Abstract
Objective:
To study the correlation and effect of sequential measurement of intraocular pressure (IOP) with Goldmann applanation tonometer (GAT), ocular response analyzer (ORA), dynamic contour tonometer (DCT), and Corvis ST.
Setting and Design:
Observational cross-sectional series from the comprehensive clinic of a tertiary eye care center seen during December 2012.
Methods:
One hundred and twenty-five study eyes of 125 patients with normal IOP and biomechanical properties underwent IOP measurement on GAT, DCT, ORA, and Corvis ST; in four different sequences. Patients with high refractive errors, recent surgeries, glaucoma, and corneal disorders were excluded so as to rule out patients with evident altered corneal biomechanics.
Statistical Analysis:
Linear regression and Bland–Altman using MedCalc software.
Results:
Multivariate analysis of variance with repeated measures showed no influence of sequence of device use on IOP (P = 0.85). Linear regression r2 between GAT and Corvis ST, Corvis ST and Goldmann-correlated IOP (IOPg), and DCT and Corvis ST were 0.37 (P = 0.675), 0.63 (P = 0.607), and 0.19 (P = 0.708), respectively. The Bland–Altman agreement of Corvis ST with GAT, corneal compensated IOP, and IOPg was 2 mmHg (−5.0 to + 10.3), −0.5 mmHg (−8.1 to 7.1), and 0.5 mmHg (−6.2 to 7.1), respectively. Intraclass correlation coefficient for repeatability ranged from 0.81 to 0.96.
Conclusions:
Correlation between Corvis ST and ORA was found to be good and not so with GAT. However, agreement between the devices was statistically insignificant, and no influence of sequence was observed.
Keywords: Corneal biomechanics, corneal viscoelasticity, intraocular pressure, Scheimpflug noncontact tonometry
The intraocular pressure (IOP) is the only “modifiable” risk factor in glaucoma, and hence the accurate assessment and monitoring of the IOP is of paramount importance. Many novel methods of measuring the IOP have been devised.[1,2,3,4] The effect of corneal thickness and biomechanics is a subject of immense interest as it is well-known that these affect the IOP measurement.[1,3,5,6,7,8] The Goldmann applanation tonometer (GAT) is considered the standard method of measuring IOP although it does not take into account the corneal biomechanical properties.[8] The influence of central corneal thickness (CCT) on applanation tonometry has been explored; however, other factors especially biomechanical properties such as viscoelastics need to be studied.[8] The dynamic contour tonometer (DCT) is a contact method of measuring the IOP. This is based on the principle that by surrounding and matching the contour of the cornea, the pressure on the outside matches the pressure on the inside. IOP measured by GAT on an average has been noted to be lower than that measured by the DCT as it is considered to be less affected by corneal biomechanical properties and maintains the natural shape of the cornea during measurement.[9,10,11] Ocular pulse amplitude (OPA) is another variable reported by DCT and is the difference between the maximum and minimum pressure of pressure pulse. OPA of glaucoma patients can vary significantly from that of normal patients.[12,13,14]
The ocular response analyzer (ORA) is a noncontact method of measuring the IOP in addition to biomechanical properties of the cornea using a dynamic bi-directional applanation process.[2] It uses a rapid pulse of air to applanate the cornea and an advanced electro-optical system to monitor its deformation. The ORA reports two IOPs: Goldmann-correlated IOP (IOPg) and corneal compensated IOP (IOPcc).[2,15,16,17,18,19] IOPcc was designed to account for the error in IOP measurement due to corneal biomechanical variations among subjects. Several studies have shown IOPcc and in a few even IOPg to overestimate GAT IOP in glaucoma patients.[15,16,17,18] In addition, ORA IOPs may also be affected by corneal thickness.[19] The Corvis ST is a newer noncontact tonometer with a Scheimfplug camera, which measures the IOP and evaluates the dynamic (viscoelastic) response of the cornea to a puff of air. The unique features of the Corvis ST are that it provides a two-dimensional image of a cross-section of the deforming cornea during applanation, measures the apical displacement of the cornea and also measures the IOP.[19,20,21] The clinical application of these newer instruments can be improved once their correlation with GAT is evaluated. Therefore, the aim of this study was to compare the IOPs measured by the different instruments (GAT, DCT, ORA, and Corvis) in a multivariate model. To further distinguish the contrasting effects on repeatability of IOP measurements, we have measured the IOP in each patient in four different sequences of measurement by the different devices. The hypothesis was that noncontact methods of measurement (using an air-puff) may introduce some form of residual deformation in the cornea due to its viscoelastic nature, which may affect the subsequent IOP measurements when the devices are used sequentially.
