Abstract
Thyroid eye disease (TED) can affect the eye in myriad ways: proptosis, strabismus, eyelid retraction, optic neuropathy, soft tissue changes around the eye and an unstable ocular surface. TED consists of two phases: active, and inactive. The active phase of TED is limited to a period of 12–18 months and is mainly managed medically with immunosuppression. The residual structural changes due to the resultant fibrosis are usually addressed with surgery, the mainstay of which is orbital decompression. These surgeries are performed during the inactive phase. The surgical rehabilitation of TED has evolved over the years: not only the surgical techniques, but also the concepts, and the surgical tools available. The indications for decompression surgery have also expanded in the recent past. This article discusses the technological and conceptual advances of minimally invasive surgery for TED that decrease complications and speed up recovery. Current surgical techniques offer predictable, consistent results with better esthetics.
Keywords: Exophthalmos, Grave's orbitopathy, optic neuropathy, orbit, orbital decompression, orbital fat, proptosis, thyroid eye disease
Graves’ disease (GD) is a systemic autoimmune disease that targets the thyroid gland, orbit and the skin. Despite advances in the treatment of endocrinologic dysfunction associated with GD, few therapies are available for the thyroid eye disease (TED). Recent progress has identified several molecular mediators and molecular interactions responsible for this process.[1] A worldwide effort to understand this enigmatic disease marks the establishment of International TED Study group.[2] We look forward to an era where we can understand the molecular basis for GD, treat it medically and avoid or reverse the sequelae that require surgical correction.
However, until then, surgery will remain an important tool in the management of patients who are unfortunate to get these mechanical and fibrotic sequelae. The surgical rehabilitation of TED has continued to evolve, restoring appearance and function to the patients. This article highlights the technological and conceptual advances that have allowed us to perform minimally invasive surgery for TED, reducing complications, and speed up recovery. The authors acknowledge that incisions, as well as surgical choices for orbital decompression vary based on personal preference and training, and this article represents our preferred approach to TED.
The Four Stages of Surgical Rehabilitation
There are four components of TED that require attention from the surgical point of view: proptosis, restrictive strabismus, eyelid abnormality (retraction), and cosmetic concerns (fat bags, rhytids, etc.). Based on these four components, the surgical management of TED involves four major stages of rehabilitation: orbital decompression, extraocular muscle surgery, eyelid repositioning, and cosmetic soft tissue redraping.[3] Not all patients require all four stages, but one may require more than one stage of surgery.
Proptosis is one of the most visible and obvious side-effects of TED that demands treatment. Traditionally, orbital decompression was performed only for extreme proptosis, or compressive optic neuropathy. There has been a gradual evolution in the indications for orbital decompression.[4] It is increasingly common and accepted to perform orbital decompression not only for extreme vision-threatening proptosis, but also cosmetically disfiguring proptosis.[5,6] Though optic neuropathy responds well to surgery, the nerve is resilient, and minor degrees of compressive optic neuropathy are probably not as emergent as we once thought.[7,8] Proptosis demands treatment not only from a cosmetic standpoint, but also to reduce the symptoms of exposure, and in rare cases, avoid the emergent complication of globe luxation. Earlier, decompression surgery was performed through large incisions and more invasive procedures, with resultant complications such as sinusitis, inferomedial globe displacement, and scarring. Today, advancements in orbital decompression allow new areas of bone removal, an awareness of orbital fat as the “first wall” for decompression, and the use of smaller incisions.[4]
The second stage constitutes the correction of diplopia, which is the most disabling side-effect of TED, and the most challenging to treat. The current day decompression surgeries focus on decreasing the rate of “new-onset” strabismus.[9,10]
Correction of eyelid abnormality constitutes the third stage, and most commonly includes correction of upper and lower eyelid retraction. There are several surgical options described for the correction of eyelid retraction.[11] The fourth stage of cosmetic concerns includes eyelid fat bags, periorbital hollows, and rhytids such as glabellar folds.[4] These are best treated toward the end of the surgical rehabilitation, addressing the esthetic concerns and restoring the normal appearance.
Timing of Surgical Correction
Surgical rehabilitation is carried out in the “inactive” phase of TED. Hence, differentiating active from inactive disease is important in TED. The congestive orbitopathy (inactive, but red eye due to vascular congestion) further adds to the confusion, and can attract unnecessary administration of steroids. It is a common myth that control of systemic thyroid abnormality (normally done by the endocrinologist) would reverse the ocular changes in TED. The systemic thyroid abnormality and TED can be considered as two separate but co-existing entities. Established sequelae of TED rarely reverse, and almost always require surgical rehabilitation by an ophthalmic plastic surgeon to restore normalcy. Normal thyroid levels are a prerequisite for surgical fitness, as general anesthesia is essential while performing orbital decompression.
