Abstract
Background:
To investigate visual and anatomical outcomes in eyes with exudative age-related macular degeneration treated with intravitreal aflibercept following prior treatment with intravitreal ranibizumab.
Materials and Methods:
Retrospective, single-center study of 192 eyes treated with 0.5 mg intravitreal ranibizumab every 4 weeks for three consecutive doses followed by a variable dose schedule. After more than 12 months of ranibizumab treatment, eyes that required ranibizumab injections at 4-week or 6-week intervals were switched to aflibercept therapy.
Results:
After 12–69 months (42 months ± 18 months, mean ± standard deviation [SD]) of treatment with intravitreal ranibizumab, 80 eyes were changed to 2 mg intravitreal aflibercept treatment with follow-up after 12–18 months (16 months ± 1 month, mean ± SD). Thirty-nine eyes had persistent macular fluid after treatment with ranibizumab. Mean logMAR visual acuity (VA) in eyes treated with ranibizumab changed by − 0.089 ± 0.310 (mean ± SD; P = 0.0003), which correlates to an approximate gain of 4.5 letters. The number of eyes with macular fluid decreased from 39 to 23 after aflibercept treatment. Mean logMAR VA in eyes with intraretinal macular fluid treated with aflibercept changed by −0.079 ± 0.134 (mean ± SD; P = 0.006), which correlates to an approximate gain of 4 letters. Mean logMAR VA in eyes with submacular fluid was not significantly different after aflibercept treatment.
Conclusion:
Eyes with persistent intraretinal macular fluid had visual and anatomic response after changing from ranibizumab to aflibercept treatment.
Keywords: Aflibercept, age-related macular degeneration, choroidal neovascularization, intraretinal fluid, ranibizumab, subretinal fluid
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness among elderly patients in the developed world.[1,2] Neovascular AMD is characterized by choroidal neovascularization (CNV), the growth of abnormal vessels in the macular region, with subsequent bleeding or fluid leakage that may result in a sudden loss of central vision. Vascular endothelial growth factors (VEGFs) drive the growth of abnormal vessels in CNV.[3,4,5,6,7]
Intravitreal administration of anti-VEGF agents has replaced photodynamic therapy as first-line treatment in CNV.[8] Ranibizumab (Lucentis; Genentech Inc., South San Francisco, California, USA) is a recombinant, humanized, monoclonal antibody antigen-binding fragment that neutralizes VEGF-A. Randomized controlled trials have demonstrated the efficacy and safety of intravitreal ranibizumab for stabilizing and improving visual acuity (VA) in CNV.[9,10,11,12,13] Some patients who have a good initial response to ranibizumab with resolution of fluid, later become resistant to further treatment and develop recurrent exudation with vision loss.[14,15]
Aflibercept (Eylea, Regeneron, Tarrytown, New York, USA) is a fusion protein that incorporates the second binding domain of the VEGF receptor 1 (VEGFR-1) and the third domain of the VEGFR-2.[16] Mathematical modeling and pharmacokinetic studies have predicted that aflibercept has a stronger binding affinity for VEGF-A than ranibizumab and also has higher trough binding levels. Therefore, aflibercept should be able to neutralize VEGF more effectively and longer than ranibizumab.[17,18,19,20]
The comparative efficacies of aflibercept and ranibizumab were investigated in the VEGF trap: Investigation of efficacy and safety in wet AMD (VIEW) 1 and VIEW 2 trials. Bimonthly injections of 2 mg aflibercept were noninferior to 0.5 mg monthly ranibizumab (VIEW 1: +7.9 letters vs. +8.1 letters; VIEW 2: +8.9 letters vs. +9.4 letters; P > 0.05 for both).[21]
Recent studies have reported the outcomes of aflibercept in eyes that received prior anti-VEGF treatment.[22,23,24,25] The current study evaluates the visual and anatomical outcomes in eyes with exudative AMD treated with intravitreal aflibercept after receiving prior treatment with ranibizumab.