Methods
This was an observational cross-sectional study performed on patients of comprehensive clinic of a tertiary eye care center located in Southern part of India after approval by the Institutional Review Board in December 2012. Informed consent was obtained from all the subjects, and the study adhered to the tenets of the declaration of Helsinki. The sample size was calculated based on a pilot study assuming Type 1 error of 0.01 and Type 2 error of 0.05 power of the study being 80% to detect a statistically significant difference. Both eyes of the patients were tested but only one eye (the right eye) of each patient was included in the study to eliminate bias, and the patients on whom all the tests could not be performed were excluded from analysis.
Both the eyes of 132 patients were examined, and IOP was estimated with all four instruments. Of these, for four patients, DCT could not be estimated with appropriate reliability, and for three patients, GAT could not be performed for logistic reasons. Hence, the right eyes of 125 subjects were included in the study. The patients included were in the age group of 18–80 years either emmetrope or had a refractive error of < 5D of myopia and 3D of hyperopia. The patients with cataract and pseudophakia that were operated more than a year ago were included. This was done to eliminate any factors that would have affected the corneal biomechanical properties significantly. Similarly, the patients with altered corneal biomechanics: Keratoconus, any other cornea problems, for example, pellucid marginal corneal degeneration, prior refractive surgery, prior cornea surgery, prior retina surgery, cataract surgery done within last 1 year, aphakia, refractive error >−5.0D and + 3.00D, astigmatism >+3D were not included in the study. Patients in whom subclinical corneal ectatic conditions or keratoconus was suspected based on refraction and clinical findings were subjected to pentacam and were excluded from the study if it showed significant abnormality.
The instruments that were used in the study were the Corvis ST (Oculus Optikger¨ate GmbH, Germany), the ORA (Reichert Inc., Germany), the DCT (Ziemer Ophthalmic Systems AG, Switzerland), and a GAT (Haag-Streit AG, Switzerland) mounted on a slit lamp. All patients underwent IOP measurements on the GAT, the DCT, the ORA, and the Corvis ST, by two experienced users of these devices. There was a gap of 5 min between each measurement on a machine. A total of two measurements each was made with GAT and DCT as these are contact tonometers, and three measurements each with the ORA and Corvis ST since these are noncontact tonometers. The machines can be classified into two groups: Static measurement (GAT and DCT) and dynamic measurement (ORA and Corvis ST). The dynamic measurement is due to the high-pressure air-puff applied on the cornea to quantify corneal biomechanics in addition to IOP measurement. There is a possibility of residual effect of applanation or air puff on subsequent IOP measurements, hence to eliminate the bias we chose four different sequences, i.e. the order in which the different devices were used for IOP measurement. Although, with four devices, there are 24 sequences possible but that would make the study very complex; hence, we limited ourselves to four sequences. The patients were randomly assigned to one of the four sequences. To eliminate diurnal variations in IOP, all measurements were completed in one visit of the patient and no follow-up measurements were needed. These four sequences were as follows:
Sequence A GAT, Corvis ST, ORA, DCT
Sequence B Corvis ST, ORA, DCT, GAT
Sequence C ORA, DCT, GAT, Corvis ST
Sequence D DCT, GAT, Corvis ST, ORA
Statistical analysis
It was performed to evaluate the effect of sequence groups using multiple analysis of variance (MANOVA) post-hoc analyses for repeated measures. Linear regression was performed between the mean values of IOPs for each patient from a device and the correlation coefficient along with the lack of fit was analyzed. Similarity of the devices was compared with Bland–Altman plots for repeated measures. Intraclass correlation coefficient (repeated measures) and interobserver (different devices) variability (coefficient of variation) were also evaluated for all the devices. All tests used a P value of 0.05 as the measure of statistical significance. MedCalc version12.5.0.0-64 bit (MedCalc software bvba) was used to perform the statistical analysis.