Imaging
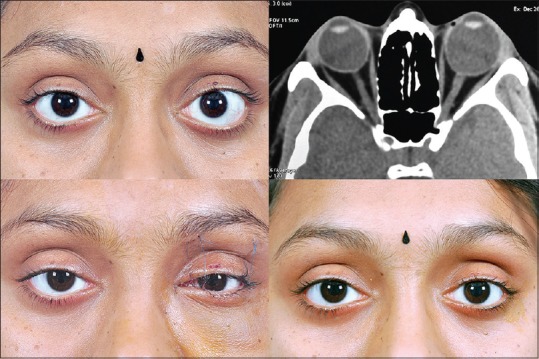
An essential step in the surgical planning of a decompression surgery is a thorough imaging study of the orbit. Either modality: Magnetic resonance imaging or computed tomography (CT) may be done. We prefer a CT scan of the orbit with coronal and axial sections at 2 mm intervals. A CT scan allows us to visualize all the paranasal sinuses, especially the ethmoidal and maxillary sinuses. It helps differentiate between a fat-predominant disease and a muscle predominant disease. This is crucial in surgical planning in terms of surgical steps as well as prognostication in terms of final proptosis reduction.[12] Additional information obtained from CT scan includes the level of cribriform plate, the thickness of orbital floor, and also the presence of infraorbital canal, all having a bearing on the complexity of the surgical technique.
The first stage: Orbital decompression
The principle of orbital decompression surgery is to expand the orbital space by widening the bony orbit and removal of excessive orbital fat. This decreases the venous congestion and mechanical pressure on the optic nerve, and also reduces proptosis. Theoretically, there are four bony orbital walls available for decompression: Medial, inferior, lateral, and superior wall. Historically, the inferior and medial walls were removed by the otorhinolaryngologists; and the deep lateral wall by the neurosurgeons. The transantral approach created unbalanced inferomedial decompression with a high incidence of consecutive strabismus, infraorbital anesthesia, and sinusitis.[6,13] The fourth wall, orbital roof decompression was initially advocated by Naffziger, but is fraught with potentially serious complications, and hence is best avoided except in extreme cases.[14]
Today, all three walls can be reached via hidden incisions and minimally invasive orbital approach by an ophthalmic plastic surgeon.[4,15] Typically, decompression begins with orbital fat removal, and if required removal of the bony wall is added as the deep lateral wall, medial wall, and floor in that order [Fig. 1]. The preference of the available walls for decompression of progressively severe disease may vary from surgeon to surgeon based on the training, access to specialized instruments, and specific factors related to an ethnic group. As a thumb-rule however, each orbital wall would provide approximately 2 mm reduction in proptosis, and fat alone would provide 2 mm reduction.
Figure 1.
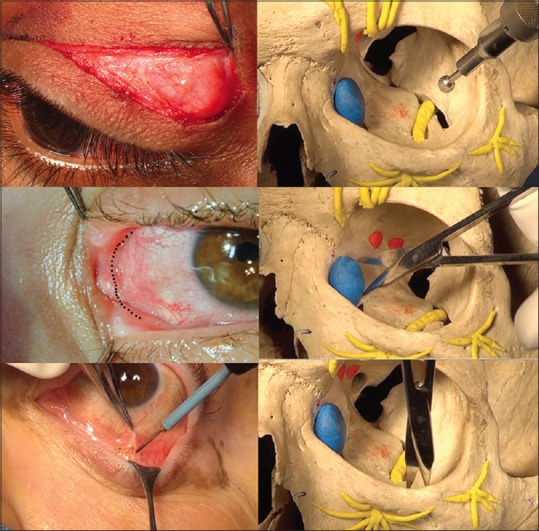
Minimally invasive surgical approaches for orbital decompression. Eyelid crease incision allows access to the deep lateral wall (top left and right). Transcaruncular incision bypasses the lacrimal apparatus and allows medial wall decompression (middle left and right). Inferior trans-conjunctival approach allows quick access to the orbital floor (bottom left and right)
Fat decompression: The conceptual “First Wall”
Removing orbital fat is a major technical and conceptual advance in orbital decompression. Initially, there was concern that cutting into the intraconal fat could worsen strabismus. However, it has been found actually to improve double vision, reduce congestive orbitopathy, proptosis, and even compressive optic neuropathy.[16] For many surgeons, removal of intraconal fat is the “first wall” of orbital decompression for mild (2–3mm) proptosis [Fig. 2]. Orbital fat, especially in patients of TED, is very vascular and after excision, the cut ends of small bleeders may retract. Therefore, hemostasis is essential, and fat removal should be meticulous and only under direct visualization.
