Abstract
Purpose:
To assess demographic and clinical characteristics of glaucoma patients in an Ophthalmologic Hospital of Jinan, China from 2003 to 2012.
Materials and Methods:
Medical charts of patients with primary open-angle glaucoma (POAG), primary angle closure glaucoma (PACG), and secondary glaucoma (SG) were reviewed. The main outcome measures of patients with glaucoma included basic demographic data (age at presentation, gender, and residence), clinical characteristics (admission date, intraocular pressure, and naked vision), and previous history (injury, cardiovascular disease, diabetes mellitus, hypertension, smoking, and alcohol consumption).
Results:
Data from 1458 glaucoma patients were reviewed, of which PACG and SG patients accounted for 45.40% and 47.19%, respectively. The average age of all patients with glaucoma increased from 56.05 years in 2003 to 57.83 years in 2012, and the proportion of patients from rural areas rose from 46.43% to 59.13% during 10-year period. Female gender, cardiovascular disease, and hypertension were associated with PACG. POAG was related to smoking and alcohol consumption. There was positive correlation between SG and history of injury and diabetes mellitus.
Conclusion:
PACG and SG are the major types of glaucoma. Gender, injury, diabetes mellitus, cardiovascular disease, hypertension, smoking, and alcohol consumption were associated with different types of glaucoma.
Keywords: Clinical characteristics, epidemiology, glaucoma, retrospective study
Glaucoma is the second leading cause of irreversible blindness worldwide.[1] Globally, an estimated 60 million people were suffered from glaucomatous optic neuropathy, and an approximate 8.4 million people became blind as the result of glaucoma.[2] In 2020, there will be 79.64 million patients with open-angle glaucoma (OAG) and primary angle closure glaucoma (PACG), with China having 21.82 million (27.40%)-the largest number in the world.[3] Among researches conducted in Mainland China, in 2003, a population-based study by Ren et al. found a glaucoma prevalence of 2.14% in rural population with an age of 50 years or more in Shaanxi Province.[4] In 2001, the Beijing Eye Study reported that the glaucoma prevalence was 3.6% in the adult population (≥40 years) of Greater Beijing with a ratio of OAG to PACG of 2.6:1 and increased with age.[5]
To some extent, the hospitalized glaucoma patients could perhaps represent those in the broader population. However, there are distinctive differences between patients with glaucoma in different areas both in Mainland China and abroad and, thus, previous epidemiological studies are not representative. Therefore, we retrospectively analyze the proportion of different types of glaucoma, demographic and clinical characteristics of the patients to provide epidemiologic reference for medical treatment and future prevention in clinics of this disease.
Materials and Methods
The medical records of 1458 patients diagnosed with glaucoma between January 2003 and December 2012 from an ophthalmologic hospital were reviewed and analyzed. Glaucoma was defined according to the International Society of Geographical and Epidemiologic Ophthalmology criteria.[6] In this definition, criteria for the category 1 cases were a vertical cup/disc diameter ratio (VCDR) or VCDR asymmetry ≥97.5 percentile in the normal population or a neuroretinal rim width (11–1 or 5–7 o’clock) ≤0.1 VCDR and a reliable glaucomatous visual defect. Criteria for the category 2 cases were a severely damaged optic disc without reliable visual field test and a VCDR or a VCDR asymmetry ≥99.5 percentile in the normal population. In the category 1 or 2 cases, there would be no other explanation for the VCDR finding (e.g., dysplastic discs or marked anisometropia) or visual field defect (e.g., cerebrovascular disease, retinal vascular disease, or macular degeneration). Criteria for category 3 cases were subjects with no visual field or optic nerve head data but with a visual acuity <3/60 and either an intraocular pressure (IOP) ≥99.5 percentile or a history of previous glaucoma filtration surgery.
Initially, according to the principle discharge diagnosis, we divided all the patients into four subgroups such as primary open-angle glaucoma (POAG), PACG, secondary glaucoma (SG), and congenial glaucoma (CG). However, patients with CG were not comparative with those of the other three diagnoses in terms of demographic and clinical data since they tended to be much younger and had little previous history involving chronic disease, and thus were excluded. In addition, this kind of glaucoma was inherited and, therefore, may have little research value in the field of epidemiology and preventive medicine. In general, patients with POAG were identified if optic nerve damage met any of the three categories of criteria above, and there was no evidence of angle closure on gonioscopy and no identifiable secondary cause. Patients with PACG were defined as having an angle in which ≥270° of the posterior trabecular meshwork could not be seen, including both acute and chronic ones.[6] Corticosteroid-induced glaucoma, neovascular glaucoma, ciliary block glaucoma, glaucomatocyclitic crisis, and glaucoma resulting from ocular trauma were classified as SG.