Results
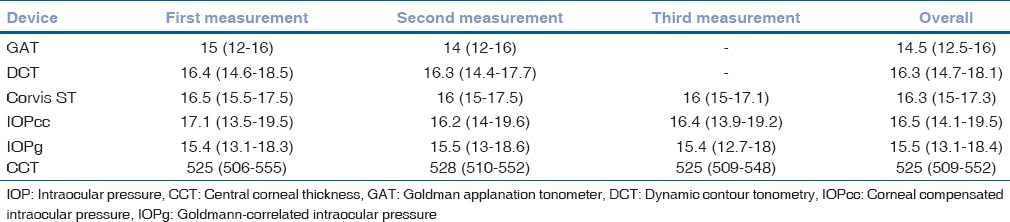
The right eyes of 125 subjects were included in the study, out of which 25 were normal volunteers and 100 were patients who presented to the outpatient department. There were 57 males and 68 females. The age ranged from 20 to 79 year; however, majority of the patients (103/125) patients were in the range of 21–50 years. Table 1 lists the median values of all measurements taken by different devices. There were 32 patients with Sequence A and 31 for each Sequence B, C, and D. MANOVA analysis with repeated measures did not yield any significant effect of sequence on IOP measured (P = 0.85). However, there was a significant difference between repeated measurements from the same device (P < 0.001) and this difference was independent of the sequence of device usage (P = 0.782). The intraclass correlation coefficient of repeatability for GAT, DCT, Corvis ST, IOPcc, and IOPg was 0.96 (95% confidence interval [CI]: 0.92–0.98), 0.81 (95% CI: 0.73–0.86), 0.91 (95% CI: 0.87–0.94), 0.90 (95% CI: 0.87–0.93), and 0.95 (95% CI: 0.93–0.96), respectively. The coefficient of variation was 3.52%, 7.18%, 11.06%, 8.68%, and 4.61% for GAT, DCT, IOPcc, IOPg, and Corvis ST, respectively.
Table 1.
Median values with 25-75% inter-quartile range (in brackets) of the intraocular pressure (mmHg) measured by all the devices and central corneal thickness (in microns) measured by the Corvis ST
The results of linear regression between IOPs measured by the different devices, without considering the sequences of device usage, are shown in Table 2. All regressions passed the lack of fit test with CCT versus GAT having the least coefficient of correlation. IOPcc correlated the best with IOPg followed by Corvis ST. Even though IOPg was supposed to be Goldmann-correlated, the present patient group did not demonstrate high coefficient of correlation. All regressions demonstrated significant presence of fit. Table 3 lists the results of Bland–Altman analysis with repeated measures. All devices consistently overestimated IOP compared to GAT. Interestingly, the difference between Corvis and ORA and between Corvis and DCT was considerably lower than the differences with GAT. The correlation plots [Figs. 1 and 2] show the correlation between IOP measured by Corvis ST and GAT, and between Corvis ST and IOPcc, respectively. The Bland–Altman plots [Figs. 3-5] show the agreement among Corvis ST and GAT, DCT and Corvis ST, and among ORA IOPcc and Corvis ST, respectively.
Table 2.
Linear regression analysis between intraocular pressure's measured with Goldman applanation tonometer, dynamic contour tonometry, ocular response analyzer, and Corvis ST
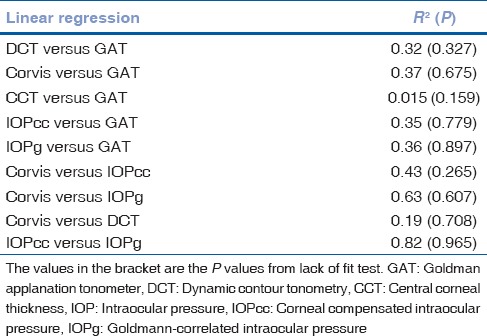
Table 3.