Materials and Methods
A retrospective observational case series was performed to study the outcome in eyes with neovascular AMD treated with intravitreal aflibercept following prior treatment with intravitreal ranibizumab. All work was conducted in accordance with the declaration of Helsinki. Patients with CNV secondary to neovascular AMD were treated with 0.5 mg intravitreal ranibizumab in one or both eyes at a retinal practice in Adelaide, South Australia. The diagnosis of AMD was based on clinical findings and confirmed using fluorescein angiography. Patients were excluded if they received prior verteporfin photodynamic therapy. The following data were collected from patient records: Age at presentation, gender, treated eye, best corrected VA measured as the number of letters on a Snellen chart and converted to a logMAR score, and retinal examination findings.
All eyes were treated with a fixed regimen of three 0.5 mg intravitreal ranibizumab injections given at 4-week intervals and were given a follow-up appointment 6 weeks after the third ranibizumab injection. Retreatment was offered in the presence of persistent intraretinal and/or submacular fluid. Eyes that required retreatment were given another course of three injections at 4-week intervals followed by an appointment 6 weeks after the third injection. Following the second course of three ranibizumab injections, these eyes received maintenance injections at 4-week, 6-week, 8-week, 10-week, or 12-week intervals depending on the time to recurrence from the last assessment that showed no signs of active CNV.
Eyes that did not require retreatment at the first follow-up appointment after the initial course of treatment were offered another appointment after 8 weeks. If there was no active CNV at this appointment, another appointment was scheduled after 10 weeks. If there was no active CNV at this appointment, a follow-up examination was scheduled after 12 weeks. Appointments were not spaced out further than 12 weeks. If eyes developed active CNV at any of the follow-up appointments, they were given a second course of three injections at 4-week intervals and maintenance injections thereafter.
After more than 12 months of ranibizumab treatment, eyes that required ranibizumab injections at 4-week or 6-week intervals were changed to aflibercept therapy. These eyes either had persistent macular fluid and were being treated at 4-week intervals or required 4-week or 6-week injection intervals to maintain a fluid-free macula. Eyes were injected with 2 mg intravitreal aflibercept at the same intervals as their ranibizumab injections. Injections were extended to 6-week then 8-week intervals if there were no signs of active CNV. Patients were continued on aflibercept for at least 12 months. All patients were asked to phone in for an appointment immediately if they experienced any visual loss, worsening of metamorphopsia, or sudden changes on Amsler grid testing.
Statistical analysis
Two-tailed paired Student's t-tests were performed to analyze visual outcomes. A Bonferroni correction was used to account for multiple comparisons. Using α =0.05, the adjusted P value was determined to be 0.007.
Results
The study included 192 patients comprising 81 men (42.2%) and 111 women (57.8%). One hundred and two right eyes and 90 left eyes received treatment. Patient ages ranged from 63 to 100 years with a mean age of 83.2 ± 7 (mean ± standard deviation [SD]). Thirty-two eyes (16.7%) experienced resolution of exudation after three initial ranibizumab injections and were observed long-term. The remaining 160 eyes (83.3%) required maintenance injections. Duration of treatment with ranibizumab before changing to aflibercept ranged from 12 months to 69 months (42 months ± 18 months, mean ± SD). Duration of treatment with aflibercept ranged from 12 months to 18 months (16 months ± 1 month, mean ± SD).
Ranibizumab treatment
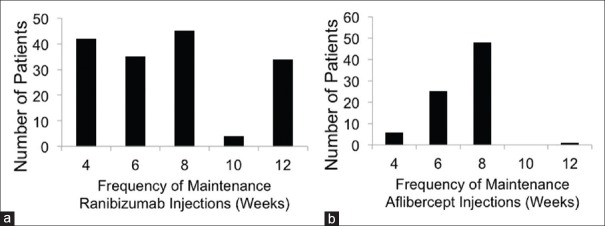
Of the 160 eyes that required maintenance injections, 30 eyes (15.6%) required retreatment 6 weeks after the initial three injections. All 160 eyes that required retreatment were managed using a maintenance regimen of injections given at 4-week, 6-week, 8-week, 10-week, or 12-week intervals. Forty-two eyes (26.3%) were maintained on 4-week injections, 35 eyes (21.9%) were maintained on 6-week injections, 45 eyes (28.1%) were maintained on 8-week injections, 4 eyes (2.5%) were maintained on 10-week injections, and 34 eyes (21.2%) were maintained on 12-week injections [Fig. 1a]. One hundred and twenty-one eyes had a fluid-free macula after 12–69 months of ranibizumab treatment; 39 eyes had persistent macular fluid. The mean interval between maintenance injections was 7.4 weeks and the median interval was 8 weeks.