This study followed the tenets of the Declaration of Helsinki for research involving human subjects and was approved by the Institutional Review Boards of the Ophthalmologic Hospital. In this retrospective study, of all available 1873 cases in archives, we excluded 415 from the analysis because of incomplete information and uncertain diagnosis. Besides, data of 2006 were absent due to some issues related to hospital construction when medical service was suspended. We excluded the case notes when they were fragmentary or the diagnoses were unclear. With respect to patients, only the information of their initial hospitalization was collected. If both eyes had the same diagnosis, we included the data from both eyes in the analysis. If one eye was diagnosed with glaucoma, we only included data of the affected eye. Data collected from the medical records of patients were the basic demographic data which included age at presentation, gender and residence, clinical characteristics such as admission date, IOP (IOP which is measured with a tonometer as part of a comprehensive eye examination), and naked vision (or unaided vision which is measured using an eye chart, optical instruments, or computerized tests), and previous history including injury, cardiovascular disease (cardiovascular disease is a class of diseases that involve the heart or blood vessels, and it includes coronary artery diseases such as angina and myocardial infarction), diabetes mellitus, hypertension (in people aged 18 years or older, hypertension is defined as a systolic and/or a diastolic blood pressure measurement consistently higher than 139 mmHg systolic and 89 mmHg diastolic), smoking, and alcohol consumption.
All data were entered into a standard Microsoft Excel 2010 (Microsoft Corporation, Washington, WA, USA) form designed to collect them, and statistical analyses were subsequently carried out using the SPSS version 19.0 (IBM Corporation, New York, NY, USA). For categorical variables, data were presented in forms of frequencies and percentages, and comparison between groups was done using Chi-square test. Continuous variables were reported as mean ± standard deviation, and the one-way analysis of variance was used to determine the significance of sociodemographic and clinical characteristics of various glaucoma subgroups. The trend in age of patients across time was examined by applying Pearson correlation analysis. To examine the association of glaucoma types with demographic or clinical variables, logistic regression analysis was conducted. Statistical significance was assigned at P < 0.05 (two-sided).
Results
A total of 1458 patients (984 right eyes and 981 left eyes) were enrolled. Their mean age was 56.73 ± 18.13 years (range: 1–93 years). Seven hundred forty-two (50.89%) patients were males while 716 (49.11%) were females, giving male to female ratio of 1.04:1. The mean ages of male and female were 53.21 ± 18.91 years and 60.37 ± 16.51 years, respectively (P < 0.001). Of them, 850 (58.30%) patients came from rural areas and the remaining 608 (41.70%) lived in cities.
The yearly amount of study subjects from the three subgroups of glaucoma is shown in Fig. 1. Among them, patients with PACG and SG outnumbered those with POAG at all years, and then accounted for the most common ones of glaucoma with a total number of 662 (45.40%) and 688 (47.19%) patients, respectively. We also analyzed the constituent ratio of the amount of patients with the three different types of glaucoma between each two successive years as well as between 2003 and 2012. The results showed that there was no statistical significance between any of those 2 years (P > 0.05).
Figure 1.
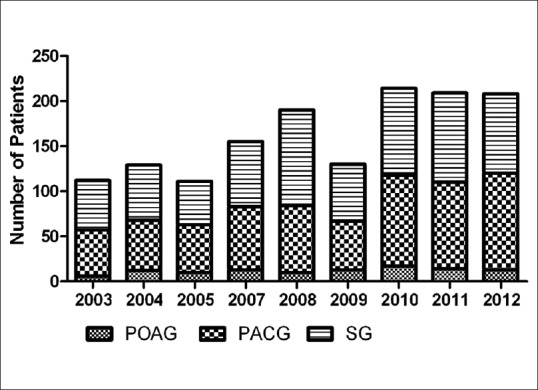
Trend of patient number of the three glaucoma subgroups, 2003–2012
The mean age of patients with PACG, POAG, and SG was 65.20 ± 11.82, 43.63 ± 18.23, and 50.94 ± 19.52 years, respectively (F = 166.206, P < 0.001). As shown in Fig. 2, the average age of all patients with glaucoma changed with respect to time, increasing from 56.05 years in 2003 to 57.83 years in 2012 (r = 0.074, P = 0.005). Likewise, the correlation between year and average age of patients with SG reached the level of statistical significance (r = 0.095, P = 0.013). A downward and upward trend of the proportion of patients coming from urban and rural areas, respectively, is shown in Fig. 3 with the former decreasing from 53.57% to 40.87% and the latter rising from 46.43% to 59.13% during the given time frame (t = 4.473, P = 0.029). We performed Chi-square test to examine changes in the proportion of the two groups between two successive years, and no statistical differences had been found among any of them (P > 0.05).