Results of Bland-Altman analysis for repeated measures
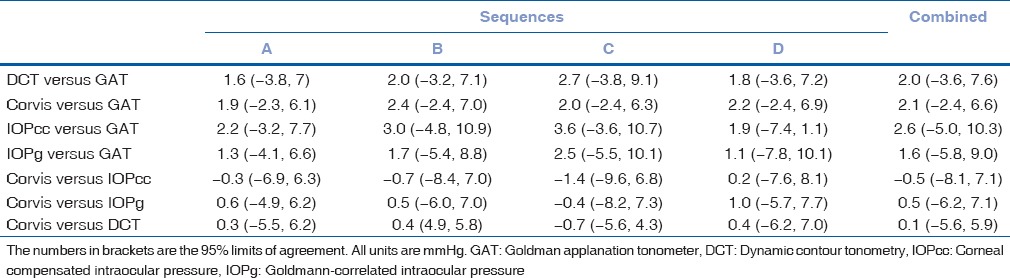
Figure 1.
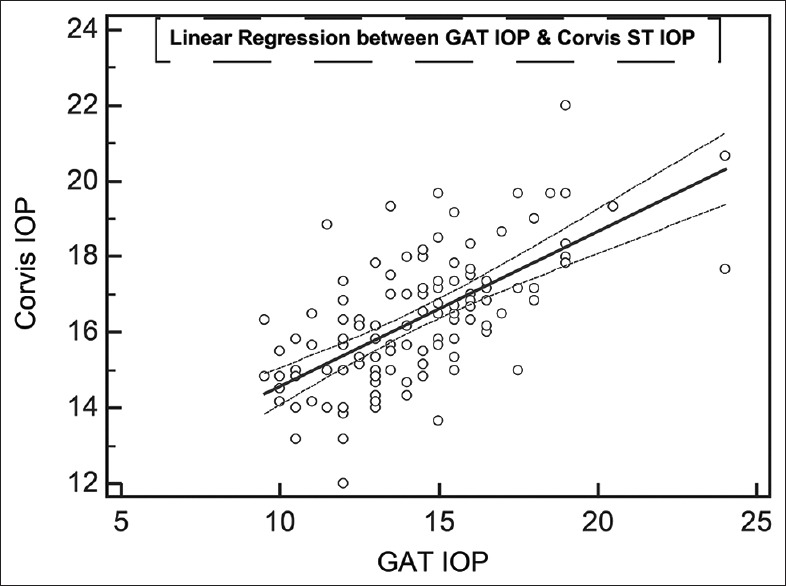
Correlation on linear regression between mean of Goldmann applanation tonometer intraocular pressure and mean of Corvis ST intraocular pressure. All numbers are in mmHg
Figure 2.
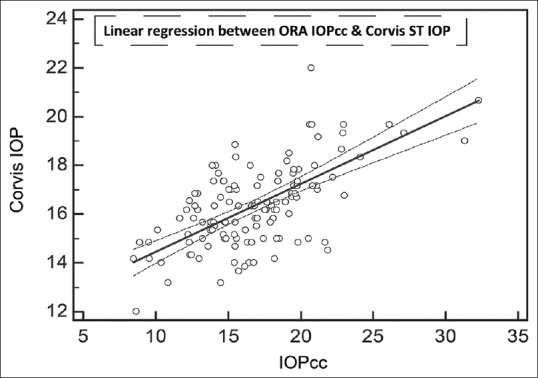
Correlation on linear regression between mean of corneal compensated intraocular pressure and mean of Corvis ST intraocular pressure. All numbers are in mmHg
Figure 3.
