was supported by our previous findings that BCG-specific γ9δ2 T cells did not respond to canarypox virus, another live vaccine also capable of inducing γ9δ2 T cell expansions (44). We now provide additional evidence for the development of pathogen-specific γ9δ2 T cells, showing that γ9δ2 T cell lines generated by repeated expansion with BCG-infected DCs do not respond to vaccinia virus-infected APC. The pathogen specificity potential of γ9δ2 T cells was further confirmed by showing that vaccinia virus-specific γ9δ2 T cells respond only to vaccinia virus-infected APC and not to BCG-infected APC.
The roles of γ9δ2 T cells in TB immunity are not limited to the direct inhibition of intracellular mycobacteria. We showed that γ9δ2 T cells could enhance the expansion of αβ T cells, and these findings were similar to the findings previously reported by other groups (45). To further confirm their M. tuberculosis-specific functions, we tested the ability of αβ T cells expanded with the help of M. tuberculosis-specific γ9δ2 T cells to inhibit intracellular mycobacteria. Our results showed that αβ T cells expanded with the help of M. tuberculosis-specific γ9δ2 T cells have increased abilities to kill intracellular mycobacteria, confirming that the interactions were antigen specific and relevant for the control of M. tuberculosis growth.
Activated γδ T cells have been reported to mediate APC functions (45–47). However, we have previously shown that soluble mediators are required for the direct inhibition of intracellular mycobacteria by γ9δ2 T cells (12). Therefore, we studied the relative roles of soluble mediators and cell-to-cell contact for the immune-enhancing effects of γ9δ2 T cells. We showed that soluble mediators are not required for the ability of γ9δ2 T cells to help M. tuberculosis-specific αβ T cells. In contrast, we demonstrated that CD40-CD40L interactions were essential for the immune-enhancing effects of γ9δ2 T cells. We tested the immune-enhancing effects of γ9δ2 T cells in mixed cell populations consisting of all other APC subsets present in total PBMC. Interestingly, the robust immune-enhancing effects of γ9δ2 T cells were seen even in the presence of other APC, indicating that these M. tuberculosis-specific γ9δ2 T cell effects were not redundant.
It is known that CD4+ αβ T cells help other immune cells which are important for TB-specific immunity (48–50). Therefore, we compared the immune-enhancing functions of γ9δ2 T cells with those of CD4+ αβ T cells. Contrary to previous reports (45), the immune-enhancing/APC functions and CD40-CD40L dependence were not unique to γ9δ2 T cells. We demonstrated that optimally preactivated CD4+ αβ T cells and γ9δ2 T cells had similar effects in promoting the expansion of autologous CD4+ αβ T cells in an M. tuberculosis antigen-specific manner. We believe that suboptimal preactivation of CD4+ αβ T cells (the use of a superantigen expected to activate only some αβ T cells) may explain the different results in our study compared with those of previously published work.
The immune-enhancing effects of γ9δ2 T cells for αβ T cell responses detected in our current work have important implications for further TB vaccine design. Primary immune responses involve the proliferation and differentiation of naive T cells after their initial antigen encounter into armed effector T cells capable of inhibiting pathogen replication and memory immune T cells that protect against secondary challenges. By enhancing primary CD4+ and CD8+ αβ T cell responses, γ9δ2 T cell induction could be useful for the priming step of new prime/boosting TB vaccine strategies. Furthermore, CD4+ αβ T cells appear to be critically important for the control of early TB infection associated with active mycobacterial replication, while CD8+ αβ T cells may be more important for the control of latent M. tuberculosis infection (51). By enhancing both memory CD4+ and CD8+ αβ T cell responses, prophylactic and/or immunotherapeutic vaccine strategies capable of stimulating γ9δ2 T cell responses may improve the overall ability of vaccine-induced immunity to prevent both primary and reactivation TB disease. The limitation of this study is that most of our results were generated using BCG as a model mycobacterium instead of M. tuberculosis. We have previously shown that stimulation of γ9δ2 T cells with natural phosphoantigens, such as isopentenyl pyrophosphate (IPP) and HMB-PP, leads to polyclonal expansion, but that these pyrophosphate-expanded γ9δ2 T cells could not inhibit intracellular mycobacterial growth (29). We are now working on lead M. tuberculosis antigens that can expand M. tuberculosis-specific γ9δ2 T cells with inhibitory activity. These compounds, once developed, will have the potential to be used as prophylactic vaccines to enhance TB immunity pre-exposure, as immunotherapies designed to clear or reduce latent TB infection, and/or as therapeutic vaccines to shorten the duration of standard TB treatment, as well as improve treatment outcomes in multidrug-resistant/extensively drug-resistant TB patients.