Figure 2.
Mild right eye proptosis (2–3 mm) managed by trans-conjunctival fat decompression, also referred to as the “first wall” in orbital decompression
Lateral wall decompression
Recently, the lateral wall decompression has emerged as a primary procedure for moderate proptosis.[17] The deep lateral wall (sphenoid bone) can be accessed through either a coronal incision or orbital approach through an eyelid crease, or swinging eyelid incision with substantially less morbidity compared to an open craniotomy [Fig. 1].[15] Removing bone from the deep lateral wall causes less consecutive strabismus, eliminates the risk of sinusitis (as with floor or medial wall decompression), effects faster resolution of postoperative strabismus and is especially useful for woody/fibrotic orbits.[17,18] Deep lateral wall decompression can be carried superiorly to include fossa for lacrimal gland and inferiorly up to the inferior orbital fissure (the “basin” of inferior orbital fissure).[17]
Medial wall decompression
The medial wall can be accessed through a hidden transcaruncular incision, a medial skin incision (Lynch), or via endonasal approach using an endoscope.[19,20] With the transcaruncular route, the access is quick, and adequate bony decompression can be achieved without complex instrumentation like an endoscope. A simple Kerrison's bone punch is adequate to achieve medial wall decompression [Fig. 2]. Therefore, for cases that require 2-4 mm correction of proptosis, the medial wall can be chosen, with or without fat decompression.
Orbital floor decompression
The orbital floor can be accessed through a hidden inferior trans-conjunctival incision, or a sub-ciliary skin incision, or trans-antral (Caldwell-Luc) approach using an endoscope. If done through trans-conjunctival incision it can be continued with transcaruncular incision medially for combined medial wall decompression or continued as swinging eyelid incision laterally for combined lateral wall decompression. Floor decompression can be achieved easily without specialized instruments [Fig. 1], but runs the risk of infra-orbital anesthesia, and hypoglobus.[13] Floor decompression if planned, should be done in a manner that preserves the infraorbital nerve to avoid anesthesia. This can be achieved using the Piezoelectric technology to avoid soft tissue damage.[21]
Three wall decompression
A three-wall decompression is usually chosen for severe grades of proptosis (exophthalmometry >26 mm), and all three walls: Lateral, medial, and floor are addressed along with intraconal fat removal [Fig. 3].
Figure 3.
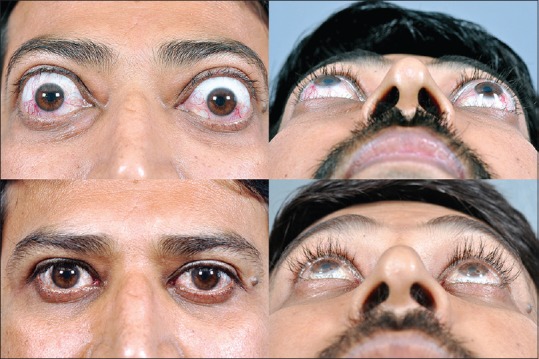
Severe bilateral proptosis managed with minimally invasive three wall decompression through hidden incisions. Note the reduction in proptosis, eyelid retraction, and also periocular soft tissue fullness
“Balanced” decompression
For moderate to severe proptosis, a balanced decompression of the medial and lateral orbital wall can reduce the occurrence of postoperative strabismus.[18,22] A balanced decompression is proposed to be equally effective as a three wall decompression.[23]
A balanced decompression is thought to prevent inferomedial displacement of the globe and produce an equal prolapse of the medial and lateral rectus muscles into the surrounding space. In performing balanced two wall decompressions, transcaruncular, or endoscopic approaches to the medial wall may be used in combination with a lateral decompression.
“Unbalanced” decompression
For moderate to severe proptosis, a decompression could be referred to as “unbalanced” if the medial wall and orbital floor are addressed. The chances of new-onset diplopia and globe dystopia are significant with the trans-antral approach, where the floor, as well as medial wall, are entirely removed. However, preservation of the inferomedial orbital strut can prevent most of the complications associated with an unbalanced orbital decompression, whether performed trans-conjunctivally or endonasally.[24,25] In addition, unbalanced decompression can be done without the need for any additional instrumentation like the mechanical drill.