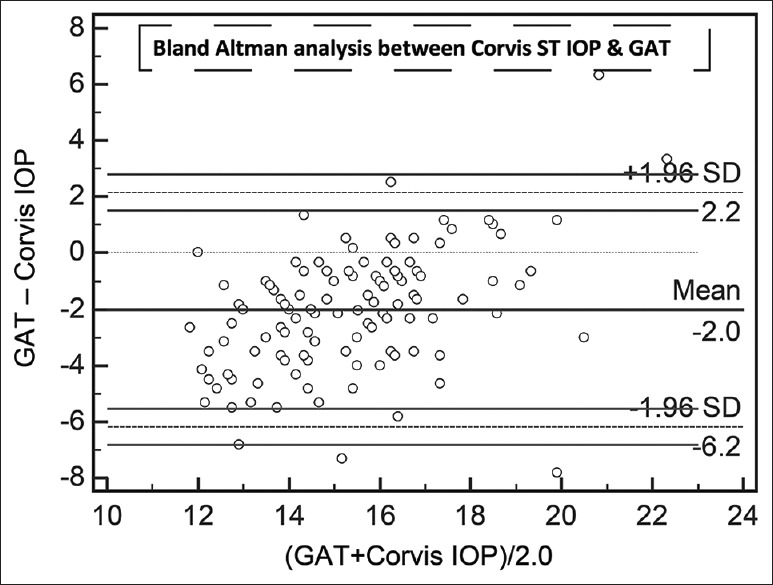
Agreement on Bland–Altman analysis between mean of Goldmann applanation tonometer intraocular pressure and mean of Corvis ST intraocular pressure. All numbers are in mmHg
Figure 5.
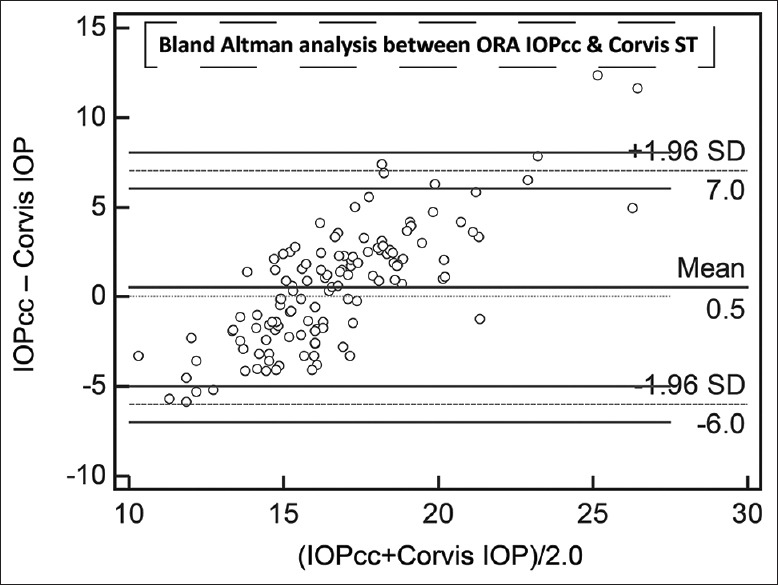
Agreement on Bland–Altman analysis between mean of corneal compensated intraocular pressure and mean of Corvis ST intraocular pressure. All numbers are in mmHg
Figure 4.
