Abstract
Background
Hyperemesis gravidarum (HG), or intractable vomiting during pregnancy, is the single most frequent cause of hospital admission in early pregnancy. HG has a major impact on maternal quality of life and has repeatedly been associated with poor pregnancy outcome such as low birth weight. Currently, women with HG are admitted to hospital for intravenous fluid replacement, without receiving specific nutritional attention. Nasogastric tube feeding is sometimes used as last resort treatment. At present no randomised trials on dietary or rehydration interventions have been performed. Small observational studies indicate that enteral tube feeding may have the ability to effectively treat dehydration and malnutrition and alleviate nausea and vomiting symptoms. We aim to evaluate the effectiveness of early enteral tube feeding in addition to standard care on nausea and vomiting symptoms and pregnancy outcomes in HG patients.
Methods/Design
The MOTHER trial is a multicentre open label randomised controlled trial (www.studies-obsgyn.nl/mother). Women ≥ 18 years hospitalised for HG between 5 + 0 and 19 + 6 weeks gestation are eligible for participation. After informed consent participants are randomly allocated to standard care with intravenous rehydration or early enteral tube feeding in addition to standard care. All women keep a weekly diary to record symptoms and dietary intake until 20 weeks gestation. The primary outcome will be neonatal birth weight. Secondary outcomes will be the 24-h Pregnancy Unique Quantification of Emesis and nausea score (PUQE-24), maternal weight gain, dietary intake, duration of hospital stay, number of readmissions, quality of life and side-effects. Also gestational age at birth, placental weight, umbilical cord plasma lipid concentration and neonatal morbidity will be evaluated. Analysis will be according to the intention to treat principle.
Discussion
With this trial we aim to clarify whether early enteral tube feeding is more effective in treating HG than intravenous rehydration alone and improves pregnancy outcome.
Trial registration
Trial registration number: NTR4197. Date of registration: October 2nd 2013.
Electronic supplementary material
The online version of this article (doi:10.1186/s12884-016-0815-1) contains supplementary material, which is available to authorized users.
Keywords: Hyperemesis, Nausea and vomiting in pregnancy, Tube feeding, Intravenous rehydration, Effectiveness, Outcomes
Background
Nausea and vomiting in pregnancy (NVP) is common, affecting 50–80 % of pregnancies [1]. Often these symptoms are mild and self-limiting and resolve without intervention in the second trimester. In other cases however, severe intractable vomiting can lead to dehydration, electrolyte disturbances and significant weight loss necessitating hospital admission. The condition of intractable vomiting during pregnancy is called hyperemesis gravidarum (HG) [2]. HG has repeatedly been associated with poor pregnancy outcome including low birth weight (LBW, <2500 g: OR 1.42), small for gestational age (OR 1.28) and prematurity (OR 1.32) [2–4]. Furthermore, HG has a major impact on maternal wellbeing and quality of life [5–7] and remains the largest single cause of hospital admission in early pregnancy [8, 9]. However, the aetiology of HG is poorly understood [10–12].
Approximately 0.8–2 % of all pregnancies are complicated by HG [2]. Currently, there are no treatments with proven efficacy available according to the latest Cochrane review on interventions for nausea and vomiting in early pregnancy [1]. Hospitalisation can be required for intravenous treatment of dehydration and electrolyte imbalance. Currently, women who suffer from HG do not receive any particular nutritional attention, although enteral tube feeding is sometimes used as a treatment of last resort [2, 13]. Enteral tube feeding effectively treats both dehydration and malnutrition in non-pregnant patients with poor intake [14] and has been shown to be safer than parenteral nutrition in pregnancy [15]. Moreover, in several small studies in women with HG, which did not employ a control group, it alleviated symptoms and was well tolerated if continued in a home setting [16–18]. There have been no controlled trials to investigate the extent to which enteral tube feeding can positively affect pregnancy outcome and maternal quality of life, nausea and vomiting symptoms or time in hospital.
At present, there is no evidence on the effectiveness and efficiency of rehydration and dietary interventions for HG. We hypothesise that enteral tube feeding in addition to standard care is a more effective treatment for HG symptoms than standard care with intravenous rehydration alone, and improves pregnancy outcome.