Figure 2.
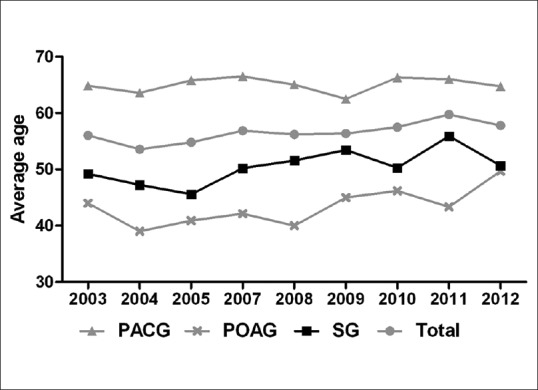
Trend of average age of patients from three glaucoma subgroups
Figure 3.
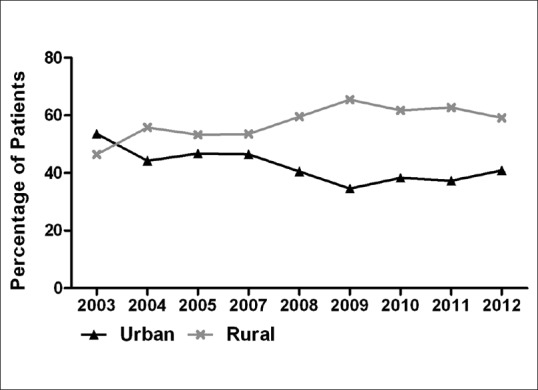
Trend of patient proportion from urban and rural areas, 2003–2012
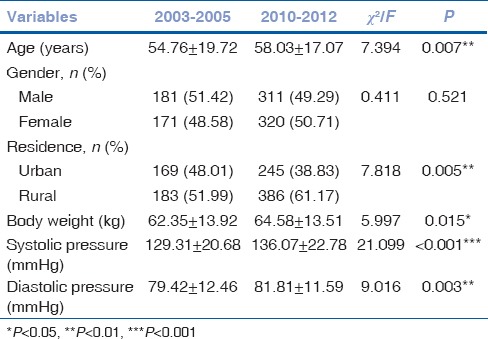
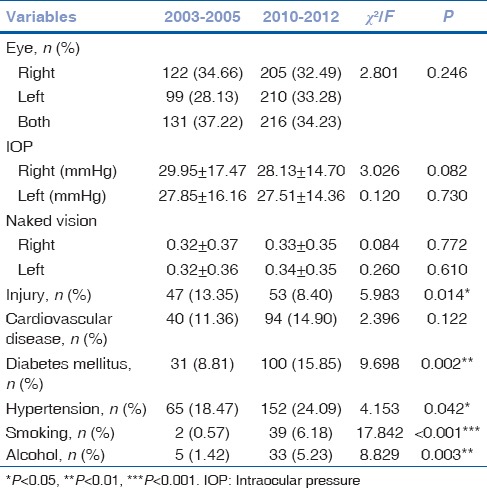
The comparison of demographic and clinical characteristics of the patients between 2003–2005 and 2010–2012 is shown in Table 1. As can be seen, all of them except gender were significantly different. With regard to age, patients hospitalized during 2010–2012 were older compared to those during 2003–2005 with their mean ages of 58.03 ± 17.07 and 54.76 ± 19.72 years, respectively (P = 0.007). Concerning residence, the proportion of patients from rural areas was higher in 2003–2005 than it was in 2010–2012 (P = 0.005). With respect to body weight, patients admitted to hospital in the previous 3 years were considerably lighter than those in the latter 3 years (P = 0.015). Similarly, the level of systolic and diastolic pressure for those hospitalized during 2003–2005 was lower than those during 2010–2012 (P < 0.001 and P = 0.003, respectively).
Table 1.
Comparison of demographic characteristics of the patients reviewed from 2003 to 2005 versus 2010 to 2012
As shown in Table 2, patients hospitalized during 2010–2012 were less likely to have a history of injury (P = 0.014). A higher percentage of those admitted in the previous 3 years tended to smoke and drink alcohol more than those in the latter 3 years (P < 0.001 and P = 0.003, respectively). Likewise, concerning diabetes mellitus and hypertension, the proportion of those in 2010–2012 was higher than their counterparts in 2003–2005 (P = 0.002 and P = 0.042, respectively). However, changes of affected eyes, IOP, and naked vision between previous and latter 3 years were not obvious and statistically significant (P > 0.05).
Table 2.