Extended indications of orbital decompression
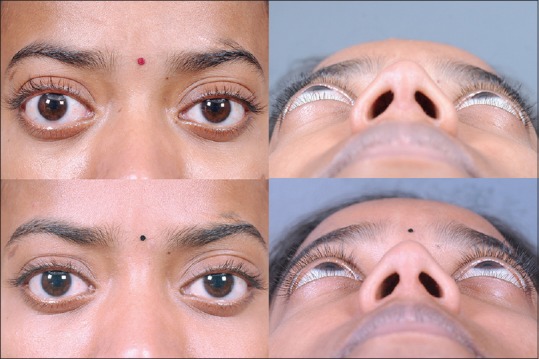
Apart from TED, orbital decompression can also be performed for pseudoproptosis due to unilateral axial myopia [Fig. 4], malar hypoplasia with the scleral show and in the case of shallow orbits such as Crouzon's disease [Fig. 5].[26] The benefit of proptosis reduction is not much in craniosynostosis, but nevertheless it can provide protection from exposure keratopathy and globe luxation that are common occurrences in these patients.
Figure 4.
Left pseudoproptosis with inferior scleral show (top left) due to unilateral axial myopia (top right). Fat decompression, lower eyelid retractor release with fat-pearl grafting and postoperative lower eyelid traction suture (bottom left) leads to a good cosmetic outcome (bottom right)
Figure 5.
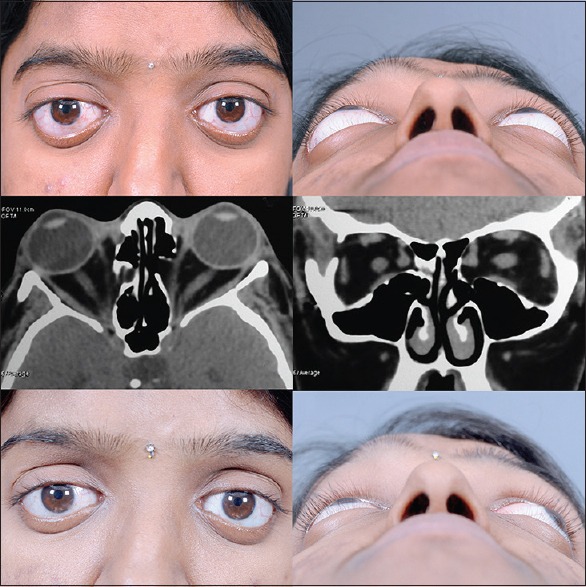
Craniosynostosis (top left and right) as the nonthyroid indication for orbital decompression. The shallow depth of the orbit and hypoplastic maxilla (middle left and right) allows limited decompression effect (bottom left and right) but nevertheless is effective in preventing globe luxation and exposure symptoms
Customized orbital decompression
Often, decompression surgery cannot be exercised as a one-size-fits-all approach, and customization is required based on the findings. For example, a case with unilateral hyperglobus [Fig. 6] can be treated with deliberate orbital floor decompression, to symmetrize globe position. Similarly, floor decompression should be included in the surgical plan of patients with the excessive inferior scleral show [Fig. 6]. Patients with only proptosis and no eyelid retraction can undergo decompression via the preferential conjunctival incision. Those with excessive upper eyelid retraction and proptosis can undergo deep lateral wall decompression via an eyelid crease incision, to simultaneously recess the levator. Similarly, patients who scar badly or have a prior history of hyperpigmentation of scars (Fitzpatrick Grades IV-VI) can undergo trans-conjunctival approach. Patients with upper eyelid fullness due to prolapsed lacrimal gland would need bone removal from superolateral orbit (fossa for lacrimal gland).
Figure 6.
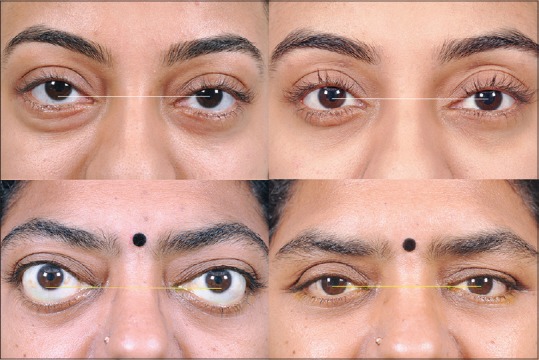
Customized orbital decompression involves intelligent choice of walls in order to better address the cosmetic deformity. This young lady with mild right proptosis and hyperglobus (top left) deliberately underwent orbital floor decompression so as to allow a “drop” in eyeball position (top right). A case of bilateral moderate proptosis with severe lower eyelid retraction (bottom left) can undergo an “unbalanced” medial and floor decompression to allow globe descent and simultaneous correction of sclera show
Our preferred choice is to opt for inferior trans-conjunctival “fat” decompression for mild cases (2 mm or less). For 2–4 mm reduction in proptosis, we prefer to add medial wall decompression as the first choice due to the speed and ease of surgical approach. For moderate reduction of 4–6 mm proptosis, either a deep lateral wall decompression or a balanced two wall decompression is preferred. We often choose the “unbalanced” two wall decompression, leaving the inferomedial orbital strut intact. For extreme requirements of correction 6–8 mm or more, we routinely perform a three wall decompression with a combination of eyelid crease and trans-conjunctival approach.