This multicentre randomised controlled trial (RCT) aims to compare early enteral tube feeding in addition to standard care, with standard care alone. Outcomes of interest are birth weight and maternal nausea and vomiting symptoms, maternal quality of life, duration of hospitalisation, weight gain and neonatal morbidity. The study is conducted within the Dutch Consortium for Studies in Obstetrics, Fertility and Gynaecology (www.studies-obsgyn.nl).
Methods/Design
Participants/ eligibility criteria
Patients ≥ 18 years of age are eligible if they have been admitted to hospital because of HG (first admission or readmission) at a gestational age between 5 + 0 and 19 + 6 weeks. Patients with singleton or multiple pregnancies are eligible. A diagnosis of HG is made if excessive nausea or vomiting necessitates hospital admission, in the absence of any other obvious cause such as drug induced vomiting or infection.
Exclusion criteria are mola hydatidosa pregnancy, non-vital pregnancy, acute infection causing vomiting (e.g. appendicitis, pyelonephritis), contraindication for enteral tube feeding (e.g. oesophageal varices, allergies to enteral tube mix compounds) or HIV infection.
Procedures, recruitment and randomisation
This study is a nationwide multicentre open label RCT conducted within the Dutch Consortium for Studies in Obstetrics, Fertility and Gynaecology, a nationwide collaboration of hospitals in the Netherlands. The staff and/or local research coordinator of the participating hospitals identifies eligible women. After counselling and reading the patient information form, patients are asked for written informed consent. Patient information is provided in Dutch and English. See Fig. 1.
Fig. 1.
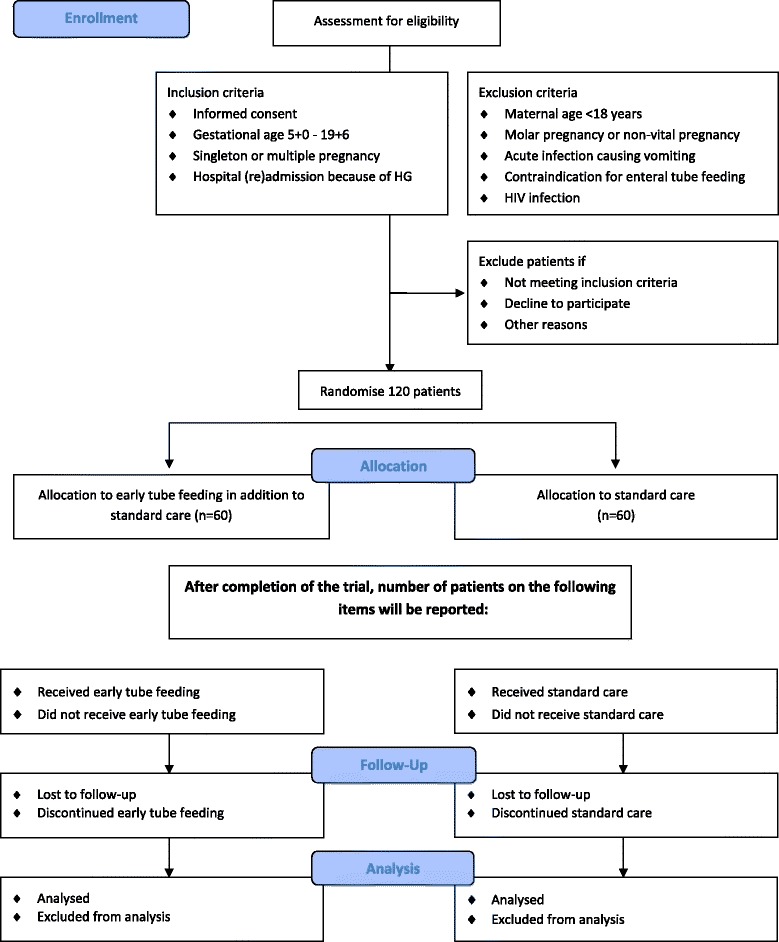
CONSORT 2010 flow diagram MOTHER trial
Randomisation is performed by a web based computerised program using permuted-block randomisation. Randomisation is allocated in a 1:1 ratio for standard care or enteral tube feeding in addition to standard care, with a block size of four. Stratification according to centre is applied.