Figure 1.
Eyes treated with ranibizumab (a) and eyes treated with aflibercept after changing from ranibizumab treatment (b) are categorized according to the frequency of maintenance injections
Aflibercept treatment
After more than 12 months of ranibizumab treatment, eyes receiving maintenance injections at 4-week and 6-week intervals were changed to aflibercept therapy. In addition, three patients receiving 8-week maintenance injections chose to change to aflibercept therapy. A total of 80 eyes were treated using aflibercept. This included 39 eyes that had persistent macular fluid after 12–69 months of ranibizumab treatment. Forty-eight eyes (60%) were managed using 8-week injections, 25 eyes (31%) were managed using 6-week injections, and 6 eyes (7.5%) were managed using 4-week injections [Fig. 1b]. One patient, who was initially treated using 4-week ranibizumab injections, was extended to 12-week aflibercept injections and experienced improved VA in the treated eye. Vision improved from logMAR 0.62 at the start of aflibercept therapy to logMAR 0.508 after 12 months of aflibercept therapy. This correlated to an approximate gain of 5.5 letters.
Before starting aflibercept therapy, 41 out of 80 eyes had a fluid-free macula, 26 out of 80 eyes had intraretinal macular fluid, 9 out of 80 eyes had submacular fluid, 2 out of 80 eyes had both intraretinal and submacular fluid, one eye had a retinal pigment epithelial detachment (PED), and one eye had a PED as well as submacular fluid. After 12–18 months of aflibercept therapy, the number of eyes with a fluid-free macula increased from 41 to 57, the number of eyes with intraretinal macular fluid decreased from 26 to 12, and the number of eyes with submacular fluid decreased from 9 to 8 [Table 1].
Table 1.
Distribution of macular fluid in eyes treated with aflibercept
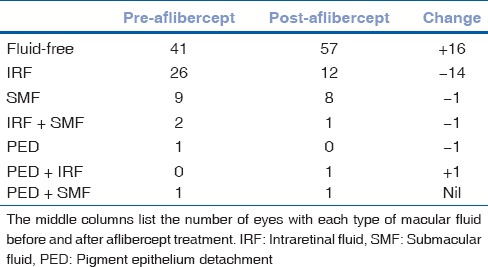
Thirty-eight out of 41 eyes maintained a fluid-free macula after aflibercept treatment. Two out of 41 eyes that had a fluid-free macula before aflibercept therapy developed intraretinal macular fluid and one eye developed submacular fluid [Table 2]. One out of 2 eyes had persistent intraretinal and submacular fluid after aflibercept therapy. One out of 2 eyes had resolution of intraretinal macular fluid with persistence of submacular fluid after aflibercept treatment. The eye with submacular fluid and PED before aflibercept therapy had stable submacular fluid and PED after aflibercept treatment. The eye with only PED before aflibercept therapy had PED with intraretinal macular fluid after aflibercept treatment.
Table 2.
Distribution of macular fluid in eyes treated with aflibercept
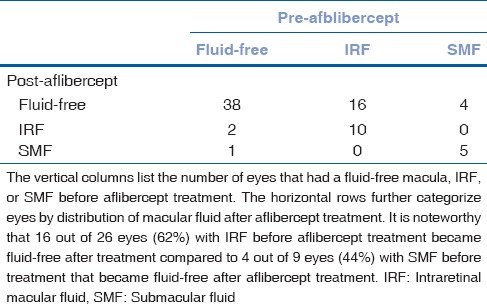
Five out of 9 eyes had persistent submacular fluid after aflibercept treatment. Four out of 9 eyes had resolution of submacular fluid with a fluid-free macula [Table 2]. Ten out of 26 eyes had persistent intraretinal macular fluid after aflibercept therapy. Sixteen out of 26 eyes had resolution of intraretinal macular fluid with a fluid-free macula after aflibercept treatment [Table 2]. There were no new PEDs during the 12–18 months of aflibercept treatment.