In conclusion, our results confirm direct protective effects of γ9δ2 T cells and uniquely demonstrate the following conclusions: (i) γ9δ2 T cells enhance the expansion of M. tuberculosis-specific αβ T cells and increase the ability of αβ T cells to inhibit intracellular mycobacteria; (ii) soluble mediators do not have a role in the immune-enhancing effects of γ9δ2 T cells despite the fact that γ9δ2 T cells inhibit intracellular M. tuberculosis through soluble mediators (12); and (iii) the immune-enhancing/APC functions of γ9δ2 T cells are dependent on cell-to-cell contact mediated through CD40-CD40L interactions, further confirming previous reports that γ9δ2 T cells can act as costimulatory APC. Importantly, these robust immune-enhancing functions were seen even in the presence of other APC, indicating that these γ9δ2 T cell effects are not redundant. Our results also demonstrate that both CD4+ αβ T cells and γ9δ2 T cells can mediate similar APC functions, indicating that the ability to process and present antigens to other M. tuberculosis-specific T cells is not unique to γ9δ2 T cells. Therefore, our results indicate that in addition to CD4+ and CD8+ αβ T cells, γ9δ2 T cells should be considered potentially important new targets for stimulation by new TB vaccines.
Funding Statement
This investigation was supported by National Institutes of Health Vaccine Treatment and Evaluation Unit contract NO1-AI-25464 (D.F.H., coinvestigator) and NIH R01-AI-48391 (D.F.H., principal investigator).
REFERENCES
- 1.Onozaki I, Raviglione M. 2010. Stopping tuberculosis in the 21st century: goals and strategies. Respirology 15:32–43. doi: 10.1111/j.1440-1843.2009.01673.x. [DOI] [PubMed] [Google Scholar]
- 2.Warndorff DK. 1996. Tuberculosis prevention: where do we go from here? Afr Health 19:21–22. [PubMed] [Google Scholar]
- 3.Fine PEM. 1989. The BCG story: lessons from the past and implications for the future. Rev Infect Dis 11:S353–S359. doi: 10.1093/clinids/11.Supplement_2.S353. [DOI] [PubMed] [Google Scholar]
- 4.Andersen P. 2001. TB vaccines: progress and problems. Trends Immunol 22:160–168. doi: 10.1016/S1471-4906(01)01865-8. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann SH. 2010. Future vaccination strategies against tuberculosis: thinking outside the box. Immunity 33:567–577. doi: 10.1016/j.immuni.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Jiao X, Lo-Man R, Guermonprez P, Fiette L, Deriaud E, Burgaud S, Gicquel B, Winter N, Leclerc C. 2002. Dendritic cells are host cells for mycobacteria in vivo that trigger innate and acquired immunity. J Immunol 168:1294–1301. doi: 10.4049/jimmunol.168.3.1294. [DOI] [PubMed] [Google Scholar]
- 7.Flesch I, Kaufmann SHE. 1987. Mycobacterial growth inhibition by interferon-activated bone marrow macrophages and differential susceptibility amongst strains of Mycobacterium tuberculosis J Immunol 138:4408–4413. [PubMed] [Google Scholar]
- 8.Feng CG, Bean AG, Hooi H, Briscoe H, Britton WJ. 1999. Increase in gamma interferon-secreting CD8+, as well as CD4+, T cells in lungs following aerosol infection with Mycobacterium tuberculosis. Infect Immun 67:3242–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliaro J, Dudal S, Liautard J, Andrault JB, Liautard JP, Lafont V. 2005. Vγ9Vδ2 T cells use a combination of mechanisms to limit the spread of the pathogenic bacteria Brucella. J Leukocyte Biol 77:652–660. doi: 10.1189/jlb.0704433. [DOI] [PubMed] [Google Scholar]