Intervention
Participants are allocated to standard care or enteral tube feeding in addition to standard care. Standard care consists of intravenous rehydration and, when considered necessary, laboratory monitoring, electrolyte and/or vitamin supplementation, antiemetic medication and dietetic advice. Type of rehydration regimen, medication and duration of hospitalisation is prescribed according to local protocol. In case of prolonged hospital admission or readmissions, tube feeding can be initiated at the decision of the attending physician.
When allocated to the intervention group, participants receive a nasogastric tube as soon as possible after randomisation, in addition to standard care. If the initial nasogastric tube is dislocated or poorly tolerated a nasoduodenal or nasojejunal insertion can also be considered. Tube feeding regimen and mix is prescribed according to local protocol. As soon as tube feeding is tolerated and participants have received safety instructions (e.g. recognising symptoms that need to be evaluated in hospital, because of potential tube blockage, dislocation or aspiration), discharge home with tube feeding is encouraged under the guidance of a hospital dietician. Energy intake per tube is continued at least until the patient is able to maintain an oral intake of 1000 cal per day for one week. According to the NICE guideline on nutrition, tube feeding in a home setting is considered to be safe [14].
Data collection
At the day of randomisation, participants fill out a questionnaire. This questionnaire consists of validated NVP symptom and NVP specific quality of life measures (24-h Pregnancy Unique Quantification of Emesis and nausea score, PUQE-24; Hyperemesis Impact of Symptoms questionnaire, HIS; Nausea and Vomiting in Pregnancy Quality of Life questionnaire, NVPQoL) [7, 19–21], psychopathology (Hospital Anxiety and Depression Scale, HADS; Symptoms CheckList 90, SCL-90) [22–25] and general health related questions (Short Form 36, SF-36; EuroQol 5 Dimensions questionnaire,EQ5D) [26, 27].
Participants fill out additional questionnaires (NVPQoL, HIS, HADS) 1 and 3 weeks after randomisation and record a diary at weekly intervals (PUQE-24, weight, medication use, dietary intake) from randomisation until 20 weeks gestation. If dietary intake has normalised from 15 weeks gestation onwards, this is no longer recorded. Six weeks postpartum (HADS, SF-36, EQ5D) and 12 months postpartum a final questionnaire is filled out (HADS, SF-36, EQ5D, SCL-90). See Table 1.
Table 1.
Time line MOTHER trial
| T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | |
|---|---|---|---|---|---|---|---|---|
| Randomisation | +1 week | + 2 weeks | + 3 weeks | + 4 weeks until GA 20 weeks | Birth | 6 weeks post-partum | 12 months post-partum | |
| Diary | ||||||||
| PUQE | X | X | X | X | X | |||
| Current weight | X | X | X | X | X | |||
| Medication use | X | X | X | X | X | |||
| Dietary intake | X | X | X | X | Xa | |||
| Questionnaires | ||||||||
| General health | X | |||||||
| NVPQoL | X | X | X | |||||
| HIS | X | X | X | |||||
| HADS | X | X | X | X | X | |||
| SF-36 | X | X | X | |||||
| EQ5D | X | X | X | |||||
| SCL-90 | X | X | ||||||
| Biobank material | ||||||||
| Maternal blood | X | |||||||
| Cord blood | X | |||||||
| Placental biopsies | X | |||||||
PUQE pregnancy unique quantification of emesis and nausea score, NVPQoL nausea and vomiting in pregnancy quality of life questionnaire, HIS hyperemesis impact of symptoms questionnaire, HADS hospital anxiety and depression scale, SF-36 short form 36, EQ5D euroQol 5 dimensions questionnaire, SCL-90 symptoms checklist 90
aIf dietary intake has normalised from GA 15 weeks onwards, this will be no longer recorded
To evaluate potential HG and birth weight predictors, detailed information on obstetric and medical history, anthropometrics (before and during pregnancy), antiemetic medication use, given treatment(s) (including intravenous and/or tube feeding regimen and tube location), laboratory results, treatment and pregnancy complications and birth outcomes are collected using a standardised Case Report Form (CRF; see Additional file 1). Research staff obtains the information needed based on medical and dietician records. Maternal demographics (ethnicity, education level, marital status), mode of conception and onset of nausea and vomiting symptoms are enquired via the questionnaire.