Visual outcome
Visual outcomes are reported in Table 3. Mean logMAR VA in 192 eyes before ranibizumab therapy was 0.741 ± 0.416 (SD). Mean logMAR VA after treatment with ranibizumab was 0.652 ± 0.430 (SD). Mean change in logMAR VA was − 0.089 ± 0.310 (SD). This correlates to an approximate gain of 4.5 letters. VA changes from baseline were found to be significantly different after eyes were treated with ranibizumab (P = 0.0003). Mean logMAR VA in 80 eyes before aflibercept therapy was 0.642 ± 0.318 (SD). Mean logMAR VA after aflibercept therapy was 0.615 ± 0.305 (SD). Mean change in logMAR VA was − 0.027 ± 0.144 (SD). VA changes from baseline were not found to be significantly different after treatment with aflibercept (P = 0.09).
Table 3.
Mean logMAR best corrected VA in eyes treated with ranibizumab and eyes treated with aflibercept
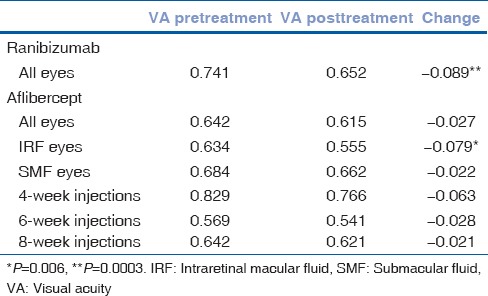
Mean logMAR VA in eyes with intraretinal macular fluid treated with aflibercept was 0.634 ± 0.301 (SD) before treatment and 0.555 ± 0.304 (SD) after treatment. Mean change in logMAR VA was − 0.079 ± 0.134 (SD). This correlates to an approximate gain of four letters. VA changes from baseline were significantly different in this group (P = 0.006). Mean logMAR VA in eyes with submacular fluid treated with aflibercept was 0.684 ± 0.301 (SD) before treatment and 0.662 ± 0.370 (SD) after treatment. VA changes from baseline were not significantly different in this group (P = 0.37).
Discussion
Twenty per cent of eyes treated with intravitreal ranibizumab had persistent fluid after 12–69 months of treatment. This is consistent with clinical trials that have identified a group of patients who have a good initial response to ranibizumab with resolution of fluid that later become resistant to further treatment and develop recurrent exudation with vision loss.[13,20]
We found anatomical improvement after 12–18 months of aflibercept injections in eyes that were previously treated with ranibizumab. The number of fluid-free eyes increased by 39% (from 41 to 57) after aflibercept treatment. There are several possible explanations for the observed anatomical benefit after changing to aflibercept, including its pharmacodynamics and the possibility of tachyphylaxis to prior treatment with ranibizumab.
Aflibercept binds all isoforms of VEGF-A and VEGF-B, as well as placental growth factor, with a significantly higher binding affinity for VEGF than either ranibizumab or bevacizumab.[16] Mathematical models predict that a single intravitreal injection of 2 mg aflibercept would last between 48 and 83 days (compared with 30 days for ranibizumab) and should be efficacious in neutralizing VEGF longer and more effectively.[17,20] It is possible that greater VEGF blockage might be helpful for a recalcitrant subset of exudative AMD eyes, as supported by the super-dose anti-VEGF trial.[26]
Previous retrospective studies of patients treated with aflibercept after changing from ranibizumab have not observed long-term visual gains.[27,28] We found eyes treated for 12–69 months with ranibizumab experienced significant VA improvement equal to 4.5 letters (P = 0.0003). In addition, eyes that had intraretinal macular fluid experienced VA improvement equal to four letters after aflibercept treatment (P = 0.006).