Comparison of clinical profiles of the patients reviewed from 2003 to 2005 versus 2010 to 2012
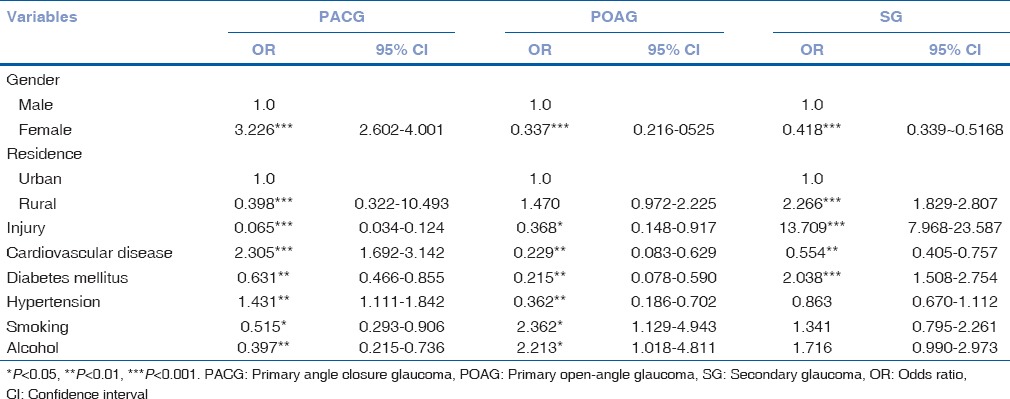
The distribution of the selected characteristics of the three glaucoma subgroups is shown in Tables 3 and 4. A higher proportion of patients with POAG and SG tended to be male and live in rural areas (P < 0.001). More of those with PACG had history of cardiovascular disease (P ≤ 0.001, odds ratio [OR] =2.305) and hypertension (P ≤ 0.001, OR = 1.431). Concerning smoking (P ≤ 0.001, OR = 2.362) and drinking alcohol (P ≤ 0.001, OR = 2.213), the proportion of those from POAG subgroup was higher. Those with SG were more likely to have a history of injury (P ≤ 0.001, OR = 13.709) and diabetes mellitus (P ≤ 0.001, OR = 2.038).
Table 3.
Comparison of demographic and clinical characteristics among patients from three glaucoma subgroups, 2003-2012
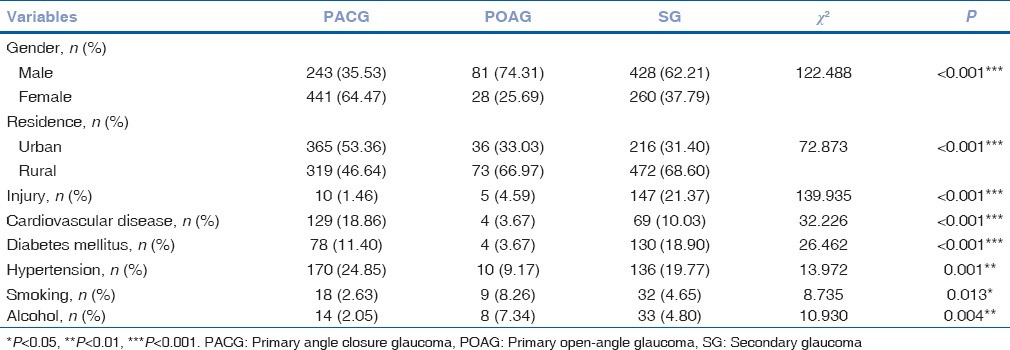
Table 4.
Associations between glaucoma types and demographic and clinical variables (univariate analysis) among patients, 2003-2012
Discussion
Our study demonstrated that patients with PACG and SG are in the majority accounting for 45.40% and 47.19%, respectively. This is consistent with findings of several previous studies in Mainland China,[4,7,8,9,10] and it is reported that the prevalence of PACG is highest in Asia.[11] Zhong et al. noted a poor diagnosis rate of POAG at even less than one-fifth compared with that of PACG.[12] POAG may be due to smaller corneal diameter, smaller anterior chamber depth (ACD), and a more anteriorly placed lens. Over the past several decades, POAG could only be diagnosed when IOP was elevated, which may lead to the underestimated proportion of POAG and the overrated proportion of PACG. In addition, some PACG patients had an acute symptomatic attack and most cases of OAG are asymptomatic and chronic, especially in the early stages, so that many of these patients remain unrecognized until symptoms occur, which leads to lower hospitalization rate compared with those suffering from PACG and SG. SG can be attributed to many factors including injury, diabetes mellitus, and uveitis, which are related to the socioeconomic development as well as changes and expansion of occupational categories. However, as PACG and SG patients may need hospitalization while those with POAG may be managed on outpatient basis, the numbers reported in this study may not represent true prevalence of various glaucomas in the population. In future, more emphasis should be attached to the screening methods and early detection of POAG as well as prophylaxis and treatment of PACG and SG in Mainland China.