Safe orbital decompression
Thought orbital decompression is fairly safe in the hands of experienced orbital surgeons, the potential complications include infra-orbital anesthesia due to nerve injury, new-onset strabismus, optic nerve injury, and sometimes cerebrospinal fluid leak.[6,9] Equipment such as the piezoelectric technology that emulsifies bone without harming the soft tissue is of great help in preventing inadvertent soft tissue trauma.[21] Bone removal around the infra-orbital nerve is safe and predictable with piezoelectric technology and allows maximal bony decompression in extreme cases. Similarly, the orbital navigation technology provides exact intra-operative localization, and safety.[27] While performing bony decompression in critical areas such as orbital apex, and medial wall, navigation technology is of prime importance to deliver a safe surgical result [Fig. 7].
Figure 7.
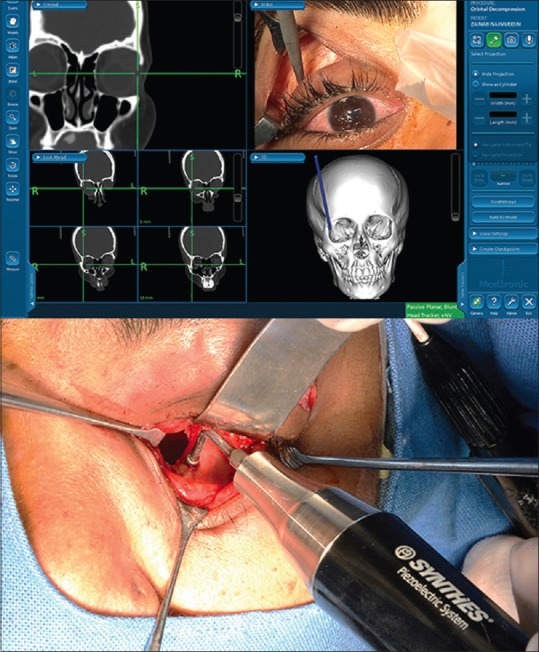
Orbital decompression surgery can be made safe by employing piezoelectric technology with preferentially dissolves bone, without damaging soft tissue. Navigation guided surgery helps accurate and safe bony decompression without potential sight threatening complications
The second stage: Strabismus surgery
Strabismus surgery is usually carried out in the inactive phase of TED, following orbital decompression surgery. However, decompression may be skipped in some patients due to the presence of minimal proptosis, or because the patient is concerned about the functional aspect of diplopia rather than esthetics. When strabismus surgery is required, the most common surgery performed is a recession of tight muscles (often using adjustable sutures) so as to maximize the area of binocular single vision [Fig. 8]. It is important to explain it to the patient, that binocular single vision may not be achievable in all directions of gaze. Patients should, therefore, be encouraged to have realistic expectations.[28] Strabismus surgery has improved over time, with a better understanding of the anatomy and physiology of the orbital connective tissue system and pulleys.[29] The oculoplastic surgeon must be aware of lower eyelid retraction induced by the recession of the inferior rectus muscle, and treat it either in the same sitting or subsequently.[30]
Figure 8.
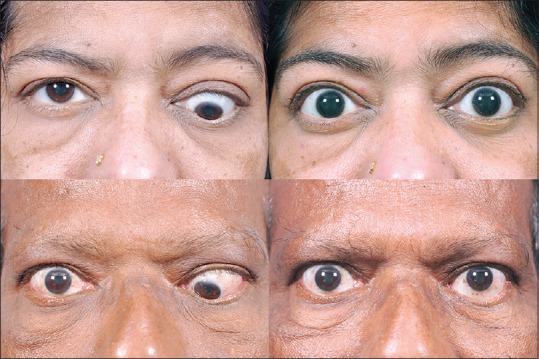
Two cases of TED with left tight inferior rectus muscle leading to severe hypotropia. Strabismus surgery (usually inferior rectus recession) results in good correction of the strabismus. Eyelid correction if required, can be preformed thereafter
The third stage: Eyelid abnormality
The advances in eyelid surgery in TED have led to the management of eyelid retraction with smaller incisions, transconjunctival approach, and fat grafting.[31,32,33] Nonsurgical treatment with hyaluronic acid gel may be useful for temporary improvement in some cases.[34] Overall, the surgical correction of eyelid retraction gives good results [Fig. 9], although there is still unpredictability and sometimes more than one stage of surgery is required.[33] Our preferred approach is trans-cutaneous levator recession (full thickness blepharotomy), due to the ease and clarity of anatomical exposure, and better eyelid crease symmetry.