In an analysis of data from the Comparison of Age-related Macular Degeneration Treatment (CATT) trial, eyes treated with ranibizumab that had intraretinal macular fluid had worse VA than eyes with submacular fluid.[29] We found patients with persistent intraretinal macular fluid after ranibizumab treatment had better anatomical outcomes after 12–18 months of aflibercept therapy compared to patients with persistent submacular fluid. Sixty-two percent of eyes with intraretinal macular fluid became fluid-free after aflibercept treatment compared to 44% of eyes with submacular fluid. As noted above, this improved anatomical outcome was also associated with significant improvement in VA. A recent study by Golbaz et al.[30] reported that intravitreal ranibizumab has greater therapeutic effect on submacular fluid. Our results suggest submacular fluid, particularly when located in the fovea, might be more susceptible to ranibizumab treatment. Conversely, intraretinal macular fluid might be more susceptible to aflibercept treatment.
The proposed mechanism of relative intraretinal macular fluid responsiveness and submacular fluid refractoriness to aflibercept may be due to the molecular size of the aflibercept molecule. Aflibercept is a large molecule (115 kDa) compared to ranibizumab (48 kDa). Ranibizumab diffuses across the retina more easily after intravitreal injection into the subretinal space and reaching the choriocapillaris.[31] Due to the large size of the aflibercept molecule, subretinal penetration after intravitreal injections is considerably reduced, which might explain the relative poor effect on submacular fluid.
Limitations of our study include the small cohort, its uncontrolled retrospective design, use of Snellen VA, and nonstandard treatment protocols. Further, fluorescein angiography was not always performed prior to change to aflibercept. The strength of the present study is the longer-term follow-up compared with similar retrospective studies. Future prospective studies could evaluate anatomical and visual outcomes of intravitreal aflibercept on different CNV subtypes including intraretinal and submacular fluid.
Conclusion
In summary, a significant proportion of eyes with exudative AMD that required 4-week or 6-week ranibizumab injections experienced improved anatomical outcomes and after 12–18 months of treatment with aflibercept. Patients with intraretinal macular fluid responded better than patients with submacular fluid after changing from ranibizumab to aflibercept treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Boyer DS, Antoszyk AN, Awh CC, Bhisitkul RB, Shapiro H, Acharya NR. MARINA Study Group. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114:246–52. doi: 10.1016/j.ophtha.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 2.Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004;291:1900–1. doi: 10.1001/jama.291.15.1900. [DOI] [PubMed] [Google Scholar]
- 3.Frank RN, Amin RH, Eliott D, Puklin JE, Abrams GW. Basic fibroblast growth factor and vascular endothelial growth factor are present in epiretinal and choroidal neovascular membranes. Am J Ophthalmol. 1996;122:393–403. doi: 10.1016/s0002-9394(14)72066-5. [DOI] [PubMed] [Google Scholar]
- 4.Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, de Jong PT. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997;81:154–62. doi: 10.1136/bjo.81.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kvanta A, Algvere PV, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996;37:1929–34. [PubMed] [Google Scholar]
- 6.Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1996;37:855–68. [PubMed] [Google Scholar]
- 7.Rakic JM, Lambert V, Devy L, Luttun A, Carmeliet P, Claes C, et al. Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3186–93. doi: 10.1167/iovs.02-1092. [DOI] [PubMed] [Google Scholar]
- 8.Ip MS, Scott IU, Brown GC, Brown MM, Ho AC, Huang SS, et al. Anti-vascular endothelial growth factor pharmacotherapy for age-related macular degeneration: A report by the American Academy of Ophthalmology. Ophthalmology. 2008;115:1837–46. doi: 10.1016/j.ophtha.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 10.Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;5(355):1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 11.Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145:239–248. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Abraham P, Yue H, Wilson L. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 2. Am J Ophthalmol. 2010;150:315–324. doi: 10.1016/j.ajo.