All patients with three glaucoma types during 2010–2012 tend to be older than those during 2003–2005 and patients with PACG were the oldest group (65.20 ± 11.82). Lee et al. reported in a study involving 200 subjects form Hong Kong, China with acute ACG that the mean age was 69.2 ± 9.9 years, which is comparable to PACG patients in our study.[13] In the study conducted by Lee et al. in Hong Kong, China, the presenting age of the 100 cases of phacomorphic glaucoma was 73.8 ± 10.6 years, which is a little higher than the patients in our research.[14] Female gender was a risk factor for PACG (OR: 3.226 [95% confidence interval (CI): 2.602–4.001]). PACG can be mainly attributed to pupil block, plateau iris configuration, crowding of peripheral anterior chamber, or a combination of these factors together. As in previous reports, age is known to be a risk factor for PACG.[15,16,17] Gradual thickening and forward position of the crystalline lens and pupil narrowing are consistent with age. In addition, older people tended to have shallower ACD and, therefore, were predisposed to PACG.[13,18,19] The association between female and PACG was the same as has been previously reported.[3,10,16,20,21] However, using univariate and multivariate linear regression models as well as receiving operator characteristic curves and the area under the curve, Hsu et al. reported that age was an independent associating factor of ACD while sex was not.[17]
In our study, we find that more patients with PACG had history of hypertension (OR: 1.341 [95% CI: 1.111–1.842]) and cardiovascular disease (OR: 2.305 [95% CI: 1.692–3.142]), which was inconsistent with previous studies.[22,23,24,25,26,27,28,29,30] Gangwani et al. reported that patients with PACG (1.1%) were found to be the second most prevalent, followed by POAG (0.5%), which is similar to the results in our study.[30] Hypertension, diabetes mellitus, and cardiovascular disorders are the most common systemic diseases seen in glaucoma subjects.[31] The effect of blood pressure on glaucoma reminds a matter of debate. Some studies have indicated that hypertension is associated with glaucoma, especially POAG;[23,24,27,29,30] however, some found that hypertension was a protective factor, reducing risk of developing glaucoma.[22,25,26,28] Blood pressure influenced IOP, a well-known risk factor for glaucoma and ocular perfusion pressure (OPP), which regulates blood flow of the optic nerve. In short-term, high blood pressure increases OPP and protects against IOP-induced ischemia. Chronic hypertension impairs ocular blood flow and autoregulation, whose cumulative effect could lead to ganglion cell loss.[32] Therefore, for glaucoma patients who suffer from hypertension at the same time, it is important to avoid both overtreatment and undertreatment to control OPP in normal limits. The influence of blood pressure on glaucoma needs further investigation.
Many epidemiological studies have investigated the relationship between OAG and diabetes mellitus,[33,34,35,36,37,38] and some of them provide evidence for a positive association.[33,35,37,38] We find more patients with SG have history of diabetes mellitus (OR: 2.038 [95% CI: 1.508–2.754]) which has not been confirmed by other researches yet. The relationship between glaucoma and diabetes mellitus is complex because different types of glaucoma may be attributed to different pathogenic mechanisms. Diabetes mellitus causes sclerosis of trabecular meshwork, which impedes the outflow of aqueous humor and then leads to elevated IOP and OAG ensue. In addition, disturbance of blood circulation in diabetes patients reduces ocular perfusion and results in optic nerve damage, which leads to the occurrence of normal tension glaucoma (NTG). In addition, lens swells under high glucose condition, giving rise to closure of anterior chamber angle and elevated IOP. And eventually, this contributes to secondary ACG. Last but not least, uncontrolled proliferative diabetic retinopathy will finally cause neovascular glaucoma.
We find that patients with POAG are more likely to be male (74.31%) and have history of alcohol consumption (OR: 2.213 [95% CI: 1.018–4.811]) and smoking (OR: 2.362 [95% CI: 1.129–4.943]). Wise et al. found a positive association between alcohol consumption and POAG[38] whereas Fan et al. reported that alcohol consumption had a protective effect on POAG.[39] Others, however, failed to found that alcohol consumption was associated with glaucoma or POAG.[40,41,42,43,44] With regard to the relationship between smoking and POAG, previous studies found either null[43,45,46,47] or positive[28,39,40,42,44,48,49,50] correlation between them. The discrepancies may result from difference in race, sample size, and statistical method. Cigarette smoking can be a risk factor for POAG since it produces ischemia and oxidative stress, as well as increases the expression of inflammation (interleukin [IL]-6) and apoptosis markers (poly-ADP-ribose polymerase 1, caspase-3) in POAG patients. As a consequence, the progression of the disease accelerates. In Mainland China, the most majority of smokers and drinkers are men, which may, to some extent, explain the relatively high proportion of male patients suffering from POAG.