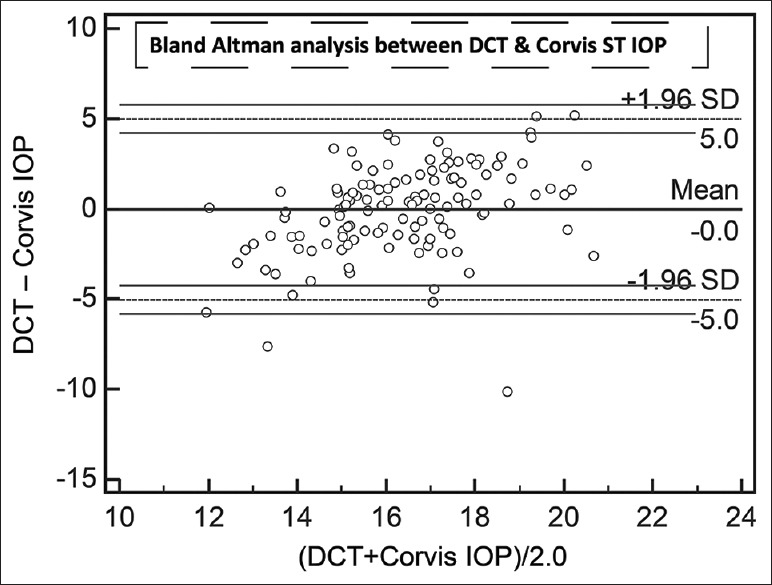
Agreement on Bland–Altman analysis between mean of dynamic contour tonometer and mean of Corvis ST intraocular pressure. All numbers are in mmHg
Discussion
An accurate measurement of IOP has always been a challenge. There was no single instrument that can measure the same in an accurate manner since IOP measurement is dependent on many factors such as the corneal thickness, corneal biomechanics, scleral rigidity, refractive error, and time of the day. Corneal biomechanical properties such as viscosity, elasticity, hydration, and curvature of the cornea were found to have significant impact on IOP measurement.[6,8] Studies have compared contact tonometers: Goldman applanation and dynamic contour tonometry.[3,4] Furthermore, studies had correlated ORA with GAT and noncontact tonometers.[15,16,17] To the best of our knowledge, this was the first study that correlated the four tonometers: Corvis ST, ORA, GAT, and DCT and that also considered the influence of sequence of testing of contact and noncontact tonometers. It was found that there was no influence of measurements with one device on measurements with next device in a sequence. The gap between the device measurements was 5 min. Hence, the current study suggested that the residual effect of corneal viscoelasticity due to applanation or indentation of cornea due to air puff may not last longer than that.
It was observed that the measurements done by machines that take into account the biomechanics had similar outputs, for example, correlation of 0.63 between Corvis IOP and IOPg. A study by Kaushik et al. had demonstrated a better correlation among GAT IOP, and corneal hysteresis (CH) as well as corneal resistance factor showing the effect of corneal biomechanics on IOP.[6] Reznicek et al. had reported a good agreement and repeatability of Corvis ST measurements of IOP and pachymetry as compared to GAT and ultrasonic pachymetry in normal and glaucoma patients.[22] However, the current study demonstrated that GAT overestimated the IOP as compared to dynamic contour tonometry, Corvis ST, and ORA, with the difference being the lowest with dynamic contour tonometry. In addition, the bias was found to be least between Corvis ST and IOPcc and also between Corvis ST and DCT. However, the limits of agreement were found to be very wide. Another explanation for the insensitivity of devices in sequential measurement of IOP could be that the inherent differences (such as technique of measurement, data processing) between tonometers were so significant that the differences due to sequence change could not be estimated. Nemeth et al. reported good repeatability with intraclass correlation of 0.87 for IOP measurement by Corvis ST.[23] Moreover, the current study showed better repeatability with intraclass correlation of 0.95 and a coefficient of repeatability equal 4.6% that was comparable to that of GAT.
It was quite evident that these instruments cannot replace each other, and it definitely indicated that the information obtained from the Corvis ST is very different than what we obtained from GAT and DCT. The Corvis ST did show a good repeatability and less interobserver variation for IOP measurements. Furthermore, the tonometers that measured corneal biomechanics, i.e., ORA and Corvis ST were very different from each other. Future studies should focus on understanding the corneal biomechanics in abnormal corneas, for example, eyes with high IOP could have stronger biomechanics due to nonlinear viscoelasticity of cornea and eyes with keratoconus or post-LASIK with relatively weaker biomechanics. There have been few studies comparing corneal resistance factor and CH on ORA to understand changes in corneal elasticity postpenetrating keratoplasty and in keratoconus.[24,25] With the capability to measure corneal biomechanical properties with Corvis ST using corneal deformation history, the study points out that instruments that measure corneal biomechanical properties correlate well with each other; however, insignificant agreement suggests that they cannot replace each other.
Financial support and sponsorship
Nil.
Conflicts of interest
The manuscript has been read and approved by all the authors, that the requirements for authorship for each one have been met, and that each author believes that the manuscript represents honest work. The manuscript has not been published or under consideration for publication anywhere else. Also, none of the authors would re-edit, submit or copyright once it is published with Indian Journal of Ophthalmology.