Figure 9.
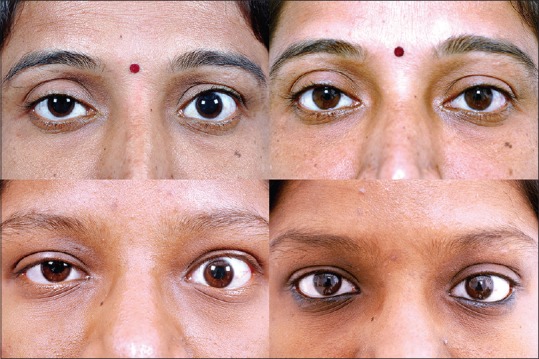
Upper eyelid retraction is often the only sign of thyroid eye disease, with no other clinical indicators. In such “silent presenters,” levator recession surgery can be performed to achieve cosmesis
The fourth stage
The fourth stage of surgical rehabilitation involves procedures to address the aesthetic concerns of periocular sequelae of GD. These include deep glabellar folds, excess periorbital fat bags, and mid-face descent leading to periorbital hollows. Botulinum toxin injection can be given into the corrugators supercilii muscles to relax the dynamic glabellar lines. Usually, a decompression surgery eliminates the lower eyelid fat bags. However, there may be cases of mild proptosis where a decompression may have been deferred, and the prolapsed fat is cosmetically bothersome. A conservative blepharoplasty or a hyaluronic acid filler injection into the tear trough improves the esthetic appearance [Fig. 10].
Figure 10.
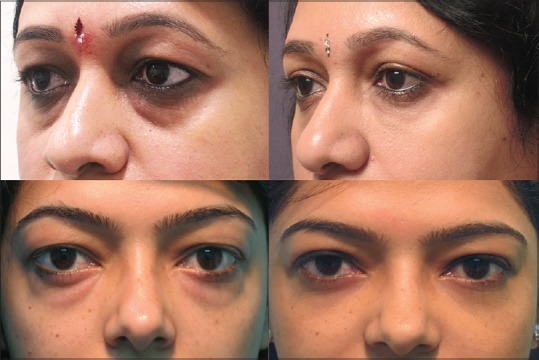
Under eye tear trough deformity in a mild case of thyroid eye disease camouflaged by hyaluronic acid filler injection, and conservative upper eyelid blepharoplasty (top left and right). Lower eyelid fat bags can be treated with trans-conjunctival blepharoplasty (bottom left and right)
Summary
The techniques for the surgical rehabilitation of patients with Graves’ orbitopathy continue to improve. We have been able to decrease the incidence of some of the worst complications-particularly, new-onset double vision. We can accomplish surgical rehabilitation through smaller incisions and less invasive procedures that hasten postoperative recovery. Less invasive techniques have expanded the indications for decompression, allowing us to operate lesser degrees of proptosis for cosmetic reasons. Despite these advances, we cannot avoid the fibrotic and structural changes that can be camouflaged and compensated, but not truly cured, by surgery. While we continue to evolve the surgical rehabilitation, we eagerly await path-breaking advances in immunotherapy, which could prevent the fibrotic changes that are currently the hallmark of TED.[35]
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Naik VM, Naik MN, Goldberg RA, Smith TJ, Douglas RS. Immunopathogenesis of thyroid eye disease: Emerging paradigms. Surv Ophthalmol. 2010;55:215–26. doi: 10.1016/j.survophthal.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Douglas RS, Goldberg RA, Smith TJ. A symposium on thyroid-associated ophthalmopathy, also known as Graves’ orbitopathy at the Jules Stein Eye Institute at the University of California, Los Angeles. Thyroid. 2008;18:931. doi: 10.1089/thy.2008.0016. [DOI] [PubMed] [Google Scholar]
- 3.Shorr N, Seiff SR. The four stages of surgical rehabilitation of the patient with dysthyroid ophthalmopathy. Ophthalmology. 1986;93:476–83. doi: 10.1016/s0161-6420(86)33712-6. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg RA. The evolving paradigm of orbital decompression surgery. Arch Ophthalmol. 1998;116:95–6. doi: 10.1001/archopht.116.1.95. [DOI] [PubMed] [Google Scholar]
- 5.Lyons CJ, Rootman J. Orbital decompression for disfiguring exophthalmos in thyroid orbitopathy. Ophthalmology. 1994;101:223–30. doi: 10.1016/s0161-6420(94)31343-1. [DOI] [PubMed] [Google Scholar]
- 6.Fatourechi V, Garrity JA, Bartley GB, Bergstralh EJ, DeSanto LW, Gorman CA. Graves ophthalmopathy. Results of transantral orbital decompression performed primarily for cosmetic indications. Ophthalmology. 1994;101:938–42. [PubMed] [Google Scholar]