The most significant drawback of our study is that it was hospital-based rather than population-based. This may, to some extent, just reflect the demographic and clinical characteristics of glaucoma patients in a specific region, instead of the broader population. In addition, the subjects were enrolled retrospectively from the medical records of a regional hospital. Besides, we included the data from both eyes in the analysis; this may create a lot of bias by doubling the effect of a single patient. In addition, the researchers in our research team, the funds we got, and the time of conducting this study were all limited, so we did not choose identical control material without glaucoma when comparing demographic and clinical characteristics among patients from three glaucoma subgroups and when assessing the associations between glaucoma types and demographic and clinical variables. Other limitations of this study include the absence of more advance glaucoma diagnostic tools such as optical coherence tomography and the absence of mentioning NTG which has a high prevalence in South Asia. In future research, a population-based participant source should be considered to avoid a potential referral bias inherent to any hospital-based study. In addition, additional researches in Mainland China are needed to establish the associations between various risk factors and different types of glaucoma.
Conclusion
Our study showed that PACG and SG are the major types of glaucoma. Besides, an upward trend can be found in the average age of glaucoma patients as well as in the proportion of those from rural area. In addition, gender, cardiovascular disease, and hypertension seem to have a role in PACG, and POAG was associated with smoking and alcohol consumption. Moreover, there was positive correlation between SG and history of injury and diabetes mellitus.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–93. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook C, Foster P. Epidemiology of Glaucoma: What's new? Can J Ophthalmol. 2012;47:223–6. doi: 10.1016/j.jcjo.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren BC, He Y, Chen L, Yang JG, Sun NX. Epidemiology of glaucoma in a rural population in Shaanxi Province. In J Ophthalmol. 2005;5:1037–42. [Google Scholar]
- 5.Wang YX, Xu L, Yang H, Jonas JB. Prevalence of Glaucoma in North China: The Beijing Eye Study. Am J Ophthalmol. 2010;150:917–24. doi: 10.1016/j.ajo.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 6.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238–42. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen W, Juerti, Abudukader The analysis in proportion of hospitalized patients with glaucoma. Journal of Xinjiang Medical University. 2009;32:177–82. [Google Scholar]
- 8.Lü ZP, Chen XM. Proportion and its changes of hospitalized patients with glaucoma in West China Hospital. Int J Ophthalmol. 2011;11:1953–6. [Google Scholar]
- 9.Zhang YL, Wang CY, Peng AM. The characteristics of proportion in hospitalized patient with glaucoma. Chin J Pract Ophthalmol. 2011;29:284–6. [Google Scholar]
- 10.Cheng JW, Cheng SW, Ma XY, Cai JP, Li Y, Wei RL. The prevalence of primary glaucoma in mainland China: A systematic review and meta-analysis. J Glaucoma. 2013;22:301–6. doi: 10.1097/IJG.0b013e31824083ca. [DOI] [PubMed] [Google Scholar]
- 11.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology. 2014 doi: 10.1016/j.ophtha.2014.05.013. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Zhong H, Li J, Li C, Wei T, Cha X, Cai N, et al. The prevalence of glaucoma in adult rural Chinese populations of the Bai nationality in Dali: The Yunnan Minority Eye Study. Invest Ophthalmol Vis Sci. 2012;53:3221–5. doi: 10.1167/iovs.11-9306. [DOI] [PubMed] [Google Scholar]
- 13.Lee JW, Wong BK, Yick DW, Wong IY, Yuen CY, Lai JS. Primary acute angle closure: Long-term clinical outcomes over a 10-year period in the Chinese population. Int Ophthalmol. 2014;34:165–9. doi: 10.1007/s10792-013-9806-7. [DOI] [PubMed] [Google Scholar]
- 14.Lee JW, Lai JS, Yick DW, Tse RK. Retrospective case series on the long-term visual and intraocular pressure outcomes of phacomorphic glaucoma. Eye (Lond) 2010;24:1675–80. doi: 10.1038/eye.2010.108. [DOI] [PubMed] [Google Scholar]