All participants in this trial are asked for informed consent of storage of maternal blood (taken with routine laboratory analysis during hospital admission for HG), cord blood and placental biopsies (taken at birth) in an obstetrical biobank (the Preeclampsia and Non-preeclampsia Database, Academic Medical Centre Amsterdam, the Netherlands). The addition of these samples will enable molecular studies in HG aetiology and consequences. Furthermore, cord blood will be used for the assessment of plasma lipids (cholesterol, HDL, LDL, triglycerides, Apolipoprotein A and B), glucose, leptin and thyroid function (TSH, fT4).
Outcome measures
Primary outcome measure
The primary outcome will be neonatal birth weight.
Secondary outcome measures
Secondary outcomes will be the validated PUQE-24 score one week after randomisation, maternal weight gain, dietary intake, HIS, NVPQoL, EQ5D, SF-36, HADS and SCL-90 scores, urinary ketones, duration of hospital stay and number of readmissions. Furthermore, gestational age at birth, preterm birth rate, small for gestational age (SGA; <10th percentile) placental weight, umbilical cord plasma lipids, neonatal hypoglycaemia, hyperbilirubinaemia and congenital anomalies will be evaluated. Lastly, we will evaluate maternal side effects of tube feeding and intravenous rehydration and reasons for discontinuation of the allocated treatment.
Follow-up of infants
A plan for long-term follow up of children is in preparation, because little is known about the long term health effects of babies born to mothers whose pregnancies were complicated by HG and we have reason to hypothesise that maternal malnutrition during early pregnancy has long term effects on the offspring’s cardiometabolic health [4]. Funding for follow-up has not yet been obtained.
Statistical issues
Sample size
The sample size is based on a difference in mean birth weight of 200 g (SD 400 g) between the intervention group and the control group, which we consider clinically relevant. With a beta of 0.2 and alpha of 0.05 and a possible 10 % loss to follow up, we need to randomise 120 participants (60 per arm). This sample size is also large enough to detect a two point reduction in PUQE-24 score 1 week after randomisation (maximum 15 points, SD 3 points) and differences in quality of life, psychopathology and general health questionnaires ≥ 10 %.
Data analysis
Data will be analysed according to the intention to treat principle. Difference in birth weight will be assessed using parametric testing. PUQE-24 score will be analysed using multivariate regression and repeated measurements ANOVA or mixed models, as will be quality of life assessments. Other secondary outcomes will be addressed in a similar manner. For non-normally distributed variables non-parametric equivalents will be used. To evaluate the potential of each of the strategies, we will also perform a per protocol analysis, taking into account only those women that were treated according to protocol.
Data safety monitoring committee
Serious Adverse Events (SAEs) are reported to the Data Safety Monitoring Committee (DSMC). The DSMC can decide, if indicated, to terminate the trial prematurely.
Ethical considerations
This trial has been approved by the ethics committee of the Academic Medical Centre Amsterdam (Reference number MEC AMC 2012_320) and by the boards of management of all participating hospitals. The trial is registered in the Dutch Trial Register, NTR4197, http://www.trialregister.nlwebcite. Date of registration: October 2nd 2013. The full protocol can also be downloaded from the study website: www.studies-obsgyn.nl/mother.
Discussion
Since HG is the largest cause of hospital admission in early pregnancy and has major consequences for maternal quality of life, with possible adverse effects on birth outcomes, evidence based treatment options are needed. Optimal treatment should be safe, reduce maternal complaints, duration of hospital stay and minimise adverse effects on offspring health. This trial will provide evidence on these subjects comparing standard care with intravenous rehydration and early enteral tube feeding in addition to standard care.