- 7.Neigel JM, Rootman J, Belkin RI, Nugent RA, Drance SM, Beattie CW, et al. Dysthyroid optic neuropathy. The crowded orbital apex syndrome. Ophthalmology. 1988;95:1515–21. doi: 10.1016/s0161-6420(88)32978-7. [DOI] [PubMed] [Google Scholar]
- 8.Ben Simon GJ, Syed HM, Douglas R, Schwartz R, Goldberg RA, McCann JD. Clinical manifestations and treatment outcome of optic neuropathy in thyroid-related orbitopathy. Ophthalmic Surg Lasers Imaging. 2006;37:284–90. doi: 10.3928/15428877-20060701-04. [DOI] [PubMed] [Google Scholar]
- 9.Shorr N, Neuhaus RW, Baylis HI. Ocular motility problems after orbital decompression for dysthyroid ophthalmopathy. Ophthalmology. 1982;89:323–8. doi: 10.1016/s0161-6420(82)34793-4. [DOI] [PubMed] [Google Scholar]
- 10.Ben Simon GJ, Syed HM, Lee S, Wang DY, Schwarcz RM, McCann JD, et al. Strabismus after deep lateral wall orbital decompression in thyroid-related orbitopathy patients using automated hess screen. Ophthalmology. 2006;113:1050–5. doi: 10.1016/j.ophtha.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Cruz AA, Ribeiro SF, Garcia DM, Akaishi PM, Pinto CT. Graves upper eyelid retraction. Surv Ophthalmol. 2013;58:63–76. doi: 10.1016/j.survophthal.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Gandhi RA, Nair AG. Role of imaging in the management of neuro-ophthalmic disorders. Indian J Ophthalmol. 2011;59:111–6. doi: 10.4103/0301-4738.77015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrity JA, Fatourechi V, Bergstralh EJ, Bartley GB, Beatty CW, DeSanto LW, et al. Results of transantral orbital decompression in 428 patients with severe Graves’ ophthalmopathy. Am J Ophthalmol. 1993;116:533–47. doi: 10.1016/s0002-9394(14)73194-0. [DOI] [PubMed] [Google Scholar]
- 14.Lund VJ, Larkin G, Fells P, Adams G. Orbital decompression for thyroid eye disease: A comparison of external and endoscopic techniques. J Laryngol Otol. 1997;111:1051–5. doi: 10.1017/s0022215100139313. [DOI] [PubMed] [Google Scholar]
- 15.Sasim IV, de Graaf ME, Berendschot TT, Kalmann R, van Isterdael C, Mourits MP. Coronal or swinging eyelid decompression for patients with disfiguring proptosis in graves’ orbitopathy?. Comparison of results in one center. Ophthalmology. 2005;112:1310–5. doi: 10.1016/j.ophtha.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 16.Kazim M, Trokel SL, Acaroglu G, Elliott A. Reversal of dysthyroid optic neuropathy following orbital fat decompression. Br J Ophthalmol. 2000;84:600–5. doi: 10.1136/bjo.84.6.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg RA, Kim A, Kerivan KM. The lacrimal keyhole, orbital door jamb, and basin of the inferior orbital fissure. Three areas of deep bone in the lateral orbit. Arch Ophthalmol. 2000;116:1618–24. doi: 10.1001/archopht.116.12.1618. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg RA, Perry JD, Hortaleza V, Tong JT. Strabismus after balanced medial plus lateral wall versus lateral wall only orbital decompression for dysthyroid orbitopathy. Ophthal Plast Reconstr Surg. 2000;16:271–7. doi: 10.1097/00002341-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Shorr N, Baylis HI, Goldberg RA, Perry JD. Transcaruncular approach to the medial orbit and orbital apex. Ophthalmology. 2000;107:1459–63. doi: 10.1016/s0161-6420(00)00241-4. [DOI] [PubMed] [Google Scholar]