- 15.He M, Foster PJ, Ge J, Huang W, Zheng Y, Friedman DS, et al. Prevalence and clinical characteristics of glaucoma in adult Chinese: A population-based study in Liwan District, Guangzhou. Invest Ophthalmol Vis Sci. 2006;47:2782–8. doi: 10.1167/iovs.06-0051. [DOI] [PubMed] [Google Scholar]
- 16.Liang Y, Friedman DS, Zhou Q, Yang XH, Sun LP, Guo L, et al. Prevalence and characteristics of primary angle-closure diseases in a rural adult Chinese population: the Handan Eye Study. Invest Ophthalmol Vis Sci. 2011;52:8672–9. doi: 10.1167/iovs.11-7480. [DOI] [PubMed] [Google Scholar]
- 17.Hsu WC, Shen EP, Hsieh YT. Is being female a risk factor for shallow anterior chamber?. The associations between anterior chamber depth and age, sex, and body height. Indian J Ophthalmol. 2014;62:446–9. doi: 10.4103/0301-4738.119344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He M, Huang W, Zheng Y, Alsbirk PH, Foster PJ. Anterior chamber depth in elderly Chinese: The Liwan eye study. Ophthalmology. 2008;115:1286–90. doi: 10.1016/j.ophtha.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Xu L, Cao WF, Wang YX, Chen CX, Jonas JB. Anterior chamber depth and chamber angle and their associations with ocular and general parameters: The Beijing Eye Study. Am J Ophthalmol. 2008;145:929–36. doi: 10.1016/j.ajo.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Garudadri C, Senthil S, Khanna RC, Sannapaneni K, Rao HB. Prevalence and risk factors for primary glaucomas in adult urban and rural populations in the Andhra Pradesh Eye Disease Study. Ophthalmology. 2010;117:1352–9. doi: 10.1016/j.ophtha.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Cheng JW, Zong Y, Zeng YY, Wei RL. The prevalence of primary angle closure glaucoma in adult Asians: A systematic review and meta-analysis. PLoS One. 2014;9:e103222. doi: 10.1371/journal.pone.0103222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leske MC, Wu SY, Nemesure B, Hennis A. Incident open-angle glaucoma and blood pressure. Arch Ophthalmol. 2002;120:954–959. doi: 10.1001/archopht.120.7.954. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell P, Lee AJ, Rochtchina E, Wang JJ. Open-angle glaucoma and systemic hypertension: The blue mountains eye study. J Glaucoma. 2004;13:319–26. doi: 10.1097/00061198-200408000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Hulsman CA, Vingerling JR, Hofman A, Witteman JC, de Jong PT. Blood pressure, arterial stiffness, and open-angle glaucoma: The Rotterdam study. Arch Ophthalmol. 2007;125:805–12. doi: 10.1001/archopht.125.6.805. [DOI] [PubMed] [Google Scholar]
- 25.Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–72. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B. BESs Study Group. Risk factors for incident open-angle glaucoma: The Barbados Eye Studies. Ophthalmology. 2008;115:85–93. doi: 10.1016/j.ophtha.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Costa VP, Arcieri ES, Harris A. Blood pressure and glaucoma. Br J Ophthalmol. 2009;93:1276–82. doi: 10.1136/bjo.2008.149047. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, Xu L, Jonas JB, Wong TY, Cui T, Li Y, et al. Major eye diseases and risk factors associated with systemic hypertension in an adult Chinese population: The Beijing Eye Study. Ophthalmology. 2009;116:2373–80. doi: 10.1016/j.ophtha.2009.05.041. [DOI] [PubMed] [Google Scholar]
- 29.Dave A, Bali SJ, Sharma R, Khurana AK, Gupta R, Dada T. Prevalence of diabetes mellitus and hypertension among Indian glaucoma patients and evaluation of systemic therapy. Int Ophthalmol. 2013;33:527–32. doi: 10.1007/s10792-013-9737-3. [DOI] [PubMed] [Google Scholar]
- 30.Gangwani RA, Chan J, Lee J, Kwong A, Lai JS. Detection of glaucoma in a cohort of chinese subjects with systemic hypertension. J Ophthalmol 2013. 2013:463710. doi: 10.1155/2013/463710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salim S, Shields MB. Glaucoma and systemic diseases. Surv Ophthalmol. 2009;55:64–77. doi: 10.1016/j.survophthal.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Zheng H, Algis JV, James AA, Bang VB. The role of blood pressure in glaucoma. Clin Exp Optom. 2011;92:133–49. doi: 10.1111/j.1444-0938.2010.00564.x. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura M, Kanamori A, Negi A. Diabetes mellitus as a risk factor for glaucomatous optic neuropathy. Ophthalmologica. 2005;219:1–10. doi: 10.1159/000081775. [DOI] [PubMed] [Google Scholar]