Acknowledgements
Funding has been obtained from the Dutch Consortium for Studies in Obstetrics, Fertility and Gynaecology and the Foreest Medical School, Medical Centre Alkmaar, the Netherlands.
Abbreviations
- CRF
case report form
- EQ5D
euroQol 5 dimensions questionnaire
- HADS
hospital anxiety and depression scale
- HG
hyperemesis gravidarum
- HIS
hyperemesis impact of symptoms questionnaire
- NICE
National Institute for Health and Care Excellence
- NVP
nausea and vomiting in pregnancy
- NVPQoL
nausea and vomiting in pregnancy quality of life questionnaire
- PUQE-24
24-h pregnancy unique quantification of emesis and nausea score
- SCL-90
symptoms checklist 90
- SF-36
short form 36
Additional files
Case Report Form MOTHER trial. (PDF 252 kb)
CONSORT 2010 checklist MOTHER trial. (DOCX 48 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RCP, TJR, BWM, JvdP, CRS, JMJB and IJG represent the MOTHER study group and were involved in conception and design of the study. MK, CJB, JJD, HAB, MMP, WMH, KWMB, HCJS, MTMF and MAO are local investigators at the participating centres and participated in the design of the study during several meetings. IJG, RCP and TJR drafted the manuscript, which follows the CONSORT 2010 checklist for reporting randomised trials (see Additional file 2). All authors read, edited and approved the final draft of the manuscript.
References
- 1.Matthews A, Haas DM, O’Mathuna DP, Dowswell T, Doyle M. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2014;3:CD007575. doi: 10.1002/14651858.CD007575.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Niebyl JR. Nausea and vomiting in pregnancy. N Engl J Med. 2010;363:1544–50. doi: 10.1056/NEJMcp1003896. [DOI] [PubMed] [Google Scholar]
- 3.Bailit JL. Hyperemesis gravidarium: epidemiologic findings from a large cohort. Am J Obstet Gynecol. 2005;193:811–4. doi: 10.1016/j.ajog.2005.02.132. [DOI] [PubMed] [Google Scholar]
- 4.Veenendaal MVE, van Abeelen AFM, Painter RC, van der Post JAM, Roseboom TJ. Consequences of hyperemesis gravidarum for offspring: a systematic review and meta-analysis. BJOG. 2011;118:1302–13. doi: 10.1111/j.1471-0528.2011.03023.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith C, Crowther C, Beilby J, Dandeaux J. The impact of nausea and vomiting on women: a burden of early pregnancy. Aust N Z J Obstet Gynaecol. 2000;40:397–401. doi: 10.1111/j.1479-828X.2000.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 6.Lacasse A, Rey E, Ferreira E, Morin C, Bérard A. Nausea and vomiting of pregnancy: what about quality of life? BJOG. 2008;115:1484–93. doi: 10.1111/j.1471-0528.2008.01891.x. [DOI] [PubMed] [Google Scholar]
- 7.Lacasse A, Berard A. Validation of the nausea and vomiting of pregnancy specific health related quality of life questionnaire. Health Qual Life Outcomes. 2008;6:32. doi: 10.1186/1477-7525-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams MM, Harlass FE, Sarno AP, Read JA, Rawlings JS. Antenatal hospitalization among enlisted servicewomen, 1987-1990. Obstet Gynecol. 1994;84:35–9. [PubMed] [Google Scholar]
- 9.Gazmararian JA, Petersen R, Jamieson DJ, Schild L, Adams MM, Deshpande AD, et al. Hospitalizations during pregnancy among managed care enrollees. Obstet Gynecol. 2002;100:94–100. doi: 10.1016/S0029-7844(02)02024-0. [DOI] [PubMed] [Google Scholar]
- 10.Verberg MFG, Gillott DJ, Al-Fardan N, Grudzinskas JG. Hyperemesis gravidarum, a literature review. Hum Reprod Update. 2005;11:527–39. doi: 10.1093/humupd/dmi021. [DOI] [PubMed] [Google Scholar]