- 20.Liao SL, Chang TC, Lin LL. Transcaruncular orbital decompression: An alternate procedure for Graves ophthalmopathy with compressive optic neuropathy. Am J Ophthalmol. 2006;141:810–8. doi: 10.1016/j.ajo.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 21.De Castro DK, Fay A, Wladis EJ, Nguyen J, Osaki T, Metson R, et al. Self-irrigating piezoelectric device in orbital surgery. Ophthal Plast Reconstr Surg. 2013;29:118–22. doi: 10.1097/IOP.0b013e31827f59d4. [DOI] [PubMed] [Google Scholar]
- 22.Shepard KG, Levin PS, Terris DJ. Balanced orbital decompression for Graves’ ophthalmopathy. Laryngoscope. 1998;108(11 Pt 1):1648–53. doi: 10.1097/00005537-199811000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Unal M, Leri F, Konuk O, Hasanreisoglu B. Balanced orbital decompression combined with fat removal in Graves ophthalmopathy: Do we really need to remove the third wall? Ophthal Plast Reconstr Surg. 2003;19:112–8. doi: 10.1097/01.IOP.0000056145.71641.F5. [DOI] [PubMed] [Google Scholar]
- 24.Kim JW, Goldberg RA, Shorr N. The inferomedial orbital strut: An anatomic and radiographic study. Ophthal Plast Reconstr Surg. 2002;18:355–64. doi: 10.1097/00002341-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Bleier BS, Lefebvre DR, Freitag SK. Endoscopic orbital floor decompression with preservation of the inferomedial strut. Int Forum Allergy Rhinol. 2014;4:82–4. doi: 10.1002/alr.21231. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg RA, Hwang MM, Garbutt MV, Shorr N. Orbital decompression for non – Graves’ orbitopathy: A consideration of extended indications for decompression. Ophthal Plast Reconstr Surg. 1995;11:245–52. [PubMed] [Google Scholar]
- 27.Wu CY, Kahana A. Stereotactic navigation with a registration mask in orbital decompression surgery: Preliminary results. Ophthal Plast Reconstr Surg. 2015 doi: 10.1097/IOP.0000000000000369. DOI: 10.1097/IOP.0000000000000369. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Harrad R. Management of strabismus in thyroid eye disease. Eye (Lond) 2015;29:234–7. doi: 10.1038/eye.2014.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demer JL. Evidence supporting extraocular muscle pulleys: Refuting the platygean view of extraocular muscle mechanics. J Pediatr Ophthalmol Strabismus. 2006;43:296–305. doi: 10.3928/01913913-20060901-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holds JB, Anderson RL, Thiese SM. Lower eyelid retraction: A minimal incision surgical approach to retractor lysis. Ophthalmic Surg. 1990;21:767–71. [PubMed] [Google Scholar]
- 31.Looi AL, Sharma B, Dolman PJ. A modified posterior approach for upper eyelid retraction. Ophthal Plast Reconstr Surg. 2006;22:434–7. doi: 10.1097/01.iop.0000240821.74843.7c. [DOI] [PubMed] [Google Scholar]
- 32.Elner VM, Hassan AS, Frueh BR. Graded full-thickness anterior blepharotomy for upper eyelid retraction. Arch Ophthalmol. 2004;122:55–60. doi: 10.1001/archopht.122.1.55. [DOI] [PubMed] [Google Scholar]
- 33.Ben Simon GJ, Mansury AM, Schwarcz RM, Modjtahedi S, McCann JD, Goldberg RA. Transconjunctival Müller muscle recession with levator disinsertion for correction of eyelid retraction associated with thyroid-related orbitopathy. Am J Ophthalmol. 2005;140:94–9. doi: 10.1016/j.ajo.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg RA, Lee S, Jayasundera T, Tsirbas A, Douglas RS, McCann JD. Treatment of lower eyelid retraction by expansion of the lower eyelid with hyaluronic Acid gel. Ophthal Plast Reconstr Surg. 2007;23:343–8. doi: 10.1097/IOP.0b013e318137aa41. [DOI] [PubMed] [Google Scholar]
- 35.Ahn ES, Subramanian PS. Treatment modalities of thyroid related orbitopathy. Indian J Ophthalmol. 2014;62:999–1002. doi: 10.4103/0301-4738.145994. [DOI] [PMC free article] [PubMed] [Google Scholar]