- 34.de Voogd S, Ikram MK, Wolfs RC, Jansonius NM, Witteman JC, Hofman A, et al. Is diabetes mellitus a risk factor for open-angle glaucoma?. The Rotterdam Study. Ophthalmology. 2006;113:1827–31. doi: 10.1016/j.ophtha.2006.03.063. [DOI] [PubMed] [Google Scholar]
- 35.Pasquale LR, Kang JH, Manson JE, Willett WC, Rosner BA, Hankinson SE. Prospective study of type 2 diabetes mellitus and risk of primary open-angle glaucoma in women. Ophthalmology. 2006;113:1081–6. doi: 10.1016/j.ophtha.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 36.Ozcura F, Aydin S. Is diabetes mellitus a risk factor or a protector for primary open angle glaucoma? Med Hypotheses. 2007;69:233–4. doi: 10.1016/j.mehy.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 37.Chopra V, Varma R, Francis BA, Wu J, Torres M, Azen SP, et al. Type 2 diabetes mellitus and the risk of open-angle glaucoma the Los Angeles Latino Eye Study. Ophthalmology. 2008;115:227–32. doi: 10.1016/j.ophtha.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wise LA, Rosenberg L, Radin RG, Mattox C, Yang EB, Palmer JR, et al. A prospective study of diabetes, lifestyle factors, and glaucoma among African-American women. Ann Epidemiol. 2011;21:430–9. doi: 10.1016/j.annepidem.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan BJ, Leung YF, Wang N, Lam SC, Liu Y, Tam OS, et al. Genetic and environmental risk factors for primary open-angle glaucoma. Chin Med J (Engl) 2004;117:706–10. [PubMed] [Google Scholar]
- 40.Kang JH, Willett WC, Rosner BA, Hankinson SE, Pasquale LR. Prospective study of alcohol consumption and the risk of primary open-angle glaucoma. Ophthalmic Epidemiol. 2007;14:141–7. doi: 10.1080/09286580601187963. [DOI] [PubMed] [Google Scholar]
- 41.Doshi V, Ying-Lai M, Azen SP, Varma R. Los Angeles Latino Eye Study Group. Sociodemographic, family history, and lifestyle risk factors for open-angle glaucoma and ocular hypertension. The Los Angeles Latino Eye Study. Ophthalmology. 2008;115:639–47. doi: 10.1016/j.ophtha.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 42.Kang JH, Wiggs JL, Rosner BA, Haines J, Abdrabou W, Pasquale LR. Endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: Interactions with hypertension, alcohol intake, and cigarette smoking. Arch Ophthalmol. 2011;129:773–80. doi: 10.1001/archophthalmol.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramdas WD, Wolfs RC, Hofman A, de Jong PT, Vingerling JR, Jansonius NM. Lifestyle and risk of developing open-angle glaucoma: the Rotterdam study. Arch Ophthalmol. 2011;129:767–72. doi: 10.1001/archophthalmol.2010.373. [DOI] [PubMed] [Google Scholar]
- 44.Chiotoroiu SM, Pop de Popa D, Ştefãniu GI, Secureanu FA, Purcãrea VL. The importance of alcohol abuse and smoking in the evolution of glaucoma disease. J Med Life. 2013;6:226–9. [PMC free article] [PubMed] [Google Scholar]
- 45.Kang JH, Pasquale LR, Rosner BA, Willett WC, Egan KM, Faberowski N, et al. Prospective study of cigarette smoking and the risk of primary open-angle glaucoma. Arch Ophthalmol. 2003;121:1762–8. doi: 10.1001/archopht.121.12.1762. [DOI] [PubMed] [Google Scholar]
- 46.Edwards R, Thornton J, Ajit R, Harrison RA, Kelly SP. Cigarette smoking and primary open angle glaucoma: a systematic review. J Glaucoma. 2008;17:558–66. doi: 10.1097/IJG.0b013e31815f530c. [DOI] [PubMed] [Google Scholar]
- 47.Wang D, Huang Y, Huang C, Wu P, Lin J, Zheng Y, et al. Association analysis of cigarette smoking with onset of primary open-angle glaucoma and glaucoma-related biometric parameters. BMC Ophthalmol. 2012;12:59. doi: 10.1186/1471-2415-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zanon-Moreno V, Garcia-Medina JJ, Zanon-Viguer V, Moreno-Nadal MA, Pinazo-Duran MD. Smoking, an additional risk factor in elder women with primary open-angle glaucoma. Mol Vis. 2009;15:2953–9. [PMC free article] [PubMed] [Google Scholar]
- 49.Graham SL, Butlin M, Lee M, Avolio AP. Central blood pressure, arterial waveform analysis, and vascular risk factors in glaucoma. J Glaucoma. 2013;22:98–103. doi: 10.1097/IJG.0b013e3182254bc0. [DOI] [PubMed] [Google Scholar]
- 50.Renard JP, Rouland JF, Bron A, Sellem E, Nordmann JP, Baudouin C, et al. Nutritional, lifestyle and environmental factors in ocular hypertension and primary open-angle glaucoma: An exploratory case-control study. Acta Ophthalmol. 2013;91:505–13. doi: 10.1111/j.1755-3768.2011.02356.x. [DOI] [PubMed] [Google Scholar]