- 10.Dieli F, Troye-Blomberg M, Ivanyi J, Fournie JJ, Bonneville M, Peyrat MA, Sireci G, Salerno A. 2000. Vγ9Vδ2 T lymphocytes reduce the viability of intracellular Mycobacterium tuberculosis. Eur J Immunol 30:1512–1519. doi:. [DOI] [PubMed] [Google Scholar]
- 11.Dieli F, Troye-Blomberg M, Ivanyi J, Fournie JJ, Krensky AM, Bonneville M, Peyrat MA, Caccamo N, Sireci G, Salerno A. 2001. Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vγ9Vδ2 T lymphocytes. J Infect Dis 184:1082–1085. doi: 10.1086/323600. [DOI] [PubMed] [Google Scholar]
- 12.Spencer CT, Abate G, Sakala IG, Xia M, Truscott SM, Eickhoff CS, Linn R, Blazevic A, Metkar SS, Peng G, Froelich CJ, Hoft DF. 2013. Granzyme A produced by γ9δ2 T cells induces human macrophages to inhibit growth of an intracellular pathogen. PLoS Pathog 9:e1003119. doi: 10.1371/journal.ppat.1003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morita CT, Parker CM, Brenner MB, Band H. 1994. TCR usage and functional capabilities of human γδ T cells at birth. J Immunol 153:3979–3988. [PubMed] [Google Scholar]
- 14.Parker CM, Groh V, Band H, Porcelli SA, Morita C, Fabbi M, Glass D, Strominger JL, Brenner MB. 1990. Evidence for extrathymic changes in the T cell receptor γδ repertoire. J Exp Med 171:1597–1612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoft DF, Brown RM, Roodman ST. 1998. Bacille Calmette-Guérin vaccination enhances human γδ T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J Immunol 161:1045–1054. [PubMed] [Google Scholar]
- 16.Wallace M, Bartz SR, Chang WL, Mackenzie DA, Pauza CD, Malkovsky M. 1996. γδ T lymphocyte responses to HIV. Clin Exp Immunol 103:177–184. doi: 10.1046/j.1365-2249.1996.d01-625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramsey JM, Tello A, Contreras CO, Ordonez R, Chirino N, Rojo J, Garcia F. 2002. Plasmodium falciparum and P. vivax gametocyte-specific exoantigens stimulate proliferation of TCRγδ+ lymphocytes. J Parasitol 88:59–68. doi: 10.2307/3285391. [DOI] [PubMed] [Google Scholar]
- 18.Lagler H, Willheim M, Traunmuller F, Wahl K, Winkler H, Ramharter M, Graninger W, Winkler S. 2003. Cellular profile of cytokine production in a patient with visceral leishmaniasis: γδ+ T cells express both type 1 cytokines and interleukin-10. Scand J Immunol 57:291–295. doi: 10.1046/j.1365-3083.2003.01223.x. [DOI] [PubMed] [Google Scholar]
- 19.Davies A, Lopez-Briones S, Ong H, O'Neil-Marshall C, Lemonnier FA, Nagaraju K, Metcalf ES, Soloski MJ. 2004. Infection-induced expansion of a MHC class Ib-dependent intestinal intraepithelial γδ T cell subset. J Immunol 172:6828–6837. doi: 10.4049/jimmunol.172.11.6828. [DOI] [PubMed] [Google Scholar]
- 20.Bertotto A, Spinozzi F, Gerli R, Bassotti G, Forenza N, Vagliasindi C, Vaccaro R. 1995. Peripheral blood γδ T cells in human listeriosis. Acta Pædiatr 84:1434–1435. [DOI] [PubMed] [Google Scholar]
- 21.Uezu K, Kawakami K, Miyagi K, Kinjo Y, Kinjo T, Ishikawa H, Saito A. 2004. Accumulation of γδ T cells in the lungs and their regulatory roles in Th1 response and host defense against pulmonary infection with Cryptococcus neoformans. J Immunol 172:7629–7634. doi: 10.4049/jimmunol.172.12.7629. [DOI] [PubMed] [Google Scholar]
- 22.Ottones F, Dornand J, Naroeni A, Liautard JP, Favero J. 2000. Vγ9Vδ2 T cells impair intracellular multiplication of Brucella suis in autologous monocytes through soluble factor release and contact-dependent cytotoxic effect. J Immunol 165:7133–7139. doi: 10.4049/jimmunol.165.12.7133. [DOI] [PubMed] [Google Scholar]