References
- 1.Salim S, Du H, Wan J. Comparison of intraocular pressure measurements and assessment of intraobserver and interobserver reproducibility with the portable ICare rebound tonometer and Goldmann applanation tonometer in glaucoma patients. J Glaucoma. 2013;22:325–9. doi: 10.1097/IJG.0b013e318237caa2. [DOI] [PubMed] [Google Scholar]
- 2.Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005;31:156–62. doi: 10.1016/j.jcrs.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 3.Shemesh G, Soiberman U, Kurtz S. Intraocular pressure measurements with Goldmann applanation tonometry and dynamic contour tonometry in eyes after IntraLASIK or LASEK. Clin Ophthalmol. 2012;6:1967–70. doi: 10.2147/OPTH.S38094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosentreter A, Athanasopoulos A, Schild AM, Lappas A, Cursiefen C, Dietlein TS. Rebound, applanation, and dynamic contour tonometry in pathologic corneas. Cornea. 2013;32:313–8. doi: 10.1097/ICO.0b013e318254a3fb. [DOI] [PubMed] [Google Scholar]
- 5.Scheler A, Spoerl E, Boehm AG. Effect of diabetes mellitus on corneal biomechanics and measurement of intraocular pressure. Acta Ophthalmol. 2012;90:e447–51. doi: 10.1111/j.1755-3768.2012.02437.x. [DOI] [PubMed] [Google Scholar]
- 6.Kaushik S, Pandav SS, Banger A, Aggarwal K, Gupta A. Relationship between corneal biomechanical properties, central corneal thickness, and intraocular pressure across the spectrum of glaucoma. Am J Ophthalmol. 2012;153:840–9. doi: 10.1016/j.ajo.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 7.Mansouri K, Leite MT, Weinreb RN, Tafreshi A, Zangwill LM, Medeiros FA. Association between corneal biomechanical properties and glaucoma severity. Am J Ophthalmol. 2012;153:419–27. doi: 10.1016/j.ajo.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: Quantitative analysis. J Cataract Refract Surg. 2005;31:146–55. doi: 10.1016/j.jcrs.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 9.Schneider E, Grehn F. Intraocular pressure measurement-comparison of dynamic contour tonometry and goldmann applanation tonometry. J Glaucoma. 2006;15:2–6. doi: 10.1097/01.ijg.0000196655.85460.d6. [DOI] [PubMed] [Google Scholar]
- 10.Pepose JS, Feigenbaum SK, Qazi MA, Sanderson JP, Roberts CJ. Changes in corneal biomechanics and intraocular pressure following LASIK using static, dynamic, and noncontact tonometry. Am J Ophthalmol. 2007;143:39–47. doi: 10.1016/j.ajo.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 11.Herdener S, Hafizovic D, Pache M, Lautebach S, Funk J. Is the PASCAL-Tonometer suitable for measuring intraocular pressure in clinical routine?. Long-and short-term reproducibility of dynamic contour tonometry. Eur J Ophthalmol. 2008;18:39–43. doi: 10.1177/112067210801800107. [DOI] [PubMed] [Google Scholar]
- 12.Punjabi OS, Ho HK, Kniestedt C, Bostrom AG, Stamper RL, Lin SC. Intraocular pressure and ocular pulse amplitude comparisons in different types of glaucoma using dynamic contour tonometry. Curr Eye Res. 2006;31:851–62. doi: 10.1080/02713680600899887. [DOI] [PubMed] [Google Scholar]
- 13.Božic M, Dukic ML, Stojkovic M. Spectral analysis of intraocular pressure pulse wave in open angle glaucomas and healthy eyes. Curr Eye Res. 2012;37:1019–24. doi: 10.3109/02713683.2012.700755. [DOI] [PubMed] [Google Scholar]