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 13.CATT Research Group. Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keane PA, Liakopoulos S, Ongchin SC, Heussen FM, Msutta S, Chang KT, et al. Quantitative subanalysis of optical coherence tomography after treatment with ranibizumab for neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:3115–20. doi: 10.1167/iovs.08-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forooghian F, Cukras C, Meyerle CB, Chew EY, Wong WT. Tachyphylaxis after intravitreal bevacizumab for exudative age-related macular degeneration. Retina. 2009;29:723–31. doi: 10.1097/IAE.0b013e3181a2c1c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, et al. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99:11393–8. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart MW, Rosenfeld PJ, Penha FM, Wang F, Yehoshua Z, Bueno-Lopez E, et al. Pharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab, and aflibercept (vascular endothelial growth factor Trap-eye) Retina. 2012;32:434–57. doi: 10.1097/IAE.0B013E31822C290F. [DOI] [PubMed] [Google Scholar]
- 18.Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15:171–85. doi: 10.1007/s10456-011-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Browning DJ, Kaiser PK, Rosenfeld PJ, Stewart MW. Aflibercept for age-related macular degeneration: A game-changer or quiet addition? Am J Ophthalmol. 2012;154:222–6. doi: 10.1016/j.ajo.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Stewart MW, Rosenfeld PJ. Predicted biological activity of intravitreal VEGF Trap. Br J Ophthalmol. 2008;92:667–8. doi: 10.1136/bjo.2007.134874. [DOI] [PubMed] [Google Scholar]
- 21.Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Pinheiro-Costa J, Costa JM, Beato JN, Freitas-da-Costa P, Brandão E, Falcão MS, et al. Switch to aflibercept in the treatment of neovascular AMD: One-year results in clinical practice. Ophthalmologica. 2015;233:155–61. doi: 10.1159/000381221. [DOI] [PubMed] [Google Scholar]
- 23.Kanesa-Thasan A, Grewal DS, Gill MK, Lyon AT, Mirza RG. Quantification of change in pigment epithelial detachment volume and morphology after transition to intravitreal aflibercept in eyes with recalcitrant neovascular AMD: 18-Month Results. Ophthalmic Surg Lasers Imaging Retina. 2015;46:638–41. doi: 10.3928/23258160-20150610-07. [DOI] [PubMed] [Google Scholar]
- 24.Gerding H. Functional and anatomic efficacy of a conversion to aflibercept in eyes with age-related macular degeneration after long-term ranibizumab treatment. Klin Monbl Augenheilkd. 2015;232:560–3. doi: 10.1055/s-0035-1545775. [DOI] [PubMed] [Google Scholar]
- 25.Moon da RC, Lee DK, Kim SH, You YS, Kwon OW. Aflibercept treatment for neovascular age-related macular degeneration and polypoidal choroidal vasculopathy refractory to anti-vascular endothelial growth factor. Korean J Ophthalmol. 2015;29:226–32. doi: 10.3341/kjo.2015.29.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown DM, Chen E, Mariani A, Major JC., Jr SAVE Study Group. Super-dose anti-VEGF (SAVE) trial: 2.0 mg intravitreal ranibizumab for recalcitrant neovascular macular degeneration-primary end point. Ophthalmology. 2013;120:349–54. doi: 10.1016/j.ophtha.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Cho H, Shah CP, Weber M, Heier JS. Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol. 2013;97:1032–5. doi: 10.1136/bjophthalmol-2013-303344. [DOI] [PubMed] [Google Scholar]
- 28.Bakall B, Folk JC, Boldt HC, Sohn EH, Stone EM, Russell SR, et al. Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol. 2013;156:15–22. doi: 10.1016/j.ajo.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Jaffe GJ, Martin DF, Toth CA, Daniel E, Maguire MG, Ying GS, et al. Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2013;120:1860–70. doi: 10.1016/j.ophtha.2013.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golbaz I, Ahlers C, Stock G, Schütze C, Schriefl S, Schlanitz F, et al. Quantification of the therapeutic response of intraretinal, subretinal, and subpigment epithelial compartments in exudative AMD during anti-VEGF therapy. Invest Ophthalmol Vis Sci. 2011;52:1599–605. doi: 10.1167/iovs.09-5018. [DOI] [PubMed] [Google Scholar]
- 31.Gaudreault J, Fei D, Beyer JC, Ryan A, Rangell L, Shiu V, et al. Pharmacokinetics and retinal distribution of ranibizumab, a humanized antibody fragment directed against VEGF-A, following intravitreal administration in rabbits. Retina. 2007;27:1260–6. doi: 10.1097/IAE.0b013e318134eecd. [DOI] [PubMed] [Google Scholar]