- 23.Li B, Rossman MD, Imir T, Oner-Eyuboglu AF, Lee CW, Biancaniello R, Carding SR. 1996. Disease-specific changes in γδ T cell repertoire and function in patients with pulmonary tuberculosis. J Immunol 157:4222–4229. [PubMed] [Google Scholar]
- 24.Li B, Bassiri H, Rossman MD, Kramer P, Eyuboglu AFO, Torres M, Sada E, Imir T, Carding SR. 1998. Involvement of the fas/fas ligand pathway in activation-induced cell death of mycobacteria-reactive human γδ T cells: a mechanism for the loss of γδ T cells in patients with pulmonary tuberculosis. J Immunol 161:1558–1567. [PubMed] [Google Scholar]
- 25.Zhou D, Lai X, Shen Y, Sehgal P, Shen L, Simon M, Qiu L, Huang D, Du GZ, Wang Q, Letvin NL, Chen ZW. 2003. Inhibition of adaptive Vγ9Vδ2+ T-cell responses during active mycobacterial coinfection of simian immunodeficiency virus SIVmac-infected monkeys. J Virol 77:2998–3006. doi: 10.1128/JVI.77.5.2998-3006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, Fourni JJ. 1994. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science 264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 27.Evans PS, Enders PJ, Yin C, Ruckwardt TJ, Malkovsky M, Pauza CD. 2001. In vitro stimulation with a non-peptidic alkylphosphate expands cells expressing Vγ2-Jδ1.2/Vδ2 T-cell receptors. Immunology 104:19–27. doi: 10.1046/j.1365-2567.2001.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Worku S, Hoft DF. 2003. Differential effects of control and antigen-specific T cells on intracellular mycobacterial growth. Infect Immun 71:1763–1773. doi: 10.1128/IAI.71.4.1763-1773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spencer CT, Abate G, Blazevic A, Hoft DF. 2008. Only a subset of phosphoantigen-responsive γ9δ2 T cells mediate protective tuberculosis immunity. J Immunol 181:4471–4484. doi: 10.4049/jimmunol.181.7.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH. 1997. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol 27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 31.Worku S, Hoft DF. 2000. In vitro measurement of protective mycobacterial immunity: antigen specific expansion of T cells capable of inhibiting intracellular growth of BCG. Clin Infect Dis 30:S257–S261. doi: 10.1086/313887. [DOI] [PubMed] [Google Scholar]
- 32.Hoft DF, Worku S, Kampmann B, Whalen CC, Ellner JJ, Hirsch CS, Brown RB, Larkin R, Li Q, Yun H, Silver RF. 2002. Investigation of the relationships between immune-mediated inhibition of mycobacterial growth and other potential surrogate markers of protective Mycobacterium tuberculosis immunity. J Infect Dis 186:1448–1457. doi: 10.1086/344359. [DOI] [PubMed] [Google Scholar]
- 33.Abate G, Eslick J, Newman FK, Frey SE, Belshe RB, Monath TP, Hoft DF. 2005. Flow-cytometric detection of vaccinia-induced memory effector CD4+, CD8+, and γδ TCR+ T cells capable of antigen-specific expansion and effector functions. J Infect Dis 192:1362–1371. doi: 10.1086/444423. [DOI] [PubMed] [Google Scholar]
- 34.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med 184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boom WH, Chervenak KA, Mincek MA, Ellner JJ. 1992. Role of the mononuclear phagocyte as an antigen-presenting cell for human γ/δ T cells activated by live Mycobacterium tuberculosis. Infect Immun 60:3480–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rojas RE, Torres M, Fournie JJ, Harding CV, Boom WH. 2002. Phosphoantigen presentation by macrophages to Mycobacterium tuberculosis-reactive Vγ9Vδ2+ T cells: modulation by chloroquine. Infect Immun 70:4019–4027. doi: 10.1128/IAI.70.8.4019-4027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. 1993. Disseminated tuberculosis in IFN-γ gene-disrupted mice. J Exp Med 178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. 1993. An essential role for IFN-gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med 178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ottenhoff TH, Kumararatne D, Casanova JL. 1998. Novel human immunodeficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunol Today 19:491–494. doi: 10.1016/S0167-5699(98)01321-8. [DOI] [PubMed] [Google Scholar]