- 11.Sandven I, Abdelnoor M, Wethe M, Nesheim B-I, Vikanes Å, Gjønnes H, et al. Helicobacter pylori infection and hyperemesis gravidarum. An institution-based case–control study. Eur J Epidemiol. 2008;23:491–8. doi: 10.1007/s10654-008-9261-3. [DOI] [PubMed] [Google Scholar]
- 12.Jueckstock JK, Kaestner R, Mylonas I. Managing hyperemesis gravidarum: a multimodal challenge. BMC Med. 2010;8:46. doi: 10.1186/1741-7015-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stokke G, Gjelsvik BL, Flaatten KT, Birkeland E, Flaatten H, Trovik J. Hyperemesis gravidarum, nutritional treatment by nasogastric tube feeding: a 10-year retrospective cohort study. Acta Obstet Gynecol Scand. 2015;94:359–67. doi: 10.1111/aogs.12578. [DOI] [PubMed] [Google Scholar]
- 14.National Institute for Health and Clinical Excellence . Nutrition support in adults: NICE guideline CG32. London: NICE Clinical Guidelines; 2006. [PubMed] [Google Scholar]
- 15.Holmgren C, Silver RM, Porter TF, Varner M, Aagaard-Tillery KM. Hyperemesis in pregnancy: an evaluation of treatment strategies with maternal and neonatal outcomes. Am J Obstet Gynecol. 2008;198:56. doi: 10.1016/j.ajog.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Gulley RM, Vander Pleog N, Gulley JM. Treatment of hyperemesis gravidarum with nasogastric feeding. Nutr Clin Pr. 1993;8:33–5. doi: 10.1177/011542659300800133. [DOI] [PubMed] [Google Scholar]
- 17.Hsu JJ, Clark-Glena R, Nelson DK, Kim CH. Nasogastric enteral feeding in the management of hyperemesis gravidarum. Obstet Gynecol. 1996;88:343–6. doi: 10.1016/0029-7844(96)00174-3. [DOI] [PubMed] [Google Scholar]
- 18.Pearce CB, Collett J, Goggin PM, Duncan HD. Enteral nutrition by nasojejunal tube in hyperemesis gravidarum. Clin Nutr. 2001;20:461–4. doi: 10.1054/clnu.2001.0484. [DOI] [PubMed] [Google Scholar]
- 19.Koren G, Boskovic R, Hard M, Maltepe C, Navioz Y, Einarson A. Motherisk-PUQE (pregnancy-unique quantification of emesis and nausea) scoring system for nausea and vomiting of pregnancy. Am J Obstet Gynecol. 2002;186(5 Suppl Understanding):S228–31. doi: 10.1067/mob.2002.123054. [DOI] [PubMed] [Google Scholar]
- 20.Magee LA, Chandra K, Mazzotta P, Stewart D, Koren G, Guyatt GH. Development of a health-related quality of life instrument for nausea and vomiting of pregnancy. Am J Obstet Gynecol. 2002;186:S232–8. doi: 10.1067/mob.2002.122604. [DOI] [PubMed] [Google Scholar]
- 21.Power Z, Campbell M, Kilcoyne P, Kitchener H, Waterman H. The hyperemesis impact of symptoms questionnaire: development and validation of a clinical tool. Int J Nurs Stud. 2009;47:67–77. doi: 10.1016/j.ijnurstu.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Derogatis LR. SCL-90: an outpatient psychiatric rating scale--preliminary report. Psychopharmacol Bull. 1973;9:13–28. [PubMed] [Google Scholar]
- 23.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 24.Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens AE, Van Hemert AM. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27:363–70. doi: 10.1017/S0033291796004382. [DOI] [PubMed] [Google Scholar]
- 25.Arrindell WA, Ettema JHM: SCL-90 (symptom checklist 90). Handleiding Bij Een multidimensionele psychopathologie-indicator. Lisse; Swets Test Publishers 2003.
- 26.The EuroQoL Group EuroQol - a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 27.Ware JE, Snow KK, Kosinski M, Grandek B. SF-36 health survey: manual and interpretation guide. Boston, MA; The Health Institute, New England Medical Center 1993.