- 14.Marjanovic I, Milic N, Martinez A. The impact of intraocular pressure reduction on retrobulbar hemodynamic parameters in patients with open-angle glaucoma. Eur J Ophthalmol. 2012;22:77–82. doi: 10.5301/EJO.2011.8311. [DOI] [PubMed] [Google Scholar]
- 15.Medeiros FA, Weinreb RN. Evaluation of the influence of corneal biomechanical properties on intraocular pressure measurements using the ocular response analyzer. J Glaucoma. 2006;15:364–70. doi: 10.1097/01.ijg.0000212268.42606.97. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-de-la-Casa JM, Garcia-Feijoo J, Fernandez-Vidal A, Mendez-Hernandez C, Garcia-Sanchez J. Ocular response analyzer versus Goldmann applanation tonometry for intraocular pressure measurements. Invest Ophthalmol Vis Sci. 2006;47:4410–4. doi: 10.1167/iovs.06-0158. [DOI] [PubMed] [Google Scholar]
- 17.Kirwan C, O’Keefe M, Lanigan B. Corneal hysteresis and intraocular pressure measurement in children using the reichert ocular response analyzer. Am J Ophthalmol. 2006;142:990–2. doi: 10.1016/j.ajo.2006.07.058. Erratum in: Am J Ophthalmol 2007;144:642. [DOI] [PubMed] [Google Scholar]
- 18.Morita T, Shoji N, Kamiya K, Hagishima M, Fujimura F, Shimizu K. Intraocular pressure measured by dynamic contour tonometer and ocular response analyzer in normal tension glaucoma. Graefes Arch Clin Exp Ophthalmol. 2010;248:73–7. doi: 10.1007/s00417-009-1169-4. [DOI] [PubMed] [Google Scholar]
- 19.Hon Y, Lam AK. Corneal deformation measurement using Scheimpflug noncontact tonometry. Optom Vis Sci. 2013;90:e1–8. doi: 10.1097/OPX.0b013e318279eb87. [DOI] [PubMed] [Google Scholar]
- 20.Hong J, Xu J, Wei A, Deng SX, Cui X, Yu X, et al. A new tonometer – The Corvis ST tonometer: Clinical comparison with noncontact and Goldmann applanation tonometers. Invest Ophthalmol Vis Sci. 2013;54:659–65. doi: 10.1167/iovs.12-10984. [DOI] [PubMed] [Google Scholar]
- 21.Faria-Correia F, Ramos I, Valbon B, Luz A, Roberts CJ, Ambrósio R., Jr Scheimpflug-based tomography and biomechanical assessment in pressure-induced stromal keratopathy. J Refract Surg. 2013;29:356–8. doi: 10.3928/1081597X-20130129-03. [DOI] [PubMed] [Google Scholar]
- 22.Reznicek L, Muth D, Kampik A, Neubauer AS, Hirneiss C. Evaluation of a novel Scheimpflug-based non-contact tonometer in healthy subjects and patients with ocular hypertension and glaucoma. Br J Ophthalmol. 2013;97:1410–4. doi: 10.1136/bjophthalmol-2013-303400. [DOI] [PubMed] [Google Scholar]
- 23.Nemeth G, Hassan Z, Csutak A, Szalai E, Berta A, Modis L., Jr Repeatability of ocular biomechanical data measurements with a Scheimpflug-based noncontact device on normal corneas. J Refract Surg. 2013;29:558–63. doi: 10.3928/1081597X-20130719-06. [DOI] [PubMed] [Google Scholar]
- 24.Goldich Y, Marcovich AL, Barkana Y, Mandel Y, Hirsh A, Morad Y, et al. Clinical and corneal biomechanical changes after collagen cross-linking with riboflavin and UV irradiation in patients with progressive keratoconus: Results after 2 years of follow-up. Cornea. 2012;31:609–14. doi: 10.1097/ICO.0b013e318226bf4a. [DOI] [PubMed] [Google Scholar]
- 25.Acar BT, Akdemir MO, Acar S. Corneal biomechanical properties in eyes with no previous surgery, with previous penetrating keratoplasty and with deep anterior lamellar keratoplasty. Jpn J Ophthalmol. 2013;57:85–9. doi: 10.1007/s10384-012-0197-5. [DOI] [PubMed] [Google Scholar]