- 40.Bodnar KA, Serbina NV, Flynn JL. 2001. Fate of Mycobacterium tuberculosis within murine dendritic cells. Infect Immun 69:800–809. doi: 10.1128/IAI.69.2.800-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen CY, Yao S, Huang D, Wei H, Halliday L, Sicard H, Zeng G, Jomaa H, Larsen MH, Jacobs WR, Wang R, Letvin N, Shen Y, Qiu L, Shen L, Chen ZW. 2013. Phosphoantigen/IL-2 expansion and differentiation of Vγ2Vδ2 T cells increases resistance to tuberculosis in nonhuman primates. PLoS Pathog 9:e1003501. doi: 10.1371/journal.ppat.1003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee J, Choi K, Olin MR, Cho SN, Molitor TW. 2004. γδ T cells in immunity induced by Mycobacterium bovis bacillus Calmette-Guérin vaccination. Infect Immun 72:1504–1511. doi: 10.1128/IAI.72.3.1504-1511.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsukaguchi K, Balaji KN, Boom WH. 1995. CD4+ αβ T cell and γδ T cell responses to Mycobacterium tuberculosis. J Immunol 154:1786–1796. [PubMed] [Google Scholar]
- 44.Worku S, Gorse GJ, Belshe RB, Hoft DF. 2001. Canarypox vaccines induce antigen specific human γδ T cells capable of IFN-γ production. J Infect Dis 184:525–532. doi: 10.1086/322792. [DOI] [PubMed] [Google Scholar]
- 45.Brandes M, Willimann K, Moser B. 2005. Professional antigen-presentation function by human γδ T cells. Science 309:264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 46.Wu Y, Wu W, Wong WM, Ward E, Thrasher AJ, Goldblatt D, Osman M, Digard P, Canaday DH, Gustafsson K. 2009. Human gamma delta T cells: a lymphoid lineage cell capable of professional phagocytosis. J Immunol 183:5622–5629. doi: 10.4049/jimmunol.0901772. [DOI] [PubMed] [Google Scholar]
- 47.Himoudi N, Morgenstern DA, Yan M, Vernay B, Saraiva L, Wu Y, Cohen CJ, Gustafsson K, Anderson J. 2012. Human gammadelta T lymphocytes are licensed for professional antigen presentation by interaction with opsonized target cells. J Immunol 188:1708–1716. doi: 10.4049/jimmunol.1102654. [DOI] [PubMed] [Google Scholar]
- 48.Serbina NV, Lazarevic V, Flynn JL. 2001. CD4(+) T cells are required for the development of cytotoxic CD8(+) T cells during Mycobacterium tuberculosis infection. J Immunol 167:6991–7000. doi: 10.4049/jimmunol.167.12.6991. [DOI] [PubMed] [Google Scholar]
- 49.Gerloni M, Xiong S, Mukerjee S, Schoenberger SP, Croft M, Zanetti M. 2000. Functional cooperation between T helper cell determinants. Proc Natl Acad Sci U S A 97:13269–13274. doi: 10.1073/pnas.230429197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsang JY, Chai JG, Lechler R. 2003. Antigen presentation by mouse CD4+ T cells involving acquired MHC class II:peptide complexes: another mechanism to limit clonal expansion? Blood 101:2704–2710. doi: 10.1182/blood-2002-04-1230. [DOI] [PubMed] [Google Scholar]
- 51.van Pinxteren LA, Cassidy JP, Smedegaard BH, Agger EM, Andersen P. 2000. Control of latent Mycobacterium tuberculosis infection is dependent on CD8+ T cells. Eur J Immunol 30:3689–3698. doi:. [DOI] [PubMed] [Google Scholar]